Abstract
The co-occurrence of obstructive sleep apnea (OSA) and chronic obstructive pulmonary disease (COPD) in the same patient, named the overlap syndrome (OS), was first described in 1985. Although the American Thoracic Society underlined the limited knowledge of OS, stated research priorities for this condition, and recommended a “screening” strategy to identify OSA in COPD patients with chronic stable hypercapnia, research studies on OS remain scarce. This review aims to summarize the current knowledge and perspectives related to OSA in COPD patients. OS prevalence is 1.0–3.6% in the general population, 3–66% in COPD patients, and 7–55% in OSA patients. OS patients may have worse sleep quality than those with OSA or COPD alone. Scoring hypopneas may be difficult in COPD patients; desaturation episodes may have origins in these patients, namely upper airway obstruction, hypoventilation during paradoxical sleep, ventilation/perfusion mismatches, and obesity. The apnea–hypopnea index is similar in OSA and OS patients. Desaturations may be greater and more prolonged in OS patients than in patients with COPD or OSA alone. Low body mass index, hyperinflation, and less collapsible airways reduce the risk of OSA in COPD patients. OSA is a risk factor for pulmonary hypertension in COPD patients. Whether OS increases mortality and morbidity risks compared to COPD or OSA alone remains to be confirmed. No guidelines currently recommend specific approaches to the treatment of OSA in patients with COPD.
Introduction
Obstructive sleep apnea (OSA) is “a breathing disorder characterized by narrowing of the upper airway that impairs normal ventilation during sleep” [Citation1]. Chronic obstructive pulmonary disease (COPD) is “a common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitations that are due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases. The chronic airflow limitation that is characteristic of COPD is caused by a mixture of small airways disease (e.g. obstructive bronchiolitis) and parenchymal destruction (emphysema)” [Citation2]. The co-occurrence of OSA and COPD in the same patient, the overlap syndrome (OS), was first described in 1985 by D.C. Flenley [Citation3]. Since then, only a relatively small number of articles have been specifically devoted to OS (). In 2013, McNicholas et al. pointed out that sleep disorders are the forgotten dimension of COPD management [Citation4]. This remains true, although the American Thoracic Society (ATS) underlined the lack of knowledge on OS, stated research priorities for this condition, and recommended a “screening” strategy to identify OSA in COPD patients with chronic stable hypercapnia [Citation5, Citation6]. This review aims to compile this knowledge and to consider some perspectives related to OSA in COPD patients.
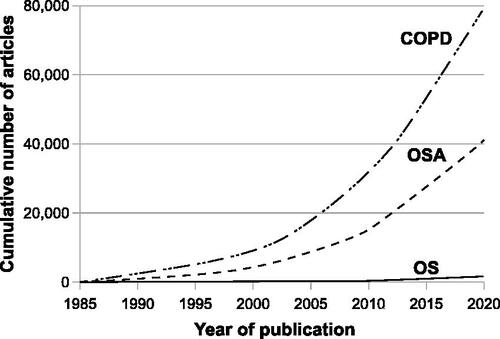
Figure 1. Cumulative number of articles published from 1985 to 2020 related to OS, OSA, and COPD. To identify the number of published articles on OS, OSA, and COPD, searches were performed in MEDLINE using the search terms “chronic obstructive pulmonary disease” AND “obstructive sleep apnea,” “obstructive sleep apnea,” and “chronic obstructive pulmonary disease,” respectively. The numbers of published articles on OS, OSA, and COPD were 7, 119, and 390, respectively in 1985; the cumulative numbers of articles on OS, OSA, and COPD from 1985 to December 2020 were 1514, 41 236, and 79 430, respectively. OS: Overlap syndrome, OSA: Obstructive sleep apnea, COPD: Chronic obstructive pulmonary disease.
Materials and methods
Literature search methodology — study biases and understanding the OS
An initial MEDLINE search was performed using the keywords “obstructive sleep apnea,” “chronic obstructive pulmonary disease,” and “overlap syndrome” for the publication years 1985 to 2020. A second MEDLINE search used additional keywords linked to each paragraph and subparagraph of this review, including “prevalence”, “clinical features”, “pulmonary function”, “diagnosis”, “guidelines”, “polysomnography”, “inflammation”, “endothelial dysfunction”, “sympathetic”, “parasympathetic”, “autonomic dysfunction”, “risk factors”, “upper airway”, “lung inflation/hyperinflation”, “arousal”, “comorbidity”, “age”, “obesity”, “smoking”, “mortality”, “cardiovascular disease”, “systemic hypertension”, “pulmonary hypertension”, “arrhythmia”, “atrial fibrillation”, “exacerbation”, “hypercapnia”, “treatment”, “positive airway pressure”, and “management”. Data from systematic reviews were highlighted.
Results
The lack of definitive answers regarding many OS-related issues is not only due to a deficit of studies but also due to study biases, including non-randomization, lack of study power, use of different definitions of respiratory events during sleep, and different methods of COPD assessment among studies. For example, the varying prevalence estimates of OSA, COPD, and OS among studies may be due to different disease definitions [Citation7]. Biases due to the presence of confounders represent a critical concern as in any clinical research study [Citation8].
Prevalence of OSA in COPD patients
The prevalence of OSA associated with excessive sleepiness in the general population is approximately 3% in women and 5% in men [Citation9–12]. The prevalence is much higher in patients with cardiovascular or metabolic disorders, i.e. 50% and 86%, respectively [Citation13–15].
The prevalence of spirometrically diagnosed COPD in 2010 was estimated at 11.7% (95% confidence interval [CI]: 8.4%–15.0%) in a systematic review of population-based studies [Citation16]; more recently, the Global Burden of Disease study found lower estimates, with a point prevalence of 3.9% worldwide [Citation17]. Differences in study methodology explain variations in prevalence estimates. In two studies of patients with cardiovascular risk factors, the prevalence of COPD was 14.5% and 27%, respectively [Citation18].
The prevalence of OS ranges from 1.0% to 3.6% in the general population, according to a systematic review [Citation7]. Several studies found a greater prevalence of OSA in patients with COPD and of COPD in patients with OSA than in the general population (from 2.9% to 65.9% and from 7.6% to 55.7%, respectively) [Citation7, Citation19, Citation20]. Conversely, among participants from the Sleep Heart Health Study, a population-based sleep cohort study, the prevalence of OSA was similar in patients with and without (predominantly mild) obstructive airway disease [Citation21]. Similarly, no association was observed between OSA and COPD in patients referred for suspected OSA [Citation22].
In sum,
In the general population, the prevalence of OSA, COPD, and OS is approximately 5%, 10%, and 2%, respectively. The prevalence of OSA and OS may be substantially higher in specific clinical settings, such as in cardiovascular patients.
Prospective comparative studies to assess the prevalence, incidence, and comorbidities of OSA in COPD patients are needed. The prevalence of OS remains to be prospectively determined in specific populations such as in patients with non-hypercapnic, GOLD stage 2-to-4 COPD and/or in COPD patients with chronic stable hypercapnia [Citation6].
Risk factors for and mechanisms of OSA in COPD patients
Non-anatomical factors contributing to OSA in COPD patients
Upper airway collapsibility and hyperinflation
The magnitude of static lung volumes’ increase and that of OSA severity seem to be inversely related. In patients (N = 53) who survived acute hypercapnic respiratory failure, the apnea–hypopnea index (AHI), representing the number of apneas and hypopneas per hour of sleep, was negatively correlated with static hyperinflation (residual volume [% predicted] to total lung capacity [% predicted] ratio; coefficient = −0.64; standard error 0.17; 95% CI −0.97 to −0.31; p < 0.001). A similar association was observed in patients with COPD only (coefficient = −0.65; standard error 0.19; 95% CI −1.03 to −0.26; p = 0.002) [Citation23]. It is of note that in this population, screening questionnaire, the STOP BANG questionnaire, did not predict OSA well [Citation23]. The link between lung hyperinflation (Box 1), emphysema severity, and OSA severity has been evaluated in smokers with obstructive airway disease (57% had OSA) [Citation24]. It was observed that the higher the gas trapping or, the more severe the emphysema, the lower the AHI. The authors suggested that gas trapping and emphysema severity contribute to the severity of OSA in COPD patients. A multicenter cross-sectional study reported that in OS patients, the more severe the airflow limitation, the lower the OSA severity (hyperinflation measurements were not reported, but patients with the lowest forced expiratory volume in the first second (FEV1) might be those with the highest hyperinflation) [Citation25]. The chronic bronchitis COPD phenotype may have a detrimental effect related to airway inflammation, mucus production, and body habitus; however, these parameters were not evaluated in the aforementioned study.
In OSA patients, the increase in lung volume is associated with a decrease in collapsibility of the upper airway (Box 2), OSA severity, and continuous positive airway pressure (CPAP) requirements [Citation26–28]. COPD patients had lower airway collapsibility, as assessed by measuring the passive critical pressure (passive Pcrit, the pharyngeal critical pressure under hypotonic conditions), than non-COPD control subjects (matched for age, sex, body mass index [BMI], and OSA). The functional residual capacity (FRC) was inversely correlated with Pcrit, i.e. the higher the FRC, the lower the Pcrit [Citation29]. The ratio of inspiratory time to respiratory cycle length (inspiratory duty cycle) increased more in COPD patients than in non-COPD control subjects. This was associated with an increased respiratory rate and a marked decrease in expiratory time (from 2.5 s to 1.5 s), both of which are the risk factors for dynamic hyperinflation [Citation29].
Respiratory drive
Respiratory centers in the brain stem command ventilation by sending neural signals. These signals are also called “output of respiratory centers” or “respiratory drive” [Citation30].
The concomitant role of respiratory drive and upper airway resistance in the development of hypoventilation from wakefulness to non-rapid eye movement (REM) sleep was studied in healthy individuals (N = 12), in patients with OSA (N = 14) and in patients with COPD (19 had COPD alone, 16 had OS) [Citation31]. The respiratory drive decreased from wakefulness to sleep in patients with COPD alone, whereas it changed only slightly in OS patients (). Non-REM sleep-related hypoventilation was associated with a reduction in neural respiratory drive in patients with COPD alone. Conversely, in patients with OS, non-REM sleep-related hypoventilation was associated with increased upper airway resistance. During sleep, the decrease in respiratory drive related to COPD could be counterbalanced in OS patients by the increase in respiratory drive associated with OSA resulting from the increased upper airway resistance [Citation31].
Figure 2. Diaphragm electromyogram (EMG) di% (left panel), ventilation (middle panel), and the VT/EMGdi (right panel) in patients with COPD alone, OS, normal subjects, and patients with OSA. EMGdi% (percentage of maximal diaphragm electromyogram) was used to assess neural respiratory drive. Upper airway resistance was inferred by the ratio of the tidal volume (VT) to EMGdi (VT/EMGdi). Ventilation decreases from wakefulness to sleep in all four groups, but the reduction is statistically significant only in patients with COPD, OS, and OSA. EMGdi decreases in patients with COPD alone but increases in patients with OSA from wakefulness to non-rapid eye movement sleep, whereas it remains the same in normal subjects and patients with OS. VT/EMGdi decreases from wakefulness to sleep in patients with OS and OSA but it changes little in normal subjects and patients with COPD alone. The decrease in ventilation is associated with a reduction of EMGdi in patients with COPD alone, whereas the reduction in ventilation is associated with decreased VT/EMGdi in patients with both OS and OSA. OSA: Obstructive sleep apnea, COPD: Chronic obstructive pulmonary disease, OS: Overlap syndrome. Adapted from [Citation31] (an open access article distributed in accordance with the Creative Commons Attribution Non Commercial [CC BY-NC 4.0] license).
![Figure 2. Diaphragm electromyogram (EMG) di% (left panel), ventilation (middle panel), and the VT/EMGdi (right panel) in patients with COPD alone, OS, normal subjects, and patients with OSA. EMGdi% (percentage of maximal diaphragm electromyogram) was used to assess neural respiratory drive. Upper airway resistance was inferred by the ratio of the tidal volume (VT) to EMGdi (VT/EMGdi). Ventilation decreases from wakefulness to sleep in all four groups, but the reduction is statistically significant only in patients with COPD, OS, and OSA. EMGdi decreases in patients with COPD alone but increases in patients with OSA from wakefulness to non-rapid eye movement sleep, whereas it remains the same in normal subjects and patients with OS. VT/EMGdi decreases from wakefulness to sleep in patients with OS and OSA but it changes little in normal subjects and patients with COPD alone. The decrease in ventilation is associated with a reduction of EMGdi in patients with COPD alone, whereas the reduction in ventilation is associated with decreased VT/EMGdi in patients with both OS and OSA. OSA: Obstructive sleep apnea, COPD: Chronic obstructive pulmonary disease, OS: Overlap syndrome. Adapted from [Citation31] (an open access article distributed in accordance with the Creative Commons Attribution Non Commercial [CC BY-NC 4.0] license).](/cms/asset/76f87762-5927-44cb-969a-2952c86d85c2/icop_a_1950663_f0002_b.jpg)
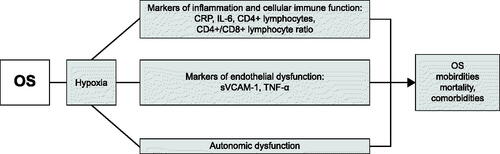
Figure 3. Markers of inflammation, endothelial dysfunction, and cellular immune function studied in OS patients. Whether these dysfunctions contribute to morbidity, mortality, and comorbidities associated with OS remain to be demonstrated. We did not speculate potential underlying mechanisms that have not been specifically elucidated in OS patients. OS: Overlap syndrome, CRP: C-reactive protein, IL-6: Interleukin-6, sVCAM-1: soluble vascular cell adhesion molecule-1, TNF-α: Tumor necrosis factor-α.
Arousal threshold
Arousal from sleep is an abrupt shift of electroencephalographic frequency that lasts for at least 3 s. Arousals may not occur. They may be initiated before, at, or after airway opening [Citation32]. They may have a beneficial effect when they contribute to reducing the work of breathing linked to obstructive apneas and hypopneas [Citation32]. They may have deleterious effects, contributing to the development of fragmented sleep, an excessive reduction in the arterial partial pressure of carbon dioxide (PaCO2), and/or a decrease in respiratory drive and upper airway muscle tone, all of which might lead to perpetuating the development of abnormal respiratory events (see reference [Citation32] for review). The arousal threshold is the level of the ventilatory drive (or respiratory effort) at which arousal from sleep occurs [Citation32]. OS patients had a higher arousal index than patients with only OSA [Citation21]. In a pilot study of patients with OS (N = 10, no comparison group), the arousal threshold was inversely linked to the functional measures of lung air trapping and static hyperinflation; the greater the hyperinflation, the lower the arousal threshold [Citation33]. A clinical score was derived to predict the arousal threshold from the data of OSA patients and healthy individuals [Citation34]. Using the same criterion in a retrospective study, a low arousal threshold was more frequently observed in OS patients with moderate/severe airflow limitation (N = 22) than in those with isolated OSA (N = 126, p = 0.016) or in those with OS with mild airflow limitation (N = 16, p = 0.026) [Citation35]. This suggests that patients with OS may have a high prevalence of a low arousal threshold. In another study, no difference was observed in the arousal threshold between a small number of patients with either OS (N = 15) or OSA (N = 15) [Citation36].
Obesity
Obesity is a cause of OSA, a confounding factor in the disease, and likely to be a consequence of OSA [Citation37]. Therefore, understanding the links among OSA, obesity, and conditions such as COPD is difficult. Patients with severe COPD (GOLD stage 3 and 4) may have a lower BMI and, therefore, be less at a risk of OSA [Citation38]. Conversely, an increased prevalence of metabolic syndrome has been reported in consecutive OS patients referred to a sleep laboratory (N = 163, 38% had metabolic syndrome) [Citation39].
Older age
In older subjects, obstructive airway disease was associated with a lower prevalence of sleep apnea (odds ratio [OR] = 0.30, 95% CI: 0.16–0.55, p = 0.0001) after adjustment for confounders (853 men, mean age 80.7 ± 4.1 years [range 73–90 years], 13.0% had obstructive airway disease, and 29.0% had sleep apnea) [Citation40]. OS was associated with greater sleep fragmentation and nocturnal oxygen desaturation compared to obstructive airway disease alone [Citation40]. A pilot prospective observational study in patients with moderate-to-severe COPD (N = 44) showed that age (as well as male sex, neck circumference, and Epworth sleepiness scale [ESS] score) did not predict OSA [Citation41].
Other potential risk factors for OSA in COPD patients
The following are the risk factors for OSA in patients with OSA alone; however, they remain to be evaluated in patients with OS. These factors include (i) the rostral fluid shift in the supine position that contributes to OSA severity [Citation42], (ii) tracheal displacement that may contribute to the maintenance of pharyngeal patency in dogs, normal subjects, and OSA patients [Citation43–45], (iii) smoking, which is associated with an increase in sleep latency and arousal frequency as well as a decrease in deep sleep stages [Citation46], although the association between smoking and OSA remains controversial [Citation47–50], and (iv) inhaled corticosteroid administration, which has been associated with a higher risk of OSA and increased upper airway collapsibility in asthma, but not in COPD, patients [Citation51, Citation52].
In sum,
In COPD patients, static hyperinflation may protect from OSA.
OSA may be a risk factor for dynamic hyperinflation in COPD patients, which may increase the work of breathing and reduce sleep efficiency.
Compared to OSA patients, OS patients may have reduced airway collapsibility, a stable respiratory drive from wakefulness to non-REM sleep, and a lower arousal threshold.
COPD patients with a lower BMI may be less at a risk of OSA.
The link between age and OS remains to be clarified.
Intermediate pathophysiological mechanisms in OS: inflammatory, endothelial, and autonomic dysfunction ()
In a cross-sectional study of COPD patients with severe comorbidities (N = 38), patients with OSA had a higher percentage of peripheral neutrophils than those with COPD in isolation [Citation53]. In a prospective observational study, patients with OS had a higher percentage of neutrophils as well as higher tumor necrosis factor (TNF)-α and interleukin (IL)-8 concentrations in the bronchoalveolar lavage fluid than patients with COPD alone [Citation54]. TNF-α concentration was correlated with the percentage of nighttime spent with O2 saturation less than 90%. Neutrophils, TNF-α, and IL-8 levels were decreased in the bronchoalveolar lavage fluid collected after 3 months of CPAP administration [Citation54]. It is suggested that OSA may increase the levels of inflammatory markers in the lungs of COPD patients, which may be improved by CPAP administration [Citation54]. TNF-α and IL-6 levels were significantly higher in OS patients than in COPD patients (however, the frequency of obese patients and of patients with metabolic syndrome were higher in the OS group than in the COPD group) [Citation55].
Inflammatory intermediate mechanisms contribute to the development of endothelial dysfunction and arterial stiffness, both of which are the risk factors for cardiovascular comorbidities [Citation56–58]. In a prospective study, the prevalence of hypertension and coronary heart disease was higher in patients with OS (N = 25) than in patients with OSA (N = 25), patients with COPD (N = 25), and healthy subjects (N = 20) [Citation59]. Compared to patients of any other group, patients with OS had higher levels of markers of endothelial dysfunction, i.e. soluble vascular cell adhesion molecule-1 and TNF-α, but lower percentages of CD4+ lymphocytes and decreased CD4+/CD8+ ratios [Citation59]. The authors suggested that patients with OS presented with more severe vascular endothelial injury, stronger inflammatory response, and decreased cellular immune function. Post-occlusive reactive hyperemia is a means of assessing endothelial dysfunction; it was similarly impaired in patients with OS, COPD, and OSA [Citation60]. In another study, patients with OS had lower levels of physical activity and higher blood levels of inflammatory biomarkers (C-reactive protein [CRP] and IL-6) compared to patients with only COPD [Citation61]. The presence and severity of comorbid bronchiectasis were assessed in patients with either OS or COPD [Citation62]. Bronchiectasis was more common in patients with OS than in those with COPD alone. The bronchiectasis severity was related to more severe hypoxia, higher blood levels of CRP, and lower CD4+/CD8+ ratio [Citation62].
Autonomic dysfunction is associated with OSA and COPD (see references [Citation63, Citation64] for review). The power of the high-frequency (HF) range derived from heart rate variability analysis represents the parasympathetic heart rate modulation. The power of the low-frequency (LF) range may represent cardiac sympathetic activity, but this is debatable [Citation65]. The LF/HF ratio is an estimation of the sympathovagal balance. OS was associated with a greater daytime sympathetic activity than either COPD or OSA alone [Citation66]. In another study, heart rate variability was measured at (i) rest, (ii) change from supine position to orthostatic position (active postural maneuver), and (iii) respiratory sinus arrhythmia maneuver (controlled breathing with cycles of 10 s [inspiration: 5 s; expiration: 5 s]) for 4 min [Citation67]. During active postural maneuvers, patients with COPD had higher parasympathetic modulation than patients with OS, whereas patients with OS presented higher sympathetic modulation during respiratory sinus arrhythmia maneuvers than patients with COPD alone [Citation67].
In sum,
Inflammation has been extensively assessed in patients with OSA or COPD. Whether inflammation exerts an additive/synergistic effect in patients with OS remains to be evaluated.
To date, few studies have observed a synergistic effect of OSA and COPD on diurnal sympathetic activity. However, this remains to be confirmed.
Clinical and functional presentation of OSA in patients with COPD
Clinical and population studies have consistently reported that patients with moderate-to-severe OSA may present with few symptoms or their symptoms, such as insomnia and marked excessive sleepiness, may be considered nonrelevant symptoms of disturbed sleep [Citation68–73]. Patients with excessive sleepiness have a higher risk of cardiovascular diseases than less sleepy patients with similar severity of OSA or OSA absence [Citation73]. COPD patients may present with delayed, fragmented, and unrefreshing sleep; poor sleep quality; and poor quality of life [Citation74–78]. In COPD patients, sleep alterations were related to older age, obesity, COPD symptoms, sleep disorders such as OSA [Citation79–83], mood disorders such as anxiety and depression [Citation78, Citation84, Citation85], and low quality of life scores [Citation78]. Conversely, in Japanese patients, sleep duration, ESS scores, and Pittsburgh Sleep Quality Index scores were similar in COPD patients and control subjects without OSA or COPD [Citation86]. Data from the Sleep Heart Health Study have shown that COPD patients with mild bronchial obstruction have minimally perturbed sleep, and in the absence of OSA, the ESS scores were similar in subjects with and without airflow limitations [Citation21]. OS patients may have worse sleep quality than those with OSA or COPD alone [Citation21]. However, sleepiness, anxiety, and depression were similar in OS patients (N = 38), OSA-only patients (N = 38), and subjects without OSA or COPD (N = 28) [Citation87]. Fatigue was greater in OS patients than in other studied groups but it did not disappear after a three-month CPAP therapy, suggesting that it was not associated with OSA [Citation87]. A cross-sectional study (1997–2017) of prospectively collected data from patients with moderate-to-severe OSA alone (N = 14,368 [87%]) or OS (N = 2098 [13%]) reported that patients with OS were minimally symptomatic [Citation88]. It remains unclear whether OS patients with excessive sleepiness have a higher risk of cardiovascular comorbidities than less sleepy OS patients with similar severity of OSA or patients with COPD alone.
Patients with OS may exhibit mild, moderate, or severe bronchial obstruction [Citation21, Citation89]. The difference in severity of bronchial obstruction within the studied populations is likely due to the differences in patients’ selection [Citation89]. OS patients may have a lower diurnal arterial partial pressure of oxygen (PaO2) and higher PaCO2 than patients with either COPD alone or OSA alone [Citation5, Citation21, Citation89–91]. In a retrospective study of OS patients, the best model for predicting PaCO2 showed that PaO2 contributed to 38%, FEV1 to 15%, and body weight to 12% of PaCO2 [Citation92]. Some authors suggest that hypercapnia in patients with OS partially depends on the combination of overweight and altered lung function. Exercise testing is a means to characterize functional capacity [Citation93, Citation94]. In a prospective study of a small number of patients with COPD (N = 15) or OS (N = 16), the presence of OSA further impaired the cardiorespiratory fitness in COPD patients [Citation95]. The greater the severity of COPD, the more negative was the impact on exercise performance [Citation95]. These findings remain to be confirmed.
Mortality and comorbidities associated with OS
Mortality
Six population studies, two of which were retrospective, reported a greater risk of death in patients with OS than in patients with either OSA or COPD alone [Citation96–101]. Aging, poorer family status, current smoking habit, serum vitamin D deficiency, cardiovascular disorders, history of cancer, diabetes, and impaired renal function were identified as the risk factors for increased all-cause mortality [Citation97, Citation99]. For example, in consecutive patients with COPD alone (N = 210), OS not treated with CPAP (N = 213), and OS treated with CPAP (N = 228), patients with OS not treated with CPAP had higher mortality (relative risk [RR] = 1.79; 95% CI: 1.16–2.77) and were more likely to suffer from severe COPD exacerbation leading to hospitalization (RR = 1.70; 95% CI: 1.21–2.38) than those with COPD alone [Citation96]. Patients with OS treated with CPAP did not have an increased risk for either outcome in comparison to patients with COPD alone [Citation96]. In another study, although not statistically significant, the presence of OS was associated with a higher likelihood of death compared to COPD alone (hazard ratio [HR] = 1.5, p = 0.160). It is of note that a decrease in lung function may be differently linked to all-cause mortality in patients with OSA and in individuals without OSA; for every 200-mL decrease in FEV1, all-cause mortality was increased by only 6.0% in patients with OSA, whereas it was increased by 11% in subjects without OSA [Citation98]. This remains to be explained. CPAP administration may be associated with improved survival [Citation96, Citation97, Citation101]. In a follow-up of patients with acute hospitalization (N = 4,980), the ten-year all-cause cumulative mortality rate of patients with nonoverlapping OSA, COPD, and OS was 60.4%, 63.0%, and 53.2%, respectively [Citation102]. After adjusting for confounders, the risk of death of OSA patients not treated with positive airway pressure was 1.34 (95% CI: 1.05–1.71) times greater than those who were treated. The risk of death was 1.78 (95% CI: 1.13–2.82) times greater in nonadherent patients than in patients who used their therapy for 70% of the nights, more than 4 h per night [Citation102].
Cardiovascular diseases
A systematic review [Citation103] reported that compared to patients with either COPD or OSA, patients with OS have an increased risk of systemic hypertension (OS vs. COPD: OR = 1.94, 95% CI: 1.49–2.52; OS vs. OSA: OR = 2.05, 95% CI: 1.57–2.68) and pulmonary hypertension (OS vs. COPD: OR = 2.96, 95% CI: 1.30–6.77; OS vs. OSA: OR = 5.93, 95% CI: 1.84–18.42). The risk for coronary heart disease and cerebrovascular disease was similar among the three groups [Citation103]. The authors suggested that OS may increase the prevalence of systemic hypertension and pulmonary hypertension. However, since the pooled outcome linked with pulmonary arterial pressure was not stable, a definitive conclusion about this link could not be drawn [Citation103].
Two studies have been published after the publication of the aforementioned systematic review [Citation104, Citation105], wherein patients with OS were compared to the control subjects and patients with OS had a greater risk for cardiovascular disease as assessed by two prediction models, the Framingham risk score and the systematic coronary risk evaluation (SCORE) [Citation104]. After adjustment for confounders, AHI and age were correlated with the Framingham risk score, whereas age, oxygen desaturation index, and FEV1 were the major determinants for SCORE. In a single-center community-based retrospective cohort (2,873 patients aged ≥65 years without atrial fibrillation in the year 2006), the incidence of new-onset atrial fibrillation over the following 2 years was 10% in the COPD group (N = 416), 6% in the OSA group (N = 60), and 21% in the OS group (N = 28, p < 0.05) [Citation105]. After adjusting for confounders, patients with OS demonstrated a significant association with new-onset atrial fibrillation (OR = 3.66, 95% CI: 1.06–6.9, p = 0.007). A retrospective analysis of data from adults without past or current atrial fibrillation (N = 268) referred for polysomnographic studies shows that incident atrial fibrillation occurred in 64 patients. After adjustment for confounders, independent predictors of incident atrial fibrillation were age-adjusted Charlson index (OR: 1.62; 95% CI: 1.3–2.0), percentage of time spent with O2 saturation below 90% (OR: 3.72, 95% CI: 1.18–11.71), and CPAP adherence (OR: 0.32, 95% CI: 0.13–0.71) [Citation106].
Finally, a cross-sectional study of prospective data from patients with moderate-to-severe OSA alone (N = 14,368 [87%]) or with OS (N = 2098 [13%]) [Citation88] reported that compared to patients with OSA alone, after controlling for confounding variables, patients with OS had a higher prevalence of heart disease, heart failure, and peripheral arterial disease [Citation88].
COPD exacerbation and hypercapnic respiratory failure
Patients with OS may have more severe exacerbations than patients with COPD alone [Citation96, Citation107]. However, OS patients treated with CPAP and patients with only COPD had a longer time to the first severe exacerbation requiring hospitalization than OS patients whose OSA was untreated [Citation96]. A retrospective cohort study of patients (N = 189,685) hospitalized for acute exacerbation of COPD reported that patients with OS had a higher risk of noninvasive positive pressure ventilation use (OR = 2.78, 95% CI: 2.63–2.95) and prolonged length of hospital stay (OR = 2.78, 95% CI: 2.63–2.95) compared to patients without OSA [Citation108]. Sleep apnea has been identified as a predictor of 30-day readmission after hospital discharge for patients with COPD [Citation109]. Patients hospitalized for COPD exacerbation (N = 545,086), diagnosed according to International Classification of Diseases-9 codes, had a seven times higher risk of pulmonary hypertension than non-hospitalized patients [Citation110]. COPD patients with pulmonary hypertension had higher rates of OSA than COPD-only patients (34% vs. 16%).
In a prospective study assessing the prevalence of comorbidities in patients with acute hypercapnic respiratory failure (N = 78), most patients (67%) exhibited COPD. Of those, 66% had undetected and untreated moderate-to-severe OSA [Citation111]. The authors suggested that surviving acute hypercapnic respiratory failure should be an opportunity to systematically assess comorbidities, including OSA. In a retrospective cohort study, initiation of CPAP therapy in elderly patients with OS was associated with a reduction in hospitalization for COPD-related conditions, but not with all-cause hospitalizations and emergency room visits [Citation112].
In sum,
In COPD patients, OSA is a risk factor for pulmonary hypertension.
The following observations remain to be confirmed:
The increased risk of mortality in patients with OS compared to that in patients with either OSA or COPD.
The influence of cardiovascular diseases, COPD, and OSA on increased mortality in patients with OS [Citation5].
The reduction of increased mortality in OS patients by CPAP administration.
Whether OSA is an added risk for cardiovascular diseases in COPD patients.
Whether OSA poses a risk for more severe COPD exacerbation.
Whether CPAP administration in OS patients reduces the risk of COPD exacerbation and short-term readmission.
OSA pretest likelihood and diagnostic management in COPD patients
Signs and symptoms of OSA have poor diagnostic predictive value for OSA and, thus, weak diagnostic utility [Citation113]. Similarly, in COPD patients, clinical symptoms poorly predict the diagnosis of OSA [Citation41]. In OSA patients without COPD, OSA risk factors include older age, male sex, larger neck circumference, and excessive sleepiness [Citation9, Citation114, Citation115]. In OS patients, they are no longer predictive of OSA [Citation41]. Conversely, the OSA diagnostic pretest probability is influenced by some comorbidities, i.e. cardiovascular disorders and hypertension in isolation are predictive of OSA in patients with COPD [Citation41].
The management of OSA diagnosis in patients with mild COPD is similar to that in non-COPD patients [Citation116, Citation117]. To date, there are no guidelines suggesting how non-hypercapnic patients with GOLD stage 2-to-4 COPD should undergo assessment for OSA. The ATS guidelines suggest that patients with COPD and stable hypercapnia undergo sleep apnea “screening” before initiation of long-term noninvasive ventilation (“conditional recommendation, very low certainty”) [Citation6]. The expert panel derived indirect estimates of sensitivity and specificity of screening tools like the STOP-BANG questionnaire from patients without COPD based on the results of meta-analysis (108 studies, 47,989 participants with suspected OSA) [Citation6]. They assumed that the STOP-BANG questionnaire characteristics were similar in patients without COPD and patients with chronic stable hypercapnic COPD, which remains to be demonstrated. Hypothesizing an OSA prevalence of 10%, the use of this questionnaire could identify only 9% of patients with true-positive results, which might be considered as a poor yield. However, it would also avoid unnecessary sleep studies in a significant proportion of patients who would exhibit true-negative results (32%). Besides, false-positive results would not be problematic since these patients could still benefit from a sleep study despite not having OSA (58%) [Citation6]. In the end, the proportion of patients with false-negative results (1%) who would not benefit from OSA care would be marginal. However, OSA may increase the risk of mortality and morbidity in patients with COPD [Citation118], which would become critical if the actual number of false-negative results were higher than that obtained by the expert panel. Another matter of debate is whether we should screen for or diagnose OSA in patients with chronic stable hypercapnic COPD? The guidelines suggest that “patients with COPD who are overweight and demonstrate cardiovascular disease … may prompt consideration of OSA screening…” [Citation6]. Screening is considered “a presumptive identification of an unrecognized disease in an apparently healthy, asymptomatic population” [Citation119, Citation120]. Thus, this term does not seem to be appropriate in this specific situation, where patients with COPD and stable hypercapnia demonstrate symptoms and can certainly not be considered as “apparently healthy.” They are also seeking medical attention because of OSA symptoms. Furthermore, the ATS guideline is discrepant with the 2017 American Academy of Sleep Medicine (AASM) clinical practice guidelines for diagnostic testing for adult OSA [Citation1]. The AASM guidelines strongly recommend (i) that “clinical tools, questionnaires and prediction algorithms not be used to diagnose OSA in adults, in the absence of polysomnography (PSG) or home sleep apnea testing” and (ii) that “polysomnography be used for the diagnosis of OSA in patients with significant cardiorespiratory disease, awake hypoventilation or suspicion of sleep related hypoventilation” (and not only from OSA screening as indicated by the ATS guidelines) [Citation1]. This position actually corresponds to a case-finding strategy. Relying primarily on polysomnography appears relevant awaiting studies on the specific yield of questionnaires in patients with COPD. Whether the identification and subsequent treatment of OSA in COPD patients provide significant clinical benefits also remains to be formally demonstrated.
In sum,
Clinical presentation of patients with OS remains to be studied in prospective comparative studies. Cluster analysis of data from patients with OS from various clinical settings may help to identify clinical phenotypes, their prevalence, and associated risks and might help to recommend which phenotypes should undergo OSA assessments.
OSA symptoms have poor diagnostic utility in patients with or without COPD. The likelihood of OSA depends on its prevalence in the clinical setting. A clinical context at risk for OSA, including metabolic and cardiovascular disorders, increases the pretest likelihood of OSA in patients with or without COPD.
The ATS clinical practice guidelines suggest that patients with COPD who have chronic stable hypercapnia should undergo screening for OSA before the initiation of long-term noninvasive ventilation (NIV) [Citation6]. No guidelines exist for the management of OSA in patients with GOLD stage 2 − 4 COPD, who have no chronic hypercapnia.
Sleep studies for OSA assessment in COPD patients
PSG findings in OS patients
According to the AASM guidelines, PSG rather than home sleep apnea testing is strongly recommended for the diagnosis of OSA in patients with a meaningful cardiorespiratory disease, e.g. COPD [Citation1]. PSG findings in OS patients include increased sleep latency and waking after sleep onset, as well as reduced total sleep time, sleep efficiency, duration of deep sleep (N3 stage), and paradoxical sleep (R stage, for REM sleep stage) [Citation7, Citation121]. Sleep efficiency may be lower in patients with OS than those with OSA [Citation7]. Polysomnographic alterations may or may not correlate with subjective clinical data [Citation86, Citation122].
The AHI is similar in OSA patients with or without COPD [Citation7]. Patients with OS present sustained desaturation episodes [Citation5] that may be deeper than in patients with either COPD or OSA [Citation7, Citation21], but this finding is controversial [Citation31]. In contrast to scoring apneas, scoring hypopneas may be difficult in COPD patients [Citation5]. A respiratory event accompanied by electrocortical arousal may be considered a hypopnea, even in the absence of significant oxygen desaturation. Desaturation episodes may have origins in these patients, namely upper airway obstruction, hypoventilation during paradoxical sleep, ventilation/perfusion mismatches, and obesity [Citation7, Citation21, Citation91, Citation96, Citation123]. Prolonged desaturation due to prolonged hypoventilation may be scored as a single hypopnea event [Citation5, Citation124, Citation125]. Oxygen desaturation contributes to the development of cardiovascular comorbidities [Citation100, Citation126, Citation127]. Therefore, it has been suggested that the total hypoxemic load and the area under the oxygen desaturation curve may have better diagnostic predictive ability than the AHI in patients with OS [Citation5]. This remains to be demonstrated. Diagnosis of hypoventilation in patients with OS and COPD may be facilitated by the monitoring of specific parameters, such as tidal volume and transcutaneous CO2.
The need for an accurate and convenient tool to diagnose OSA in COPD patients
The accuracy of a home portable device was assessed for the diagnosis of OSA in patients with COPD with a high pretest likelihood of OS. The home portable device was used on two different nights: at home and at the sleep laboratory concomitantly with polysomnography [Citation128]. The home portable device assessed nasal airflow, respiratory effort, body position, oxygen saturation, and heart rate [Citation128]. An acceptable intraclass correlation was observed between the two devices (r = 0.61, p < 0.0001). However, the high failure rate in the recordings (63%) may limit, according to the authors, the use of the home portable device to assess OSA in COPD patients [Citation128]. WatchPat is a home sleep apnea testing device that assesses peripheral arterial tone, pulse rate, oxygen saturation, actigraphy, snoring recording, and body position [Citation129]. After a concomitant evaluation of OSA by WatchPat during in-laboratory PSG in COPD patients, the sensitivity values of WatchPAT at an AHI (events/h) cutoff of ≥5, ≥15, and ≥30 were all ≥88.9%. The specificity was high in patients with severe OSA (95.8%) but relatively low in patients with mild and moderate OSA (55% and 65%, respectively) [Citation129]. WatchPat showed only fair-to-moderate agreement with PSG in the detection of wake and sleep stages in COPD patients (overall Cohen’s agreement between WatchPat and PSG in detecting all sleep stages was 63%, kappa: 0.418) [Citation130]. Nox-T3 is a portable monitor that records nasal pressure and rib cage and abdominal movements by inductance plethysmography, snoring, body position, and heart rate and oxygen saturation by pulse oximetry [Citation131]. It was assessed in OS patients who underwent unattended home testing followed by an in-laboratory PSG with simultaneous portable monitor recording [Citation131]. Using an AHI ≥5 events/h to diagnose OSA, home testing had 95% sensitivity, 78% specificity, 88% positive predictive value, and 89% negative predictive value compared to PSG [Citation131]. Oxygen saturation levels assessed with the portable monitor were lower than those measured during in-laboratory PSG [Citation131]. Wrist actigraphy was compared to PSG in a systematic review [Citation132]. In patients with chronic conditions, including COPD, wrist actigraphy overestimated total sleep time and sleep efficiency and underestimated sleep onset latency and wake after sleep onset [Citation132]. Two studies did not identify an oximetry-derived criterion useful for the diagnosis of OSA in patients with COPD [Citation133, Citation134].
In sum,
AHI is similar in OSA patients with or without COPD. In OS patients, desaturation may be greater and more prolonged than in patients with either COPD or OSA.
A desaturation episode may or may not be associated with a hypopnea event, making it difficult to score hypopneas in COPD patients.
PSG is currently the gold standard for assessing OSA in COPD patients. For OSA diagnosis, a tool is needed that is accurate and more convenient than PSG.
Treatment of OSA in COPD patients
The treatment of OSA in OS patients depends on the patient, the severity of his/her condition, and the presence of comorbidities such as cardiovascular and metabolic diseases. COPD should be optimally treated; however, this has only a modest effect on sleep quality [Citation135–137]. In patients with no meaningful comorbidities, including COPD, the AASM recommends as OSA treatment (i) the initiation of positive airway pressure using either auto-adjusting positive airway pressure at home or in-laboratory positive airway pressure titration, and (ii) ongoing treatment with auto-adjusting positive airway pressure or CPAP [Citation138]. There are currently no guidelines on whether OS patients with GOLD stage 2-to-4, non-hypercapnic COPD should be treated as an OSA patient or with some specific approaches related to the presence of COPD. COPD patients with stable chronic hypercapnia may benefit from NIV. Currently, ATS suggests only screening for OSA in COPD patients with stable hypercapnia [Citation6]. This strategy might be reevaluated in future when new data on screening tools from the COPD population with and without hypercapnia will be available. There is no guideline on how to treat OS patients with chronic stable hypercapnia. The potential beneficial effects of treating sleep apnea and good adherence to this treatment on mortality and hospitalization for a COPD-related condition have been discussed above.
CPAP administration may have short- and long-term effects [Citation139]. The short-term effects include the suppression of abnormal respiratory events such as apneas and hypopneas, the narrowing of the upper airway, and the increase in end-expiratory lung volume. The long-term effect, such as the lowering of daytime PaCO2, may be the result of a ventilatory adaptation to CPAP administration, a resetting of ventilatory drive requiring weeks or months to appear [Citation139–141]. Even when taking into consideration various study biases, in OS patients, CPAP administration is associated with better sleep quality, improved daytime arterial blood gas status, lower mortality, and decreased rates of COPD-linked hospitalization compared to no specific OSA treatment [Citation96, Citation112, Citation142, Citation143]. In OS patients, the set-up of NIV, such as bi-level and tri-level positive airway pressure ventilation, should be adapted to the presence of OSA [Citation144, Citation145].
The efficacy and drawbacks of oral appliances in OS patients remain to be evaluated.
In sum,
In OS patients with GOLD stage 1 COPD, positive airway pressure may be
Initiated with auto-adjusting positive airway pressure or in-laboratory titration.
Administered using either auto-adjusting or CPAP.
No guidelines exist that recommend specific approaches to treat OS patients with GOLD stage 2-to-4 COPD.
Several research priorities have been proposed [Citation5, Citation117], including randomized, prospective comparisons of positive airway pressure treatments, including bi-level, CPAP, or other modalities.
Conclusion
This review briefly summarizes the current knowledge and some perspectives related to OSA in COPD patients. While no convincing evidence shows that COPD patients are at a greater risk for developing OSA, an interaction exists between the two conditions. For example, OS patients may have worse sleep symptoms than patients with either OSA or COPD [Citation21]; they may present sustained desaturation episodes that may be deeper than in patients with either OSA or COPD [Citation21]; in COPD patients, the greater the lung hyperinflation, the lower the AHI [Citation24]. Although there are studies on the prevalence of OSA, this subject has not been prospectively determined in patients with various COPD phenotypes and severity levels. This may help to better understand the link between OS and comorbidities, and to suggest diagnostic strategies adapted to specific populations. Another critical issue is the assessment of hypopnea, and more generally, the definition of the disease. As oxygen desaturation plays a critical pathophysiological role, it has been suggested to define hypopnea, including variables such as the total hypoxemia load and the area under the oxygen desaturation curve [Citation5]. This remains to be done. Pathophysiological studies may improve our knowledge of intermediate mechanisms and risk factors for OSA in COPD patients. Whereas OS patients with no meaningful COPD are managed as patients with OSA alone, no guidelines currently recommend a specific management for OSA patients with GOLD stage 2-to-4 COPD. In brief, most pathophysiological, clinical, epidemiological, and therapeutic questions of OS remain to be elucidated [Citation146].
Declaration of interest
The authors report no conflict of interest. Alain Lurie reports no conflict of interest for coauthoring this review. Outside the submitted work, Alain Lurie reports non-financial support from Linde, France and ISIS Paris-Est, France. Nicolas Roche reports no conflict of interest for coauthoring this review.
Box 1. Hyperinflation in chronic obstructive pulmonary disease
Box 2. Upper airway collapsibility
References
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. DOI:https://doi.org/10.5664/jcsm.6506
- Global Initiative for Chronic Obstructive Lung Disease – GOLD. Global strategy for the diagnosis, management, and prevention of Chronic Obstructive Pulmonary Disease (2021 Report). Global Initiative for Chronic Obstructive Lung Disease; 2020. Available at: https://goldcopd.org/. (Accessed 22 November 2020).
- Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med. 1985;6(4):651–661. DOI:https://doi.org/10.1016/S0272-5231(21)00402-0
- McNicholas WT, Verbraecken J, Marin JM. Sleep disorders in COPD: the forgotten dimension. Eur Respir Rev. 2013;22(129):365–375. DOI:https://doi.org/10.1183/09059180.00003213
- Malhotra A, Schwartz AR, Schneider H, et al. Research priorities in pathophysiology for sleep-disordered breathing in patients with chronic obstructive pulmonary disease. An official American Thoracic Society research statement. Am J Respir Crit Care Med. 2018;197(3):289–299. DOI:https://doi.org/10.1164/rccm.201712-2510ST
- Macrea M, Oczkowski S, Rochwerg B, et al. Long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(4):e74–e87. DOI:https://doi.org/10.1164/rccm.202006-2382ST
- Shawon MSR, Perret JL, Senaratna CV, et al. Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: a systematic review. Sleep Med Rev. 2017;32:58–68. DOI:https://doi.org/10.1016/j.smrv.2016.02.007
- Collen J, Lettieri C, Wickwire E, et al. Obstructive sleep apnea and cardiovascular disease, a story of confounders!. Sleep Breath. 2020;24(4):1299–1313. DOI:https://doi.org/10.1007/s11325-019-01945-w
- Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. DOI:https://doi.org/10.1056/NEJM199304293281704
- Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119(1):62–69. DOI:https://doi.org/10.1378/chest.119.1.62
- Ip MSM, Lam B, Tang LCH, et al. A community study of sleep-disordered breathing in middle-aged Chinese women in Hong Kong: prevalence and gender differences. Chest. 2004;125(1):127–134. DOI:https://doi.org/10.1378/chest.125.1.127
- Reddy EV, Kadhiravan T, Mishra HK, et al. Prevalence and risk factors of obstructive sleep apnea among middle-aged urban Indians: a community-based study. Sleep Med. 2009;10(8):913–918. DOI:https://doi.org/10.1016/j.sleep.2008.08.011
- Foster GD, Sanders MH, Millman R, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32(6):1017–1019. DOI:https://doi.org/10.2337/dc08-1776
- Jehan S, Myers AK, Zizi F, et al. Obesity, obstructive sleep apnea and type 2 diabetes mellitus: epidemiology and pathophysiologic insights. Sleep Med Disord. 2018;2(3):52–58.
- Sarkar P, Mukherjee S, Chai-Coetzer CL, et al. The epidemiology of obstructive sleep apnoea and cardiovascular disease. J Thorac Dis. 2018;10(Suppl 34):S4189–S4200. DOI:https://doi.org/10.21037/jtd.2018.12.56
- Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015;5(2):020415. DOI:https://doi.org/10.7189/jogh.05-020415
- Soriano JB, Kendrick PJ, Paulson KR, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596. DOI:https://doi.org/10.1016/S2213-2600(20)30105-3
- Onishi K, Yoshimoto D, Hagan GW, et al. Prevalence of airflow limitation in outpatients with cardiovascular diseases in Japan. Int J Chron Obstruct Pulmon Dis. 2014;9:563–568.
- Lacedonia D, Carpagnano GE, Patricelli G, et al. Prevalence of comorbidities in patients with obstructive sleep apnea syndrome, overlap syndrome and obesity hypoventilation syndrome. Clin Respir J. 2018;12(5):1905–1911. DOI:https://doi.org/10.1111/crj.12754
- Schreiber A, Cemmi F, Ambrosino N, et al. Prevalence and predictors of obstructive sleep apnea in patients with chronic obstructive pulmonary disease undergoing inpatient pulmonary rehabilitation. COPD. 2018;15(3):265–270. DOI:https://doi.org/10.1080/15412555.2018.1500533
- Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167(1):7–14. DOI:https://doi.org/10.1164/rccm.2203046
- Tveit RL, Lehmann S, Bjorvatn B. Prevalence of several somatic diseases depends on the presence and severity of obstructive sleep apnea. PLoS One. 2018;13(2):e0192671. DOI:https://doi.org/10.1371/journal.pone.0192671
- Adler D, Dupuis-Lozeron E, Janssens JP, et al. Obstructive sleep apnea in patients surviving acute hypercapnic respiratory failure is best predicted by static hyperinflation. PLoS One. 2018;13(10):e0205669. DOI:https://doi.org/10.1371/journal.pone.0205669
- Krachman SL, Tiwari R, Vega ME, et al. Effect of emphysema severity on the apnea-hypopnea index in smokers with obstructive sleep apnea. Annals Ats. 2016;13(7):1129–1135. DOI:https://doi.org/10.1513/AnnalsATS.201511-765OC
- Zhu J, Zhao Z, Nie Q, et al. Effect of lung function on the apnea-hypopnea index in patients with overlap syndrome: a multicenter cross-sectional study. Sleep Breath. 2020;24(3):1059–1066. DOI:https://doi.org/10.1007/s11325-019-01961-w
- Tagaito Y, Isono S, Remmers JE, et al. Lung volume and collapsibility of the passive pharynx in patients with sleep-disordered breathing. J Appl Physiol (1985). 2007;103(4):1379–1385. DOI:https://doi.org/10.1152/japplphysiol.00026.2007
- Heinzer RC, Stanchina ML, Malhotra A, et al. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172(1):114–117. DOI:https://doi.org/10.1164/rccm.200404-552OC
- Heinzer RC, Stanchina ML, Malhotra A, et al. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax. 2006;61(5):435–439. DOI:https://doi.org/10.1136/thx.2005.052084
- Biselli P, Grossman PR, Kirkness JP, et al. The effect of increased lung volume in chronic obstructive pulmonary disease on upper airway obstruction during sleep. J Appl Physiol (1985). 2015;119(3):266–271. DOI:https://doi.org/10.1152/japplphysiol.00455.2014
- Whitelaw WA, Derenne JP. Airway occlusion pressure. J Appl Physiol (1985). 1993;74(4):1475–1483. DOI:https://doi.org/10.1152/jappl.1993.74.4.1475
- He B-T, Lu G, Xiao S-C, et al. Coexistence of OSA may compensate for sleep related reduction in neural respiratory drive in patients with COPD. Thorax. 2017;72(3):256–262. DOI:https://doi.org/10.1136/thoraxjnl-2016-208467
- Eckert DJ, Younes MK. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol (1985). 2014;116(3):302–313. DOI:https://doi.org/10.1152/japplphysiol.00649.2013
- Messineo L, Lonni S, Magri R, et al. Lung air trapping lowers respiratory arousal threshold and contributes to sleep apnea pathogenesis in COPD patients with overlap syndrome. Respir Physiol Neurobiol. 2020;271:103315. DOI:https://doi.org/10.1016/j.resp.2019.103315
- Edwards BA, Eckert DJ, McSharry DG, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190(11):1293–1300. DOI:https://doi.org/10.1164/rccm.201404-0718OC
- Yamaguchi Y, Shiota S, Kusunoki Y, et al. Polysomnographic features of low arousal threshold in overlap syndrome involving obstructive sleep apnea and chronic obstructive pulmonary disease. Sleep Breath. 2019;23(4):1095–1100. DOI:https://doi.org/10.1007/s11325-019-01786-7
- Orr JE, Schmickl CN, Edwards BA, et al. Pathogenesis of obstructive sleep apnea in individuals with the COPD + OSA overlap syndrome versus OSA alone. Physiol Rep. 2020;8(3):e14371. DOI:https://doi.org/10.14814/phy2.14371
- Kuvat N, Tanriverdi H, Armutcu F. The relationship between obstructive sleep apnea syndrome and obesity: A new perspective on the pathogenesis in terms of organ crosstalk. Clin Respir J. 2020;14(7):595–604. DOI:https://doi.org/10.1111/crj.13175
- Steveling EH, Clarenbach CF, Miedinger D, et al. Predictors of the overlap syndrome and its association with comorbidities in patients with chronic obstructive pulmonary disease. Respiration. 2014;88(6):451–457. DOI:https://doi.org/10.1159/000368615
- Archontogeorgis K, Voulgaris A, Papanas N, et al. Metabolic syndrome in patients with coexistent obstructive sleep apnea syndrome and chronic obstructive pulmonary disease (overlap syndrome). Metab Syndr Relat Disord. 2020;18(6):296–301. DOI:https://doi.org/10.1089/met.2019.0126
- Zhao YY, Blackwell T, Ensrud KE, et al. Sleep apnea and obstructive airway disease in older men: outcomes of sleep disorders in older men study. Sleep. 2016;39(7):1343–1351. DOI:https://doi.org/10.5665/sleep.5960
- Soler X, Liao S-Y, Marin JM, et al. Age, gender, neck circumference, and Epworth sleepiness scale do not predict obstructive sleep apnea (OSA) in moderate to severe chronic obstructive pulmonary disease (COPD): The challenge to predict OSA in advanced COPD. PLoS One. 2017;12(5):e0177289. DOI:https://doi.org/10.1371/journal.pone.0177289
- Perger E, Jutant E-M, Redolfi S. Targeting volume overload and overnight rostral fluid shift: A new perspective to treat sleep apnea. Sleep Med Rev. 2018;42:160–170. DOI:https://doi.org/10.1016/j.smrv.2018.07.008
- Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol (1985). 1988;65(5):2124–2131. DOI:https://doi.org/10.1152/jappl.1988.65.5.2124
- Hillman DR, Walsh JH, Maddison KJ, et al. The effect of diaphragm contraction on upper airway collapsibility. J Appl Physiol (1985). 2013;115(3):337–345. DOI:https://doi.org/10.1152/japplphysiol.01199.2012
- Tong J, Jugé L, Burke PG, et al. Respiratory-related displacement of the trachea in obstructive sleep apnea. J Appl Physiol (1985). 2019;127(5):1307–1316. DOI:https://doi.org/10.1152/japplphysiol.00660.2018
- Zhang L, Samet J, Caffo B, et al. Cigarette smoking and nocturnal sleep architecture. Am J Epidemiol. 2006;164(6):529–537. DOI:https://doi.org/10.1093/aje/kwj231
- Newman AB, Nieto FJ, Guidry U, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154(1):50–59. DOI:https://doi.org/10.1093/aje/154.1.50
- Kashyap R, Hock LM, Bowman TJ. Higher prevalence of smoking in patients diagnosed as having obstructive sleep apnea. Sleep Breath. 2001;5(4):167–172. DOI:https://doi.org/10.1055/s-2001-18805
- Hoflstein V. Relationship between smoking and sleep apnea in clinic population. Sleep. 2002;25(5):519–524.
- Mieczkowski B, Ezzie ME. Update on obstructive sleep apnea and its relation to COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:349–362. DOI:https://doi.org/10.2147/COPD.S42394
- Teodorescu M, Consens FB, Bria WF, et al. Predictors of habitual snoring and obstructive sleep apnea risk in patients with asthma. Chest. 2009;135(5):1125–1132. DOI:https://doi.org/10.1378/chest.08-1273
- Teodorescu M, Xie A, Sorkness CA, et al. Effects of inhaled fluticasone on upper airway during sleep and wakefulness in asthma: a pilot study. J Clin Sleep Med. 2014;10(2):183–193. DOI:https://doi.org/10.5664/jcsm.3450
- Macrea M, Campbell S, Martin T, et al. The peripheral neutrophils in subjects with COPD-OSA overlap syndrome and severe comorbidities: a feasible inflammatory biomarker?Adv Clin Exp Med. 2018;27(12):1677–1682. DOI:https://doi.org/10.17219/acem/75904
- Wang Y, Hu K, Liu K, et al. Obstructive sleep apnea exacerbates airway inflammation in patients with chronic obstructive pulmonary disease. Sleep Med. 2015;16(9):1123–1130. DOI:https://doi.org/10.1016/j.sleep.2015.04.019
- Zhou W, Li C-L, Cao J, et al. Metabolic syndrome prevalence in patients with obstructive sleep apnea syndrome and chronic obstructive pulmonary disease: Relationship with systemic inflammation. Clin Respir J. 2020;14(12):1159–1165. DOI:https://doi.org/10.1111/crj.13253
- Endothelial dysfunction in adults with obstructive sleep apnea. Adv Cardiol. 2011;46:139–170.
- Phillips CL, Butlin M, Wong KK, et al. Is obstructive sleep apnoea causally related to arterial stiffness? A critical review of the experimental evidence. Sleep Med Rev. 2013;17(1):7–18. DOI:https://doi.org/10.1016/j.smrv.2012.03.002
- Szucs B, Szucs C, Petrekanits M, et al. Molecular characteristics and treatment of endothelial dysfunction in patients with COPD: a review article. IJMS. 2019;20(18):4329. DOI:https://doi.org/10.3390/ijms20184329
- Wang J, Li X, Hou W-J, et al. Endothelial function and T-lymphocyte subsets in patients with overlap syndrome of chronic obstructive pulmonary disease and obstructive sleep apnea. Chin Med J (Engl). 2019;132(14):1654–1659. DOI:https://doi.org/10.1097/CM9.0000000000000312
- Khare P, Talwar A, Trivedi A, et al. Vascular responses to post occlusive reactive hyperemia and systemic inflammation in overlap syndrome of chronic obstructive pulmonary disease and obstructive sleep apnea. Indian J Physiol Pharmacol. 2016;60(2):155–166.
- Fitzgibbons CM, Goldstein RL, Gottlieb DJ, et al. Physical activity in overlap syndrome of COPD and obstructive sleep apnea: relationship with markers of systemic inflammation. J Clin Sleep Med. 2019;15(7):973–978. DOI:https://doi.org/10.5664/jcsm.7874
- Yang X, Tang X, Cao Y, et al. The bronchiectasis in COPD-OSA overlap syndrome patients. COPD. 2020;15:605–611. DOI:https://doi.org/10.2147/COPD.S243429
- Hemodynamic and autonomic changes in adults with obstructive sleep apnea. Adv Cardiol. 2011;46:171–195.
- Mohammed J, Meeus M, Derom E, et al. Evidence for autonomic function and Its influencing factors in subjects with COPD: a systematic review. Respir Care. 2015;60(12):1841–1851. DOI:https://doi.org/10.4187/respcare.04174
- Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–381.
- Taranto-Montemurro L, Messineo L, Perger E, et al. Cardiac sympathetic hyperactivity in patients with chronic obstructive pulmonary disease and obstructive sleep apnea. COPD. 2016;13(6):706–711. DOI:https://doi.org/10.1080/15412555.2016.1199668
- Zangrando KTL, Trimer R, de Carvalho LCS, Jr, et al. Chronic obstructive pulmonary disease severity and its association with obstructive sleep apnea syndrome: impact on cardiac autonomic modulation and functional capacity. Int J Chron Obstruct Pulmon Dis. 2018;13:1343–1351. DOI:https://doi.org/10.2147/COPD.S156168
- Zinchuk AV, Jeon S, Koo BB, et al. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax. 2018;73(5):472–480. DOI:https://doi.org/10.1136/thoraxjnl-2017-210431
- Ye L, Pien GW, Ratcliffe SJ, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J. 2014;44(6):1600–1607. DOI:https://doi.org/10.1183/09031936.00032314
- Keenan BT, Kim J, Singh B, et al. Recognizable clinical subtypes of obstructive sleep apnea across international sleep centers: a cluster analysis. Sleep. 2018;41(3):zsx214. DOI:https://doi.org/10.1093/sleep/zsx214
- Kim J, Keenan BT, Lim DC, et al. Symptom-based subgroups of Koreans with obstructive sleep apnea. J Clin Sleep Med. 2018;14(3):437–443. DOI:https://doi.org/10.5664/jcsm.6994
- Koch H, Schneider LD, Finn LA, et al. Breathing disturbances without hypoxia are associated with objective sleepiness in sleep apnea. Sleep. 2017;40(11):zsx152. DOI:https://doi.org/10.1093/sleep/zsx152
- Mazzotti DR, Keenan BT, Lim DC, et al. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200(4):493–506. DOI:https://doi.org/10.1164/rccm.201808-1509OC
- Budhiraja R, Parthasarathy S, Budhiraja P, et al. Insomnia in patients with COPD. Sleep. 2012;35(3):369–375. DOI:https://doi.org/10.5665/sleep.1698
- Ding B, Small M, Bergström G, et al. A cross-sectional survey of night-time symptoms and impact of sleep disturbance on symptoms and health status in patients with COPD. COPD. 2017;12:589–599. DOI:https://doi.org/10.2147/COPD.S122485
- Shah NM, Murphy PB. Chronic obstructive pulmonary disease and sleep: an update on relevance, prevalence and management. Curr Opin Pulm Med. 2018;24(6):561–568. DOI:https://doi.org/10.1097/MCP.0000000000000527
- Zeidler MR, Martin JL, Kleerup EC, et al. Sleep disruption as a predictor of quality of life among patients in the subpopulations and intermediate outcome measures in COPD study (SPIROMICS). Sleep. 2018;41(5):zsy044. DOI:https://doi.org/10.1093/sleep/zsy044
- Shah A, Ayas N, Tan W-C, et al. Sleep quality and nocturnal symptoms in a community-based COPD cohort. COPD. 2020;17(1):40–48. DOI:https://doi.org/10.1080/15412555.2019.1695247
- Agusti A, Hedner J, Marin JM, et al. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183–194. DOI:https://doi.org/10.1183/09059180.00004311
- Omachi TA, Blanc PD, Claman DM, et al. Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Med. 2012;13(5):476–483. DOI:https://doi.org/10.1016/j.sleep.2011.12.007
- Nunes DM, de Bruin VMS, Louzada FM, et al. Actigraphic assessment of sleep in chronic obstructive pulmonary disease. Sleep Breath. 2013;17(1):125–132. DOI:https://doi.org/10.1007/s11325-012-0660-z
- Dignani L, Toccaceli A, Lucertini C, et al. Sleep and quality of life in people with COPD: a descriptive-correlational study. Clin Nurs Res. 2016;25(4):432–447. DOI:https://doi.org/10.1177/1054773815588515
- Spina G, Spruit MA, Alison J, et al. Analysis of nocturnal actigraphic sleep measures in patients with COPD and their association with daytime physical activity. Thorax. 2017;72(8):694–701. DOI:https://doi.org/10.1136/thoraxjnl-2016-208900
- Fukuchi Y, Nishimura M, Ichinose M, et al. COPD in Japan: the Nippon COPD Epidemiology study. Respirology. 2004;9(4):458–465. DOI:https://doi.org/10.1111/j.1440-1843.2004.00637.x
- Rodríguez-Mañero M, López-Pardo E, Cordero A, et al. A prospective study of the clinical outcomes and prognosis associated with comorbid COPD in the atrial fibrillation population. COPD. 2019;14:371–380. DOI:https://doi.org/10.2147/COPD.S174443
- Azuma M, Chin K, Yoshimura C, et al. Associations among chronic obstructive pulmonary disease and sleep-disordered breathing in an urban male working population in Japan. Respiration. 2014;88(3):234–243. DOI:https://doi.org/10.1159/000366064
- Economou N-T, Ilias I, Velentza L, et al. Sleepiness, fatigue, anxiety and depression in chronic obstructive pulmonary disease and obstructive sleep apnea - overlap - syndrome, before and after continuous positive airways pressure therapy. PLoS One. 2018;13(6):e0197342. DOI:https://doi.org/10.1371/journal.pone.0197342
- Adler D, Bailly S, Benmerad M, et al. Clinical presentation and comorbidities of obstructive sleep apnea-COPD overlap syndrome. PLoS One. 2020;15(7):e0235331. DOI:https://doi.org/10.1371/journal.pone.0235331
- Weitzenblum E, Chaouat A, Kessler R, et al. Overlap syndrome: obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(2):237–241. DOI:https://doi.org/10.1513/pats.200706-077MG
- Alford NJ, Fletcher EC, Nickeson D. Acute oxygen in patients with sleep apnea and COPD. Chest. 1986;89(1):30–38. DOI:https://doi.org/10.1378/chest.89.1.30
- Chaouat A, Weitzenblum E, Krieger J, et al. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151(1):82–86. DOI:https://doi.org/10.1164/ajrccm.151.1.7812577
- Resta O, Barbaro MPF, Brindicci C, et al. Hypercapnia in overlap syndrome: possible determinant factors. Sleep Breath. 2002;06(1):11–18. DOI:https://doi.org/10.1055/s-2002-23151
- Mendelson M, Marillier M, Bailly S, et al. Maximal exercise capacity in patients with obstructive sleep apnoea syndrome: a systematic review and meta-analysis. Eur Respir J. 2018;51(6):1702697. DOI:https://doi.org/10.1183/13993003.02697-2017
- Stickland MK, Butcher SJ, Marciniuk DD, et al. Assessing exercise limitation using cardiopulmonary exercise testing. Pulm Med. 2012;2012:824091. DOI:https://doi.org/10.1155/2012/824091
- de Carvalho Junior LCS, Trimer R, Zangrando KL, et al. Overlap syndrome: the coexistence of OSA further impairs cardiorespiratory fitness in COPD. Sleep Breath. 2020;24(4):1451–1462. DOI:https://doi.org/10.1007/s11325-019-02002-2
- Marin JM, Soriano JB, Carrizo SJ, et al. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. DOI:https://doi.org/10.1164/rccm.200912-1869OC
- Stanchina ML, Welicky LM, Donat W, et al. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med. 2013;09(08):767–772. DOI:https://doi.org/10.5664/jcsm.2916
- Putcha N, Crainiceanu C, Norato G, et al. Influence of lung function and sleep-disordered breathing on all-cause mortality. A community-based study. Am J Respir Crit Care Med. 2016;194(8):1007–1014. DOI:https://doi.org/10.1164/rccm.201511-2178OC
- Du W, Liu J, Zhou J, et al. Obstructive sleep apnea, COPD, the overlap syndrome, and mortality: results from the 2005-2008 National Health and Nutrition Examination Survey. Int J Chron Obstruct Pulmon Dis. 2018;13:665–674. DOI:https://doi.org/10.2147/COPD.S148735
- Kendzerska T, Leung RS, Aaron SD, et al. Cardiovascular outcomes and all-cause mortality in patients with obstructive sleep apnea and chronic obstructive pulmonary disease (overlap syndrome). Ann Am Thorac Soc. 2019;16(1):71–81. DOI:https://doi.org/10.1513/AnnalsATS.201802-136OC
- Jaoude P, El-Solh AA. Predictive factors for COPD exacerbations and mortality in patients with overlap syndrome. Clin Respir J. 2019;13(10):643–651. DOI:https://doi.org/10.1111/crj.13079
- Ioachimescu OC, Janocko NJ, Ciavatta M-M, et al. Obstructive Lung Disease and Obstructive Sleep Apnea (OLDOSA) cohort study: 10-year assessment. J Clin Sleep Med. 2020;16(2):267–277. DOI:https://doi.org/10.5664/jcsm.8180
- Xu J, Wei Z, Wang X, et al. The risk of cardiovascular and cerebrovascular disease in overlap syndrome: a meta-analysis. J Clin Sleep Med. 2020;16(7):1199–1207. DOI:https://doi.org/10.5664/jcsm.8466
- Voulgaris A, Archontogeorgis K, Papanas N, et al. Increased risk for cardiovascular disease in patients with obstructive sleep apnoea syndrome-chronic obstructive pulmonary disease (overlap syndrome). Clin Respir J. 2019;13(11):708–715. DOI:https://doi.org/10.1111/crj.13078
- Ganga HV, Nair SU, Puppala VK, et al. Risk of new-onset atrial fibrillation in elderly patients with the overlap syndrome: a retrospective cohort study. J Geriatr Cardiol. 2013;10(2):129–134.
- Akinnusi M, El-Masri AR, Lawson Y, et al. Association of overlap syndrome with incident atrial fibrillation. Intern Emerg Med. 2021;16(3):633–642. DOI:https://doi.org/10.1007/s11739-020-02469-y
- Gothi D, Gupta SS, Kumar N, et al. Impact of overlap syndrome on severity of acute exacerbation of chronic obstructive pulmonary disease. Lung India. 2015;32(6):578–583. DOI:https://doi.org/10.4103/0970-2113.168132
- Hirayama A, Goto T, Faridi MK, et al. Association of obstructive sleep apnoea with acute severity of chronic obstructive pulmonary disease exacerbation: a population-based study. Intern Med J. 2018;48(9):1150–1153. DOI:https://doi.org/10.1111/imj.14016
- Glaser JB, El-Haddad H. Exploring novel Medicare readmission risk variables in chronic obstructive pulmonary disease patients at high risk of readmission within 30 days of hospital discharge. Annals Ats. 2015;12(9):1288–1293. DOI:https://doi.org/10.1513/AnnalsATS.201504-228OC
- Medrek SK, Sharafkhaneh A, Spiegelman AM, et al. Admission for COPD exacerbation is associated with the clinical diagnosis of pulmonary hypertension: results from a retrospective longitudinal study of a veteran population. COPD. 2017;14(5):484–489. DOI:https://doi.org/10.1080/15412555.2017.1336209
- Adler D, Pépin J-L, Dupuis-Lozeron E, et al. Comorbidities and subgroups of patients surviving severe acute hypercapnic respiratory failure in the intensive care unit. Am J Respir Crit Care Med. 2017;196(2):200–207. DOI:https://doi.org/10.1164/rccm.201608-1666OC
- Singh G, Agarwal A, Zhang W, et al. Impact of PAP therapy on hospitalization rates in Medicare beneficiaries with COPD and coexisting OSA. Sleep Breath. 2019;23(1):193–200. DOI:https://doi.org/10.1007/s11325-018-1680-0
- Flemons WW, Whitelaw WA. Clinical features of obstructive sleep apnea syndrome. In: McNicholas WT, Phillipson EA, editors. Breathing Disorders in Sleep. London: WB Saunders; 2002. p. 64–85.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. DOI:https://doi.org/10.1164/rccm.2109080
- Sharma SK, Kurian S, Malik V, et al. A stepped approach for prediction of obstructive sleep apnea in overtly asymptomatic obese subjects: a hospital based study. Sleep Med. 2004;5(4):351–357. DOI:https://doi.org/10.1016/j.sleep.2004.03.004
- Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276.
- McNicholas WT, Hansson D, Schiza S, et al. Sleep in chronic respiratory disease: COPD and hypoventilation disorders. Eur Respir Rev. 2019;28(153):190064. DOI:https://doi.org/10.1183/16000617.0064-2019
- McNicholas WT. COPD-OSA overlap syndrome: evolving evidence regarding epidemiology, clinical consequences, and management. Chest. 2017;152(6):1318–1326. DOI:https://doi.org/10.1016/j.chest.2017.04.160
- Bonita R, Beaglehole R, Kjellström T. Basic epidemiology. 2nd ed. Geneva: World Health Organization; 2006.
- NIH News in Health: To Screen or Not to Screen? [Internet] Bethesda (MD): National Institutes of Health; c2017. Available from: https://newsinhealth.nih.gov/2017/03/screen-or-notscreen.
- Parish JM. Sleep-related problems in common medical conditions. Chest. 2009;135(2):563–572. DOI:https://doi.org/10.1378/chest.08-0934
- Cormick W, Olson LG, Hensley MJ, et al. Nocturnal hypoxaemia and quality of sleep in patients with chronic obstructive lung disease. Thorax. 1986;41(11):846–854. DOI:https://doi.org/10.1136/thx.41.11.846
- Lacedonia D, Carpagnano GE, Aliani M, et al. Daytime PaO2 in OSAS, COPD and the combination of the two (overlap syndrome). Respir Med. 2013;107(2):310–316. DOI:https://doi.org/10.1016/j.rmed.2012.10.012
- Kambam JR, Chen LH, Hyman SA. Effect of short-term smoking halt on carboxyhemoglobin levels and P50 values. Anesth Analg. 1986;65(11):1186–1188.
- Chowdhuri S, Quan SF, Almeida F, et al. An official American Thoracic Society research statement: impact of mild obstructive sleep apnea in adults. Am J Respir Crit Care Med. 2016;193(9):e37–e54. DOI:https://doi.org/10.1164/rccm.201602-0361ST
- Punjabi NM, Newman AB, Young TB, et al. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med. 2008;177(10):1150–1155. DOI:https://doi.org/10.1164/rccm.200712-1884OC
- Turnbull CD, Sen D, Kohler M, et al. Effect of supplemental oxygen on blood pressure in obstructive sleep apnea (SOX). A randomized continuous positive airway pressure withdrawal trial. Am J Respir Crit Care Med. 2019;199(2):211–219. DOI:https://doi.org/10.1164/rccm.201802-0240OC
- Oliveira MG, Nery LE, Santos-Silva R, et al. Is portable monitoring accurate in the diagnosis of obstructive sleep apnea syndrome in chronic pulmonary obstructive disease?Sleep Med. 2012;13(8):1033–1038. DOI:https://doi.org/10.1016/j.sleep.2012.06.011
- Jen R, Orr JE, Li Y, et al. Accuracy of WatchPAT for the diagnosis of obstructive sleep apnea in patients with chronic obstructive pulmonary disease. COPD. 2020;17(1):34–39. DOI:https://doi.org/10.1080/15412555.2019.1707789
- Holmedahl NH, Fjeldstad O-M, Engan H, et al. Validation of peripheral arterial tonometry as tool for sleep assessment in chronic obstructive pulmonary disease. Sci Rep. 2019;9(1):19392. DOI:https://doi.org/10.1038/s41598-019-55958-2
- Chang Y, Xu L, Han F, et al. Validation of the Nox-T3 portable monitor for diagnosis of obstructive sleep apnea in patients with chronic obstructive pulmonary disease. J Clin Sleep Med. 2019;15(04):587–596. DOI:https://doi.org/10.5664/jcsm.7720
- Conley S, Knies A, Batten J, et al. Agreement between actigraphic and polysomnographic measures of sleep in adults with and without chronic conditions: A systematic review and meta-analysis. Sleep Med Rev. 2019;46:151–160. DOI:https://doi.org/10.1016/j.smrv.2019.05.001
- Scott AS, Baltzan MA, Wolkove N. Examination of pulse oximetry tracings to detect obstructive sleep apnea in patients with advanced chronic obstructive pulmonary disease. Can Respir J. 2014;21(3):171–175. DOI:https://doi.org/10.1155/2014/948717
- Lajoie A-C, Sériès F, Bernard S, et al. Reliability of home nocturnal oximetry in the diagnosis of overlap syndrome in COPD. Respiration. 2020;99(2):132–139. DOI:https://doi.org/10.1159/000505299
- Ryan S, Doherty LS, Rock C, et al. Effects of salmeterol on sleeping oxygen saturation in chronic obstructive pulmonary disease. Respiration. 2010;79(6):475–481. DOI:https://doi.org/10.1159/000235619
- McNicholas WT, Calverley PMA, Lee A, et al. Long-acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPD. Eur Respir J. 2004;23(6):825–831. DOI:https://doi.org/10.1183/09031936.04.00085804
- McDonnell LM, Hogg L, McDonnell L, et al. Pulmonary rehabilitation and sleep quality: a before and after controlled study of patients with chronic obstructive pulmonary disease. NPJ Prim Care Respir Med. 2014;24:14028.
- Patil SP, Ayappa IA, Caples SM, et al. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2019;15(2):335–343. DOI:https://doi.org/10.5664/jcsm.7640
- Piper AJ, Wang D, Yee BJ, et al. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax. 2008;63(5):395–401. DOI:https://doi.org/10.1136/thx.2007.081315
- Sandhya AS, Esquinas AM, Prajapat B. Predictors of CPAP failure in OSA/COPD overlap: in which direction one may look: Daytime hypercapnia and nocturnal hypoxia are independent predictors of early CPAP failure in patients with the OSA-COPD overlap syndrome. Sleep Med. 2017;37:223–224. DOI:https://doi.org/10.1016/j.sleep.2017.06.020
- Toraldo DM, De Nuccio F, Nicolardi G. Fixed-pressure nCPAP in patients with obstructive sleep apnea (OSA) syndrome and chronic obstructive pulmonary disease (COPD): a 24-month follow-up study. Sleep Breath. 2010;14(2):115–123. DOI:https://doi.org/10.1007/s11325-009-0291-1
- Wang T-Y, Lo Y-L, Lee K-Y, et al. Nocturnal CPAP improves walking capacity in COPD patients with obstructive sleep apnoea. Respir Res. 2013;14(1):66. DOI:https://doi.org/10.1186/1465-9921-14-66
- Schreiber A, Surbone S, Malovini A, et al. The effect of continuous positive airway pressure on pulmonary function may depend on the basal level of forced expiratory volume in 1 second. J Thorac Dis. 2018;10(12):6819–6827. DOI:https://doi.org/10.21037/jtd.2018.10.103
- McNicholas WT. Comorbid obstructive sleep apnoea and chronic obstructive pulmonary disease and the risk of cardiovascular disease. J Thorac Dis. 2018;10(Suppl 34):S4253–S4261. DOI:https://doi.org/10.21037/jtd.2018.10.117
- Su M, Huai D, Cao J, et al. Auto-trilevel versus bilevel positive airway pressure ventilation for hypercapnic overlap syndrome patients. Sleep Breath. 2018;22(1):65–70. DOI:https://doi.org/10.1007/s11325-017-1529-y
- Vanfleteren LE, Beghe B, Andersson A, et al. Multimorbidity in COPD, does sleep matter?Eur J Intern Med. 2020;73:7–15. DOI:https://doi.org/10.1016/j.ejim.2019.12.032
- O’Donnell DE, Laveneziana P. The clinical importance of dynamic lung hyperinflation in COPD. COPD. 2006;3(4):219–232. DOI:https://doi.org/10.1080/15412550600977478
- Gold AR, Schwartz AR. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest. 1996;110(4):1077–1088. DOI:https://doi.org/10.1378/chest.110.4.1077
