Abstract
The acute exacerbations of COPD (AECOPD) are one of the main causes of hospitalization and morbimortality in the adult population. There are not many tools available to predict the clinical course of these patients during exacerbations. Our goal was to estimate the clinical utility of C Reactive Protein (CRP), Mean Platelet Volume (MPV), eosinophil count and neutrophil/lymphocyte ratio (NLR) as in-hospital prognostic factors in patients with AECOPD. A prospective cohort study was conducted in patients who consulted three reference hospitals in the city of Medellín for AECOPD and who required hospitalization between 2017 and 2020. A multivariate analysis was performed to estimate the effect of biomarkers in the two primary outcomes: the composite outcome of in-hospital death and/or admission to the ICU and hospital length-of-stay. A total of 610 patients with a median age of 74 years were included; 15% were admitted to the ICU and 3.9% died in the hospital. In the multivariate analysis adjusted for confounding variables, the only marker significantly associated with the risk of dying or being admitted to the ICU was the NLR > 5 (OR: 3; CI95%: 1.5; 6). Similarly, the NLR > 5 was also associated to a lower probability of being discharged alive from the institution (SHR: 0.73; CI95%: 0.57; 0.94) and, therefore, a longer hospital stay. It was found that a neutrophil/lymphocyte ratio greater than 5 is a strong predictor of mortality or ICU admissions and a longer hospital stay in patients hospitalized with AECOPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death in the world, after ischemic heart disease and cerebrovascular disease. Most of them occur in middle- and low-income countries and, contrary to what happens with the cardiovascular disease, deaths from COPD are raising, possibly attributed to the increase in the life expectancy of the population and exposures to agents related to the disease. In our country, COPD is a prevalent disease, with Medellín, Antioquia, being the city with the highest prevalence compared to others in the country [Citation1].
Exacerbations are the main cause of disease morbidity and mortality. In the last decade in United States it is estimated that there are around 600,000 hospitalizations per year for COPD, which also translates into a high consumption of economic resources in the health system [Citation2]. To date, there are not many tools available to predict the clinical behavior of these patients during exacerbations, and although some prediction scales have been designed in the United Kingdom, these have not been validated in our setting [Citation3].
In recent years, biomarkers – such as the neutrophil/lymphocyte ratio, CRP, the eosinophil count, mean platelet volume (MPV) and others, have been increasingly studied as surrogate markers that reflect the degree of inflammation of patients with multiple diseases [Citation4–9]. Considering that inflammation is a very important component in AECOPD and that biomarkers are low-cost and easily accessible tools, it is interesting to know their usefulness in predicting clinically relevant outcomes. In a study carried out in Iran, it was found that a neutrophil/lymphocyte ratio greater than or equal to four (4) increased the risk of dying in hospital (Odds Ratio [OR]: 3.58; CI: 1.69 − 7.69) [Citation10]. In other descriptive studies, it was found that a low eosinophil count was associated with higher ICU mortality in these patients [Citation11].
For this reason, our goal was to establish the association between specific values in biomarkers and clinically relevant adverse outcomes in patients hospitalized with AECOPD.
Methodology
Design
Prospective cohort study with in-hospital follow-up. This study is an analysis derived from the research cohort “Radiological findings in patients with COPD exacerbation as determinants or morbidity and mortality. A cohort study,” coordinated by the University of Antioquia Clinical Epidemiology Academic Group (GRAEPIC, in Spanish).
Setting
The study recruited patients admitted between November 1, 2017 and February 15, 2020, in the Emergency Services of Hospital San Vicente Fundación, Hospital Pablo Tobón Uribe and IPS Universitaria – Clínica León XIII in Medellín, Colombia. According to the original protocol, after confirming the admission of each patient with a diagnosis of AECOPD, an interview was conducted and they were asked to sign the informed consent for their participation. Subsequently, a case report form was filled out containing basic information on their illness, vital signs upon admission, comorbidities, risk factors, treatments received and basic laboratory data. Finally, a daily follow-up of their medical history was carried out until the day of discharge or death. The study was approved by the Ethics Committee of the institution (Comité de Bioética, Instituto de Investigaciones Médicas, Universidad de Antioquia) and the Ethic Committee of the participant Hospitals approved this research.
Participants
Patients with a known diagnosis of COPD (given by clinical and epidemiological characteristics and/or spirometry showing a forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) of <70%) who were admitted due to its exacerbation, were included. The diagnosis of AECOPD was made by the attending physician and was confirmed independently by the researchers, established by the presence of at least two of the following criteria: increased dyspnea, increased sputum volume or increased purulence that is beyond daily variability. The episode did not have another cause, such as asthma, pulmonary edema, pneumonia or others (diagnoses rules out by the medical history, the initial chest X-Ray and the laboratory data) that explained the symptoms. Additionally, were excluded those who had previously participated in the study, those who had a non-resuscitation order, or those with incomplete data in the clinical history, which prevents evaluating the outcome.
Variables
As exposure variables, the following were included in the analysis: mean platelet volume (MPV: femtoliters), C-reactive protein (CRP: milligrams/milliliter), eosinophil count (cells/microliter) and the neutrophil/lymphocyte ration (NLR: index). According to the literature, the factors considered as possible confounders were age, comorbidity according to the Charlson Index, severity of dyspnea according to the Medical Research Council (mMRC), blood pH values, hemodynamic instability defined as mean arterial pressure values less than 60 mmHg, alteration of consciousness, alterations in oxygenation according to PaFiO2 values, history of exacerbations in the last year, and disease severity according to the need for antibiotics and/or steroids. The outcome variables were the composite outcome of hospital mortality or admission to the ICU, and hospital length-of-stay (LOS).
Data source and measurement
The variables were taken from the initial clinical record and during the period of hospital stay. The exposure variables were taken from the initial laboratory data, which – in the three institutions – were taken with state-of-the-art technology. Laboratory tests were taken before the application of any medication.
Biases
When there were doubts about the inclusion/exclusion criteria in any participant candidate to enter the study, the case was reviewed and jointly analyzed by the researchers (Internists, Internal Medicine residents, Radiology students), who determined the inclusion in or exclusion from the study. Subsequently, each of the data collected on the forms was reviewed. Every two weeks, an administrative review of the database was conducted to reveal extreme values, inconsistencies or findings that suggested an error in the collection.
Sample size
The sample size and the period of recruitment were dependent on the considerations of the original cohort, so the recruitment was carried out conveniently, with a total of 610 patients with a complete follow-up.
Quantitative variables
The MPV was analyzed with its value in integers. For dichotomous analysis, the value >10 femtoliters [Citation12] was taken. The CRP was analyzed in whole numbers; for the dichotomous analysis, the value >2 mg/dL was taken. The absolute eosinophil count was measured in cells per microliter; for the dichotomous analysis, the value >150 cel/µL [Citation13–16] was taken. The neutrophil/lymphocyte ratio (NLR) was taken based on the division between the absolute count of neutrophils and that of lymphocytes; for the dichotomous analysis, the value > 5 [Citation17–22] was taken.
The cutoff values for MPV and CRP were selected from normal values in a healthy population and extrapolated to the disease in study [Citation23]. The eosinophil count was selected from previous exacerbated COPD studies and the alteration of which is related to a good or bad prognosis [Citation24, Citation25]. Furthermore, the neutrophil/lymphocyte ratio was selected taking into account clinical criteria based on some references in the literature [Citation24, Citation26].
Statistical methods
Continuous variables were expressed as mean or median and the respective standard deviation (SD) or interquartile ranges (IQR), which were compared by means of the Mann-Whitney U test; the categorical variables were presented as proportions, compared by Pearson's Chi square test or Fisher's exact test. To estimate the association of the different biomarkers with composite outcome (in-hospital mortality and ICU admission), univariate logistic-regression models were made with each of the exposures (biomarkers), then a multivariate analysis was performed adjusted for potential confounding variables. Finally, a sensitivity analysis was performed with the imputation of the missing data (pH and PaFIO2 values, using 12 independent variables and the outcome variables with a Monte Carlo Markov Chain (MCMC) model with 100 iterations) [Citation27]. The association measure used for composite outcome was the OR, with its respective 95% confidence interval (95%CI). For the association with LOS, since hospital mortality is a competing risk, it was used a Cox’s competitive risks regression model to determine the hazard of the sub-distribution of being discharged alive during hospitalization. Consequently, a Sub-Hazard Ratio (SHR), with its respective 95% CI was used.
Results
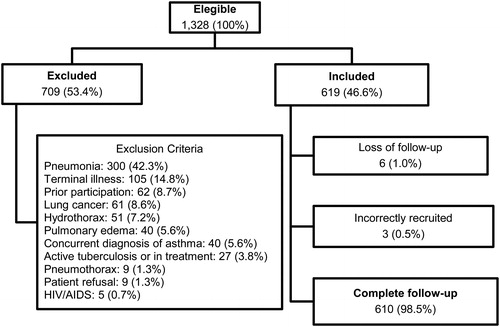
Of the 1,328 eligible patients, 709 (53.4%) were excluded; of the 619 patients included, an administrative transfer was made in six (1.0%) and three (0.5%) were incorrectly recruited, for a final analysis in 610 participants (). The median age was 74 years and 319 (52.3%) were women (). describes the vital signs on admission and the clinical characteristics of the patients, including the treatment received.
Table 1. Demographic characteristics and antecedents according to prognosis.
Table 2. Clinical and care characteristics according to prognosis.
There were missing values of the mean platelet volume (MPV) in five patients; neutrophil/lymphocyte ratio (NLR) in six patients; and C-reactive protein (CRP) in 57 patients. A total of 542 patients had complete data for full analysis and the sensitivity analysis with multiple imputation was performed with the 610 recruited patients.
Of the total cohort, 92 patients (15%) were admitted to the ICU and 24 (3.9%) died in the hospital, for a total of 116 patients (19%) with the composite outcome. The median hospital stay in patients who died or entered the ICU was 10 days (IQR: 1 to 17 days); in survivors, it was six days (IQR: 3 to 8 days).
Main results
In the univariate analyses, all biomarkers showed a significant association with the composite outcome, both in the form of continuous and dichotomous variables. However, in the multivariate analysis after adjustment for confounding variables, the only marker significantly associated with the risk of dying or being admitted to the ICU was NLR > 5 (OR = 3; CI 95% = 1.5; 6) (). This result was not modified with the sensitivity analysis.
Table 3. Logistic regression for mortality or ICU admission of the different biomarkers.
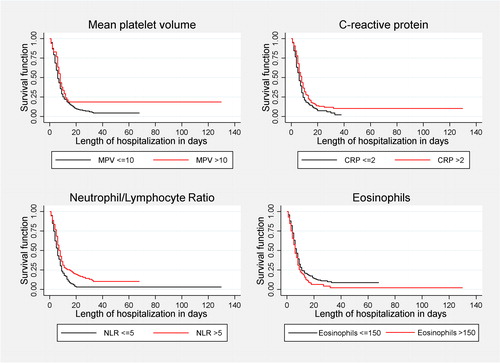
Univariate analyses showed all biomarkers had significant association with the outcome of being discharged alive from the hospital. However, after multivariate analysis and adjustment for confounding variables, the only marker significantly associated with this outcome was NLR > 5 (SHR = 0.73; CI 95% = 0.57; 0.94). In other words, NLR > 5 decreases the “risk” of being discharged alive from the hospital by 27% and, therefore, increases the relative length of hospital stay approximately 37%, compared to patients with an index ≤ 5 (; ).
Table 4. Cox regression for hospital stay with competing risk of inpatient death.
Discussion
Our hypothesis about several biomarkers as prognostic factors is AECOPD could be confirmed only for the neutrophil/lymphocyte ratio, since for the other biomarkers the results were not statistically significant. The neutrophil/lymphocyte ratio is a biomarker that offers more information than each one of its variables analyzed separately: through neutrophilia, an inflammatory response associated with greater tissue destruction is expressed due to the alteration of the protease/antiprotease balance, as well as the response to bacterial agents; while lymphopenia expresses a decrease in the immune response produced by a greater degree of inflammation, the presence of malnutrition or a poor general condition [Citation10, Citation28]. Therefore, a high neutrophil/lymphocyte ratio reflects high inflammation and less immunity, which can lead to worse clinical outcomes. This biomarker has been studied in multiple diseases due to its easy availability and low cost and its first use was in malignant diseases [Citation29]; however, for more than a decade, the search for it was begun in other conditions, such as autoimmune, cardiovascular and infectious diseases [Citation9, Citation26, Citation30, Citation31]. The increase in neutrophils and the decrease in lymphocytes in the submucosa of the bronchial epithelium, are known in patients with a greater severity of COPD [Citation32]. It has also been found that an increase in the neutrophil/lymphocyte ratio is related to an Increase in the risk of exacerbation and hospitalization due to respiratory causes [Citation33]. In accordance with our findings – where a neutrophil/lymphocyte ratio greater than five was associated with a three-fold increase in the risk of hospital mortality or admission to the ICU, three retrospective cohorts carried out in Iran and China [Citation10, Citation34, Citation35] found that an elevated neutrophil/lymphocyte ratio was associated with a 2- to 6.2-fold increase in hospital and short-term mortality. One study in Spain published in the Journal found mean NLR 3,8 abnormally elevated in patients with AECOPD [Citation36]. Additionally, a case-control study of 368 patients performed in Shanghai [Citation37] found an association of NLR and higher mortality (OR 3.58; 95%CI: 2.28-5.24). Similarly, other reports [Citation38, Citation39] also found a relationship between an elevation of the neutrophil/lymphocyte ratio and a longer hospital stay.
On the other hand, we found a trend toward lower hospital mortality or admission to the ICU (OR: 0.5; 95% CI: 0.3-1.1), as well as a shorter hospital stay (SHR: 1.21; 95% CI: 0.95- 1.53) in patients with an eosinophil count greater than 150/µL, which were not statistically significant. Results reported in cohort studies in Turkey [Citation8, Citation11], China [Citation14], Germany [Citation40] and in Australia [Citation41], shows that high eosinophil count in the setting of an AECOPD is associated with a shorter hospital LOS[Citation8, Citation11, Citation32–34, , ] and other favorable outcomes, such as a lower requirement for noninvasive mechanical ventilation [Citation14] and fewer hospital readmissions at 6 months [Citation8]. Turkey cohort study was the only one that showed lower hospital mortality (12.9% vs. 24.9%; p-value 0.034) in eosinophilic group (peripheral blood eosinophil higher than 2%) [Citation11], but they analyzed only patients admitted to ICU. The German and the Australian cohorts did not show decrease in survival neither in re-hospitalization, but showed decrease in hospital LOS. In the German cohort, median LOS in days was 9.18 (95% CI: 5- 14.9) for patients with eosinophilic count < 100/μl vs. 7 days (95% CI: 5- 12) for eosinophilic count 100–300/μl (p < 0.01). In a similar way, the Australian cohort showed a decrease in LOS and a more frequent hospital discharge within five days (89% vs 42%; p < 0.001) in the patients with eosinophil count > 150/μL.
To date, there are no studies in the literature on the behavior of MPV in AECOPD and relevant clinical outcomes. Erden et al [Citation42] found in a case-control study higher MPV values in AECOPD, compared to stable COPD and healthy individuals. We found that an MPV greater than 10 femtoliters had a tendency to increase the probability of dying or requiring critical care (OR: 1.9; 95% CI: 0.9-4.2), and a longer hospital stay (SHR: 0.72; 95% CI: 0.51-1.01), although ultimately it was not statistically significant. It is hypothesized that marked inflammation and hypoxia on the bone marrow could induce platelets of greater volume, which would associate an increase in MPV with worse outcomes [Citation42].
The advantages of our study lie in its prospective and multi-center nature, being the first in our setting with these characteristics. Additionally, clinically relevant outcomes in daily clinical practice were evaluated. On the other hand, as limitations, we are aware that in 68 patients there were missing data; however, the results were not modified in the sensitivity analysis. In addition to the lack of calculation of sample size and because this was a study nested in a previous cohort, the sample size may underpowered to obtain a statistically significant association with other biomarkers, data that must be confirmed in studies with a larger population. Other weaknesses to be taken into account are the lack of medium- and long-term follow-up of the patients after discharge, as well as the recruitment of patients without verified spirometry data. In this study setting, and consistent with others and with our health system, COPD diagnosis was made by previous clinical records, as spirometry values are usually not available in the Emergency Department [Citation1, Citation8, Citation43, , ].
Conclusion
A neutrophil/lymphocyte ratio greater than 5 is a strong predictor of hospital mortality and/or ICU admission, as well as of a longer hospital stay in patients with AECOPD. Additionally, as exploratory results, the mean platelet volume greater than 10 and the eosinophil count <150 could have a similar behavior, but we were unable to demonstrate statistical significance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Caballero A, Torres-Duque CA, Jaramillo C, et al. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study). Chest. 2008;133(2):343–349. DOI:10.1378/chest.07-1361
- Snow V, Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine, Lascher S, Mottur-Pilson C. Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest P, the American College of Physicians-American Society of Internal M. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2001;134(7):595–599. DOI:10.7326/0003-4819-134-7-200104030-00015
- Echevarria C, Steer J, Heslop-Marshall K, et al. Validation of the DECAF score to predict hospital mortality in acute exacerbations of COPD. Thorax. 2016;71(2):133–140. DOI:10.1136/thoraxjnl-2015-207775
- Asiimwe AC, Brims FJ, Andrews NP, et al. Routine laboratory tests can predict in-hospital mortality in acute exacerbations of COPD. Lung. 2011;189(3):225–232. DOI:10.1007/s00408-011-9298-z
- Patel AR, Hurst JR, Wedzicha JA. The potential value of biomarkers in diagnosis and staging of COPD and exacerbations. Semin Respir Crit Care Med. 2010;31(3):267–275. DOI:10.1055/s-0030-1254067
- Kim CH, Kim SJ, Lee MJ, et al. An increase in mean platelet volume from baseline is associated with mortality in patients with severe sepsis or septic shock. PLoS One. 2015;10(3):e0119437. DOI:10.1371/journal.pone.0119437
- Ulasli SS, Ozyurek BA, Yilmaz EB, et al. Mean platelet volume as an inflammatory marker in acute exacerbation of chronic obstructive pulmonary disease. Pol Arch Med Wewn. 2012;122(6):284–290. DOI:10.20452/pamw.1284
- Duman D, Aksoy E, Agca MC, et al. The utility of inflammatory markers to predict readmissions and mortality in COPD cases with or without eosinophilia. Int J Chron Obstruct Pulmon Dis. 2015;10:2469–2478. DOI:10.2147/COPD.S90330
- Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. 2016;14(5):573–577. DOI:10.1586/14779072.2016.1154788
- Rahimirad S, Ghaffary MR, Rahimirad MH, et al. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute exacerbation of chronic obstructive pulmonary disease. Tuberk Toraks. 2017;64(1):25–31. DOI:10.5578/tt.27626
- Salturk C, Karakurt Z, Adiguzel N, et al. Does eosinophilic COPD exacerbation have a better patient outcome than non-eosinophilic in the intensive care unit? Int J Chron Obstruct Pulmon Dis. 2015;10:1837–1846. DOI:10.2147/COPD.S88058
- Steiropoulos P, Papanas N, Nena E, et al. Mean platelet volume and platelet distribution width in patients with chronic obstructive pulmonary disease: the role of comorbidities. Angiology. 2013;64(7):535–539. DOI:10.1177/0003319712461436
- Wu CW, Lan CC, Hsieh PC, et al. Role of peripheral eosinophilia in acute exacerbation of chronic obstructive pulmonary disease. WJCC. 2020;8(13):2727–2737. DOI:10.12998/wjcc.v8.i13.2727
- Wu HX, Zhuo KQ, Cheng DY. Peripheral blood eosinophil as a biomarker in outcomes of acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:3003–3015. DOI:10.2147/COPD.S226783
- Brusselle G, Pavord ID, Landis S, et al. Blood eosinophil levels as a biomarker in COPD. Respir Med. 2018;138:21–31. DOI:10.1016/j.rmed.2018.03.016
- You Y, Shi GC. Blood eosinophils and clinical outcome of acute exacerbations of chronic obstructive pulmonary disease: A systematic review and meta-analysis. Respiration. 2021;100(3):228–237. DOI:10.1159/000510516
- Koh CH, Bhoo-Pathy N, Ng KL, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113(1):150–158. DOI:10.1038/bjc.2015.183
- Charles KA, Harris BD, Haddad CR, et al. Systemic inflammation is an independent predictive marker of clinical outcomes in mucosal squamous cell carcinoma of the head and neck in oropharyngeal and non-oropharyngeal patients. BMC Cancer. 2016;16:124. DOI:10.1186/s12885-016-2089-4
- Murphy C, Turner N, Wong HL, et al. Examining the impact of regular aspirin use and PIK3CA mutations on survival in stage 2 colon cancer. Intern Med J. 2017;47(1):88–98. DOI:10.1111/imj.13312
- Pine JK, Morris E, Hutchins GG, et al. Systemic neutrophil-to-lymphocyte ratio in colorectal cancer: the relationship to patient survival, tumour biology and local lymphocytic response to tumour. Br J Cancer. 2015;113(2):204–211. DOI:10.1038/bjc.2015.87
- Mallappa S, Sinha A, Gupta S, et al. Preoperative neutrophil to lymphocyte ratio >5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis. 2013;15(3):323–328. DOI:10.1111/codi.12008
- Hung HY, Chen JS, Yeh CY, et al. Effect of preoperative neutrophil-lymphocyte ratio on the surgical outcomes of stage II colon cancer patients who do not receive adjuvant chemotherapy. Int J Colorectal Dis. 2011;26(8):1059–1065. DOI:10.1007/s00384-011-1192-x
- Orkin S, Nathan D, Ginsburg D, et al. Nathan and Oski's Hematology and Oncology of Infancy and Childhood E-Book. Eighth edition. [Internet]. Saunders; 2014. [cited 2020 Mar 8]. available at https://books.google.com.co/books?id=gjWaBQAAQB AJ&pg=PA952&lpg=PA952&dq=normal±mean±platelet±volume± 10±femtoliters&source=bl&ots=Lpjcb1JxhW&sig=ACfU3U3LSjfkK hvvrAG-idjb2-CsIhx4qw&hl=es&sa=X&ved=2ahUKEwia9d2uwovo AhUGKa0KHXeiAsMQ6AEwEXoECAoQAQ#v=onepage&q=normal mean platelet volume 10 femtoliters&f=false.
- Kolsum U, Donaldson GC, Singh R, et al. Blood and sputum eosinophils in COPD; relationship with bacterial load. Respir Res. 2017;18(1):88. DOI:10.1186/s12931-017-0570-5
- Singh D, ECLIPSE investigators, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697–1700. DOI:10.1183/09031936.00162414
- Argeny S, Stift A, Bergmann M, et al. Prognostic value of preoperative neutrophil-to-lymphocyte ratio in Crohn's disease. Wien Klin Wochenschr. 2018;130(11-12):398–403. DOI:10.1007/s00508-018-1322-3
- Li KH. Imputation using markov chains. J Stat Comput Simul. 1988;30(1):57–79. DOI:10.1080/00949658808811085
- Paliogiannis P, Fois AG, Sotgia S, et al. Neutrophil to lymphocyte ratio and clinical outcomes in COPD: recent evidence and future perspectives. Eur Respir Rev. 2018;27(147):170113. DOI:10.1183/16000617.0113-2017
- Faria SS, Fernandes PC, Jr., Silva MJ, et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience. 2016;10:702. DOI:10.3332/ecancer.2016.702
- Curbelo J, Rajas O, Arnalich B, et al. Neutrophil count percentage and neutrophil-lymphocyte ratio as prognostic markers in patients hospitalized for community-acquired pneumonia. Arch Bronconeumol. 2019;55(9):472–477. DOI:10.1016/j.arbres.2019.02.005
- Soliman WM, Sherif NM, Ghanima IM, et al. Neutrophil to lymphocyte and platelet to lymphocyte ratios in systemic lupus erythematosus: Relation with disease activity and lupus nephritis. Reumatol Clin. 2020;16(4):255–261. DOI:10.1016/j.reuma.2018.07.008
- Caramori G, Casolari P, Barczyk A, et al. COPD immunopathology. Semin Immunopathol. 2016;38(4):497–515. DOI:10.1007/s00281-016-0561-5
- Lee H, Um SJ, Kim YS, et al. Association of the Neutrophil-to-lymphocyte ratio with lung function and exacerbations in patients with chronic obstructive pulmonary disease. PLoS One. 2016;11(6):e0156511. DOI:10.1371/journal.pone.0156511
- Yao C, Liu X, Tang Z. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. COPD. 2017; 12:2285–2290. DOI:10.2147/COPD.S141760
- Liu J, Liu J, Zou Y. Relationship between neutrophil-lymphocyte ratio and short-term prognosis in the chronic obstructive pulmonary patients with acute exacerbation. Biosci Rep. 2019;39(5):BSR20190675. DOI:10.1042/BSR20190675
- Gava G, Nunez A, Esquinas C, et al. Analysis of blood biomarkers in patients with Chronic Obstructive Pulmonary Disease (COPD) and with Asthma-COPD Overlap (ACO). COPD. 2020;17(3):306–310. DOI:10.1080/15412555.2020.1761314
- Xiong W, Xu M, Zhao Y, et al. Can we predict the prognosis of COPD with a routine blood test? Int J Chron Obstruct Pulmon Dis. 2017;12:615–625. DOI:10.2147/COPD.S124041
- Chis AF, Pop CM. Correlations between neutrophil to lymphocyte ratio, blood eosinophils and clinical characteristics in chronic obstructive pulmonary disease. Med Pharm Rep. 2020;93(2):169–174. DOI:10.15386/mpr-1412
- Rajasurya V, Gudivada D. Neutrophil lymphocyte ratio in predicting outcome for hospitalized patients with acute exacerbation of COPD. Chest. 2019;156(4):A159. DOI:10.1016/j.chest.2019.08.233
- Greulich T, Tuffers J, Mager S, et al. High eosinophil blood counts are associated with a shorter length of hospital stay in exacerbated COPD patients - a retrospective analysis. Respir Res. 2020;21(1):106. DOI:10.1186/s12931-020-01365-5
- MacDonald MI, Osadnik CR, Bulfin L, et al. Low and high blood eosinophil counts as biomarkers in hospitalized acute exacerbations of COPD. Chest. 2019;156(1):92–100. DOI:10.1016/j.chest.2019.02.406
- Erden E, Dokuyucu R, Demirköse M, et al. Assessment of mean platelet volume in chronic obstructive pulmonary disease during stable period and acute exacerbation. J Clin Exp Invest. 2013;4(4):483–487. DOI:10.5799/ahinjs.01.2013.04.0329
- Gil Y, Torres C, Figueredo M, et al. Estimación de la prevalencia de EPOC en Colombia a partir del Registro Individual de Prestaciones de Servicios de Salud (RIPS). Rev Colomb Neumol. 2019;31(1):5–15. DOI:10.30789/rcneumologia.v31.n1.2019.325