Abstract
Community-acquired pneumonia (CAP) is a major contributor to hospitalization for acute exacerbations of chronic obstructive pulmonary disease (AECOPD). The clinical manifestations of AECOPD with and without CAP are confusing. The difference in the survival or readmission rate of AECOPD with or without CAP remains controversial. A prospective cohort study was conducted to evaluate the clinical and laboratory characteristics and in-hospital outcomes of patients who were consecutively hospitalized due to AECOPD from May 2015 to December 2019. Grouping was based on chest computed tomography findings. Multivariable logistic regression was used to explore the predictors for early identification between CAP exacerbations and non-CAP exacerbations. Kaplan–Meier analysis was used to compare the cumulative survival rate and readmission rate for a 12-month follow-up between the two groups. A total of 378 patients with AECOPD were enrolled, including 200 patients with CAP and 178 patients without CAP. The presence of pleuritic pain, usage of ICS, and elevated levels of C-reactive protein and procalcitonin on admission were the predictors for the early discrimination between AECOPD with and without CAP. During a 1-year follow-up, the cumulative survival rate was lower in patients with AECOPD with CAP than in those with AECOPD without CAP (13.0% vs. 3.37%; HR: 4.099; 95% CI, 2.049–8.199; p < 0.001), but the readmission rate was similar in both groups. Patients with first-time exacerbation due to CAP were more likely to experience subsequent pneumonic exacerbation. CAP is frequent among patients hospitalized for AECOPD and associated with increased mortality and successive pneumonic exacerbation.
Introduction
Community-acquired pneumonia (CAP) is a major contributor to hospitalization for acute exacerbations of chronic obstructive pulmonary disease (AECOPD). A population analysis of 40,000 patients with COPD over > 10 years reported that 8% experienced at least one CAP episode, producing an incidence rate of 22.4 per 1,000 person years [Citation1]. The microenvironment present in the lung may modify the progression of inflammatory response by inducing a different macrophage-activation phenotype in AECOPD with or without CAP [Citation2]. Thus, CAP should be regarded as an etiology of AECOPD but not a frequent comorbidity or overlap disease.
Several risk factors, such as old age [Citation3], comorbidities [Citation4], low body mass index (BMI) [Citation5], smoking status [Citation6], progression of airflow obstruction [Citation7], frequency, and proximity of previous exacerbations [Citation8], for hospitalization have been identified in patients with AECOPD. In addition, recent data have highlighted the role of inhaled corticosteroid (ICS) use [Citation9] and elevated eosinophils [Citation10] in the promotion of AECOPD with CAP. However, limited data regarding the predictors for the early identification between CAP and non-CAP exacerbations are available.
Few previous studies reported that the mortality calculated on hospitalization and at 30 days, 90 days and 1 year and on readmission at 90 days and 1 year was similar among patients with CAP + COPD and AECOPD [Citation11]. Other studies reached diametrically opposite conclusions, showing that first-time pneumonic COPD exacerbations had higher 30-day mortality than non-pneumonic exacerbations, and pneumonia was predicted to have increased mortality associated with a second exacerbation and up to a seventh or greater exacerbation [Citation12]. Although AECOPD with CAP may be expected to have a worse outcome than AECOPD without CAP, this expectation has not been confirmed by most prospective studies.
This study aimed to compare the cumulative survival rate and readmission rate between the two groups of AECOPD with and without CAP for a 12-month follow-up. Whether a difference existed in the subsequent pneumonic exacerbations between the two groups was also investigated. In addition, the predictors associated with AECOPD with CAP requiring hospital admission were identified.
Methods
Study cohort and definition
A prospective study was conducted among adult patients with AECOPD who were consecutively hospitalized in a respiratory center of the Eastern Hospital of the First Affiliated of Sun Yat-sen University (a 626-bed tertiary hospital) from May 2015 to December 2019. The enrolled patients were previously diagnosed with COPD confirmed by spirometry, based on the criteria established by the Global Initiative for Chronic Obstructive Lung Disease (GOLD). The study was approved by the Ethics Committees of the First Affiliated Hospital, Sun Yat-sen University, in accordance with the Declaration of Helsinki. A written informed consent was obtained from all patients. An exacerbation was defined as an increase in or a new onset of ≥ two respiratory symptoms (cough, sputum, dyspnea, wheezing, and chest tightness) that resulted in the patient’s attending physician to change the initial treatment. CAP was defined as symptoms or signs of pneumonia at hospital arrival or within 2 days after admission and new pulmonary exudation or consolidation on chest computed tomography (CT) scan without other explanation. Every patient was performed a chest CT scan immediately after admission or at emergency. Chest CT scans were interpreted by a radiologist and a pulmonologist.
The main exclusion criteria were diagnosis of asthma; bronchiectasis; active tuberculosis; pulmonary embolism; spontaneous pneumothorax; diffuse pulmonary fibrosis disease; untreated malignancy, including lung cancer; severe cardiovascular disorder, including acute left heart failure and myocardial infarction (within 3 months); septic shock; renal insufficiency requiring hemodialysis; or neurological diseases, including cerebral infarction and hemorrhage (within 3 months).
Data collection
Demographic details; underlying diseases; prescribed medication history; clinical manifestations; lung function tests; laboratory findings; and outcomes, including length of stay (LOS) in hospital, and use of noninvasive and invasive mechanical ventilation (NIMV and IMV) were collected. If a patient was admitted to the hospital more than once during the study period, only the first admission was included.
Follow-up
At the follow-up visit at 1 year from discharge, the initial readmission due to a new occurrence of symptoms/signs of AECOPD was recorded by using the same criteria. Patients readmitted for reasons other than AECOPD were not considered. The survival days from the first admission were also investigated. The cumulative survival rate and readmission rates for 1 year were compared between AECOPD with CAP and without CAP. Then, the difference in the readmission rates due to CAP between both groups was further analyzed.
Statistical analysis
All statistical analyses were performed using SPSS (version 25.0), R program (version 1.4.110), and GraphPad Prism (version 8.0). Categorical variables were presented as numbers (percentages) and analyzed using χ2 test. Continuous variables with normal distribution were shown as means (standard deviation) and analyzed using t test. Continuous variables with skewed distribution were shown as median (interquartile ranges) and analyzed using Mann–Whitney U test. The variables were classified. Least absolute shrinkage and selection operator (LASSO) regression was performed using the “glmnet” package of R software to select the best potential predictive variables. Logistic regression was used for multivariable analyses to explore the predictors for the early identification of AECOPD with CAP. Cumulative survival and readmission rates were expressed using Kaplan–Meier approach and the log-rank test. Cox proportional hazard ratio model was used to determine the hazards ratio (HR) and 95% confidence interval (CI) between appropriate factors on death and readmission. A two-sided p < 0.05 was considered statistically significant.
Results
Demographic characteristics
During the study period, 477 patients with AECOPD were admitted, and 378 patients were eligible based on the inclusion criteria. Finally, a total of 378 patients with AECOPD were enrolled in this study, including 200 patients with CAP and 178 patients without CAP (). The demographic characteristics are shown in . No difference was found on age, sex, underling diseases, smoking status, BMI, COPD categories, and lung functional parameters between both groups. However, the usage of ICS was greater in the AECOPD with CAP group than in the AECOPD without CAP group (47.5% vs. 37.1%, p = 0.041).
Figure 1. Study flow diagram. AECOPD, acute exacerbation of chronic obstructive pulmonary disease; CAP, community-acquired pneumonia.
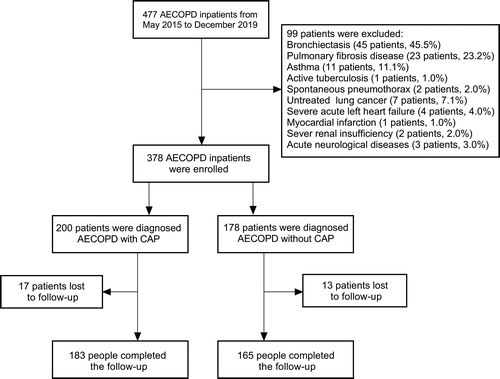
Table 1. General characteristics of AECOPD patients with and without CAP.
Clinical features, laboratory findings, and in-hospital outcomes
Fever (35.0% vs. 20.2%, p = 0.001) and pleuritic pain (13.0% vs. 3.4%, p = 0.001) were more common in the AECOPD with CAP group than in the AECOPD without CAP group. The laboratory findings on hospital admission are summarized in . Compared with the AECOPD without CAP group, the AECOPD with CAP group showed elevated white blood cell (WBC) and neutrophil counts and decreased lymphocyte, eosinophil, and red blood cell counts. The AECOPD with CAP group also showed lower levels of albumin, PaO2, and PaO2/FiO2 and higher levels of N terminal pro B-type natriuretic peptide (NT-proBNP), D-dimer, C-reactive protein (CRP), and procalcitonin (PCT) than the other group. The in-hospital outcomes, including LOS and NIMV and IMV use, were more unfavorable in the AECOPD with CAP group than in the other group.
Table 2. Clinical characteristics, laboratory findings and outcomes of AECOPD patients with and without CAP.
Predictors for AECOPD with CAP
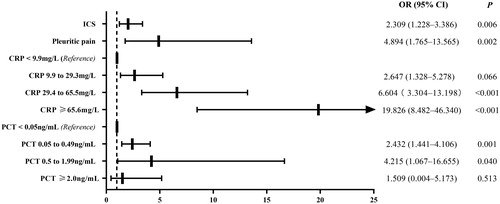
Multivariable logistic analysis indicated that the presence of pleuritic pain (odds ratio [OR], 4.894; 95% confidence interval [CI]: 1.765–13.565; p = 0.002) and the usage of ICS (OR, 2.039; 95% CI: 1.228–3.386; p = 0.006) were the clinical predictors for the early discrimination between AECOPD with CAP and AECOPD without CAP. The CRP on admission, with levels categorized by quartiles, (<9.9 mg/L, first quartile; 9.9–29.3 mg/L, second quartile; 29.4–65.5 mg/L, third quartile; ≥ 65.6 mg/L, fourth quartile) showed an increasing OR in the prediction of AECOPD with CAP (OR, 2.647; 95% CI, 1.328–5.278; p = 0.006; OR, 6.604; 95% CI, 3.304–13.198; p < 0.001; and OR, 19.826; 95% CI, 8.482–46.340; p < 0.001 in the second, third, and fourth quartiles, respectively). Moreover, the PCT with ranges of 0.05–0.49 and 0.50–1.99 ng/mL on admission were associated with CAP exacerbation (OR, 2.432; 95% CI, 1.441–4.106; p = 0.001; and OR, 4.215; 95% CI, 1.067–16.655; p = 0.040), as shown in .
Figure 2. Predictors for the early discrimination between AECOPD with CAP and AECOPD without CAP. The filled rectangles and solid lines represent OR values and 95% CIs. Hosmer-Lemeshow goodness-of-fit test: χ2, 4.545; P, 0.715. ICS, inhaled corticosteroid; CRP, C-reactive protein; PCT, procalcitonin.
Cumulative survival, readmission, and CAP readmission rates
During 1-year follow-up, the cumulative survival rate was lower in the AECOPD with CAP group than in the AECOPD without CAP group (13.0% vs. 3.37%; HR: 4.099; 95% CI, 2.049–8.199; p < 0.001). However, the readmission rate was similar in both groups (41.0% vs. 44.4%; HR: 1.051; 95% CI, 0.773–1.430; p = 0.530). The AECOPD readmission between the two groups was further compared, and the results showed that patients with first-time exacerbation due to CAP were more likely to experience successive pneumonic exacerbation (58.5% vs. 31.3%; HR: 2.621; 95% CI, 1.647–4.170; p < 0.001), as shown in .
Figure 3. Kaplan–Meier analysis was used for cumulative survival, readmission and CAP readmission rates. (A) The cumulative survival rate was lower in the AECOPD with CAP group than in the AECOPD without CAP group (13.0% vs. 3.37%; HR: 4.099; 95% CI, 2.049–8.199; p < 0.001). (B) The readmission rate was similar between the AECOPD with CAP group and the AECOPD without CAP group (41.0% vs. 44.4%; HR: 1.051; 95% CI, 0.773–1.430; p = 0.530). (C) The patients with first-time exacerbation due to CAP were more likely to experience successive pneumonic exacerbation (58.5% vs. 31.3%; HR: 2.621; 95% CI, 1.647–4.170; p < 0.001).
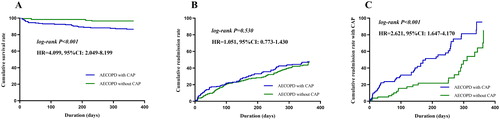
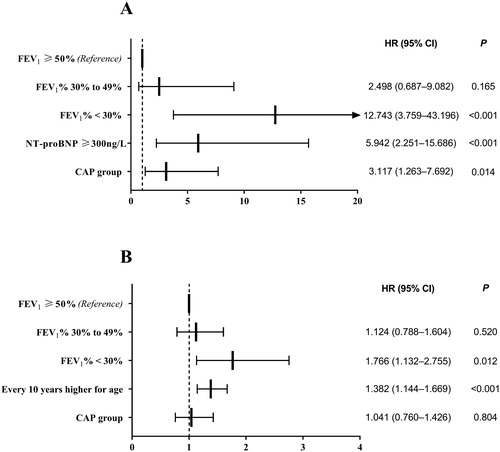
Figure 4. Cox model was used to determine the risk factors influencing death and readmission. (A) The AECOPD with CAP group (HR: 3.117; 95% CI: 1.263–7.692; p = 0.014), FEV1% < 30% (HR: 12.743; 95% CI: 3.759–43.196; p < 0.001) and NT-proBNP ≥ 300 ng/L (HR: 5.942; 95% CI: 2.251–15.686; p < 0.001) were associated with death. (B) The FEV1% < 30% (HR: 1.766; 95% CI: 1.132–2.755; p = 0.012) and every 10 years higher for age (HR: 1.382; 95% CI: 1.144–1.669; p < 0.001) were risk factors for readmission. FEV1, forced expiratory volume in 1 s; NT-proBNP, N terminal pro B-type natriuretic peptide.
Risk factors influencing death and readmission
Cox models showed that AECOPD with CAP group related to death (HR: 3.117; 95% CI: 1.263–7.692; p = 0.014) was not associated with readmission (HR: 1.041; 95% CI: 0.760–1.426; p = 0.804). The other risk factors associated with death were FEV1% < 30% (HR: 12.743; 95% CI: 3.759–43.196; p < 0.001) and NT-proBNP ≥ 300 ng/L (HR: 5.942; 95% CI: 2.251–15.686; p < 0.001). In addition, FEV1% < 30% (HR: 1.766; 95% CI: 1.132–2.755; p = 0.012) and every 10 years higher for age (HR: 1.382; 95% CI: 1.144–1.669; p < 0.001) were risk factors for readmission as shown in .
Discussion
CAP is one of the most common causes of hospitalization among patients with AECOPD. Whether AECOPD and CAP should be considered as separate entities with distinct clinical implications or whether CAP merely represents a more invasive presentation of AECOPD remains controversial. Different systemic inflammatory responses have been reported in accordance with the presence/absence of CAP in acute events, indicating that they are different immuno-pathological profile influences on AECOPD.
Approximately one-third of patients with AECOPD hospitalized for first-time exacerbation also received a diagnosis of pneumonia [Citation13, Citation14], and they were labeled depending on the presence of an infiltration or consolidation on chest X-ray. However, the considerable X-ray abnormalities remain debatable because of a considerable risk of missing or over-diagnosing CAP. In such setting, chest CT increases the confidence of practitioner in diagnosing CAP [Citation15]. Therefore, the proportion of diagnosis of CAP in patients with AECOPD based on chest CT was increased in the present study (accounting for more than 50%).
Patients with AECOPD with or without CAP had different clinical characteristics. Certain abnormalities were more specific to predict CAP (e.g. chills, pleuritic pain, and sputum purulence) [Citation11]. Fever was highly prevalent in both groups, and its presence at admission was not discriminatory [Citation16, Citation17]. The results of the present study also showed that pleuritic pain was distinctively predictive for patients with AECOPD with CAP. Previous studies found ICS to be another predictor for CAP-related acute exacerbation [Citation18, Citation19].
CAP produces a stronger inflammatory response than AECOPD in patients with COPD in terms of WBC, CRP, and PCT levels, especially in the early phase [Citation11, Citation20]. The inflammatory response appeared unaffected by the disease severity, the current pharmacotherapy (especially ICS), and the presence of an infective etiology in non-pneumonic exacerbations [Citation11], suggesting that this response was an innate immune response of different inflammatory phenotypes [Citation2, Citation21]. In this cohort, a CRP concentration of ≥ 9.9 mg/L was a sensitive biomarker for predicting CAP in patients with AECOPD. PCT, as a single test at admission, exhibited approximately the same diagnostic accuracy as CRP and WBC in diagnosing pneumonia in patients hospitalized with AECOPD [Citation20]. Besides, PCT was better than CRP, and the neutrophil/lymphocyte ratio was used to predict bacterial infection in hospitalized patients with AECOPD [Citation22]. The logistic regression model in the present study also confirmed that elevated PCT on admission (<0.05 ng/mL as references: 0.05–0.49 and 0.50–1.99 ng/mL) showed an increasing OR in the prediction of AECOPD with CAP. However, PCT ≥ 2.0 ng/mL was not meaningful, probably because this range suggested severe systematic but not specifically local inflammatory response.
The occurrence of AECOPD negatively affects COPD progression, whereas CAP may result in diametrically opposite clinical outcomes. Several studies comparing AECOPD with and without CAP failed to demonstrate the differences in LOS [Citation11, Citation17, Citation23]; treatment failure [Citation17, Citation23]; the use of invasive mechanical ventilation [Citation17]; ICU admission [Citation11, Citation17]; readmission at 90 days, 6 months, and 1 year [Citation11, Citation16, Citation24]; and mortality during hospitalization at 1 and 5 years [Citation11, Citation14, Citation16, Citation17, Citation25]. However, other studies reported that some markers of adverse events were more prevalent in pneumonic exacerbations than in non-pneumonic exacerbations, especially in-hospital mortality [Citation13, Citation23, Citation26, Citation27], and short- or long-term mortality [Citation12, Citation24, Citation26–28]. Several potential methodological criticisms of published studies may explain the conflicting outcomes, including highly heterogeneous with respect to comparator groups (e.g. coexisting respiratory comorbidities or severe AECOPD admitted in ICU), study design (e.g. retrospective or prospective study), and data source (e.g. from audit data or from health care resource databases).
After adjusting for other potential confounders, the AECOPD with CAP group had an estimate of 3.1-fold increased risk of death in 1-year follow-up compared with those without CAP. In general, hospitalization for CAP in patients with COPD is associated with the severity of airflow obstruction [Citation29, Citation30]. The present study revealed an increased risk of 1-year death and readmission in patients with AECOPD with severe airflow limitation, as marked by FEV1% < 30%. Baseline NT-proBNP in COPD is an independent predictor of respiratory exacerbations, even in individuals without overt cardiac disease [Citation31]. Increased NT-proBNP levels measured at the time of a COPD exacerbation have been associated with increased need for intensive care [Citation32] and increased mortality [Citation33–36].
This study is a prospective study of comparator groups with similar demographic characteristics. The enrolled patients were previously diagnosed with COPD confirmed by spirometry and excluded for other respiratory and systematic comorbidities that may influence acute exacerbations. The patients were followed up until 1 year after discharge. Those with initial exacerbation due to CAP were more likely to have successive pneumonic exacerbation. However, this study has several limitations. First, because it was performed in a single institution, selection bias may have influenced the significance of the findings. Thus, a multicenter study is required to validate the results. Second, the follow-up period should be prolonged to investigate the long-term outcomes. Third, the dynamic changes in some indicators, such as WBC, CRP, and PCT, could help predict the clinical outcomes. Fourth, chest X-ray was only performed in some patients. Determining the predictors of CAP via CT scan in patients with AECOPD with normal chest X-ray is meaningful.
In conclusion, the presence of pleuritic pain, the usage of ICS, and the elevated CRP and PCT on admission were predictors for the early discrimination between AECOPD with and without CAP. The calculative survival rate was lower in the AECOPD with CAP group than in the AECOPD without CAP group, but the readmission rate between the two groups did not significantly differ during the 1-year follow-up. Patients with first-time exacerbation due to CAP were more likely to experience successive pneumonic exacerbation.
Declaration of interest
The authors declare no conflict of interests.
Additional information
Funding
References
- Müllerova H, Chigbo C, Hagan GW, et al. The natural history of community-acquired pneumonia in COPD patients: a population database analysis. Respir Med. 2012;106(8):1124–1133. DOI:10.1016/j.rmed.2012.04.008
- Gutierrez P, Closa D, Piñer R, et al. Macrophage activation in exacerbated COPD with and without community-acquired pneumonia. Eur Respir J. 2010;36(2):285–291. DOI:10.1183/09031936.00118909
- Hunter LC, Lee RJ, Butcher I, et al. Patient characteristics associated with risk of first hospital admission and readmission for acute exacerbation of chronic obstructive pulmonary disease (COPD) following primary care COPD diagnosis: a cohort study using linked electronic patient records. BMJ Open. 2016;6(1):e009121–9. DOI:10.1136/bmjopen-2015-009121
- Hogea SP, Tudorache E, Fildan AP, et al. Risk factors of chronic obstructive pulmonary disease exacerbations. Clin Respir J. 2020;14(3):183–197. DOI:10.1111/crj.13129
- Schembri S, Anderson W, Morant S, et al. A predictive model of hospitalisation and death from chronic obstructive pulmonary disease. Respir Med. 2009;103(10):1461–1467. DOI:10.1016/j.rmed.2009.04.021
- Dransfield MT, Kunisaki KM, Strand MJ, COPDGene Investigators, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(3):324–330. DOI:10.1164/rccm.201605-1014OC
- Müllerová H, Shukla A, Hawkins A, et al. Risk factors for acute exacerbations of COPD in a primary care population: a retrospective observational cohort study. BMJ Open. 2014;4(12):e006171. DOI:10.1136/bmjopen-2014-006171
- Cardoso J, Coelho R, Rocha C, et al. Prediction of severe exacerbations and mortality in COPD: the role of exacerbation history and inspiratory capacity/total lung capacity ratio. Int J Chron Obstruct Pulmon Dis. 2018;13:1105–1113. DOI:10.2147/COPD.S155848
- Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(3):CD010115.
- Vedel-Krogh S, Nordestgaard BG, Lange P, et al. Blood eosinophil count and risk of pneumonia hospitalisations in individuals with COPD. Eur Respir J. 2018;51(5):1800120. DOI:10.1183/13993003.00120-2018
- Huerta A, Crisafulli E, Menéndez R, et al. Pneumonic and nonpneumonic exacerbations of COPD: inflammatory response and clinical characteristics. Chest. 2013;144(4):1134–1142. DOI:10.1378/chest.13-0488
- Søgaard M, Madsen M, Løkke A, et al. Incidence and outcomes of patients hospitalized with COPD exacerbation with and without pneumonia. Int J Chron Obstruct Pulmon Dis. 2016;11:445–465.
- Steer J, Norman EM, Afolabi OA, et al. Dyspnoea severity and pneumonia as predictors of in-hospital mortality and early readmission in acute exacerbations of COPD. Thorax. 2012;67(2):117–121. DOI:10.1136/thoraxjnl-2011-200332
- Andreassen SL, Liaaen ED, Stenfors N, et al. Impact of pneumonia on hospitalizations due to acute exacerbations of COPD. Clin Respir J. 2014;8(1):93–99. DOI:10.1111/crj.12043
- Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192(8):974–982. DOI:10.1164/rccm.201501-0017OC
- Yu S, Fang Q, Li Y. Independent factors associated with pneumonia among hospitalized patients with acute exacerbations of chronic obstructive pulmonary disease. Medicine (Baltimore). 2018;97(42):e12844. DOI:10.1097/MD.0000000000012844
- Boixeda R, Bacca S, Elias L, et al. Pneumonia as comorbidity in chronic obstructive pulmonary disease (COPD). Differences between acute exacerbation of COPD and pneumonia in patients with COPD. Arch Bronconeumol. 2014;50(12):514–520. DOI:10.1016/j.arbres.2014.02.001
- Tashkin DP, Miravitlles M, Celli BR, et al. Concomitant inhaled corticosteroid use and the risk of pneumonia in COPD: a matched-subgroup post hoc analysis of the UPLIFT® trial. Respir Res. 2018;19(1):196. DOI:10.1186/s12931-018-0874-0
- Dransfield MT, Crim C, Criner GJ, et al. Risk of exacerbation and pneumonia with single inhaler triple versus dual therapy in IMPACT. Ann Am Thorac Soc. 2021;18(5):788–798. DOI:10.1513/AnnalsATS.202002-096OC
- Titova E, Christensen A, Henriksen AH, et al. Comparison of procalcitonin, C-reactive protein, white blood cell count and clinical status in diagnosing pneumonia in patients hospitalized with acute exacerbations of COPD: a prospective observational study. Chron Respir Dis. 2019;16:1479972318769762. DOI:10.1177/1479972318769762
- Taylor AE, Finney-Hayward TK, Quint JK, et al. Defective macrophage phagocytosis of bacteria in COPD. Eur Respir J. 2010;35(5):1039–1047. DOI:10.1183/09031936.00036709
- Tanriverdi H, Örnek T, Erboy F, et al. Comparison of diagnostic values of procalcitonin, C-reactive protein and blood neutrophil/lymphocyte ratio levels in predicting bacterial infection in hospitalized patients with acute exacerbations of COPD. Wien Klin Wochenschr. 2015;127(19–20):756–763. DOI:10.1007/s00508-014-0690-6
- Lu Z, Cheng Y, Tu X, et al. Community-acquired pneumonia and survival of critically ill acute exacerbation of COPD patients in respiratory intensive care units. COPD. 2016;11:1867–1872. DOI:10.2147/COPD.S113510
- Shin B, Kim SH, Yong SJ, et al. Early readmission and mortality in acute exacerbation of chronic obstructive pulmonary disease with community-acquired pneumonia. Chron Respir Dis. 2019;16:147997231880948. DOI:10.1177/1479972318809480
- Stenfors N, Liaaen ED, Henriksen AH. No difference in long term survival in patients hospitalized for pneumonic versus non-pneumonic acute exacerbations of COPD. Clin Respir J. 2018;12(3):1305–1306. DOI:10.1111/crj.12636
- Myint PK, Lowe D, Stone RA, et al. U.K. National COPD Resources and Outcomes Project 2008: patients with chronic obstructive pulmonary disease exacerbations who present with radiological pneumonia have worse outcome compared to those with non-pneumonic chronic obstructive pulmonary disease exacerbations. Respiration. 2011;82(4):320–327. DOI:10.1159/000327203
- Kim HC, Choi SH, Huh JW, et al. Different pattern of viral infections and clinical outcomes in patient with acute exacerbation of chronic obstructive pulmonary disease and chronic obstructive pulmonary disease with pneumonia. J Med Virol. 2016;88(12):2092–2099. DOI:10.1002/jmv.24577
- Sharafkhaneh A, Spiegelman AM, Main K, et al. Mortality in patients admitted for concurrent COPD exacerbation and pneumonia. COPD. 2017;14(1):23–29. DOI:10.1080/15412555.2016.1220513
- Mannino DM, Davis KJ, Kiri VA. Chronic obstructive pulmonary disease and hospitalizations for pneumonia in a US cohort. Respir Med. 2009;103(2):224–229. DOI:10.1016/j.rmed.2008.09.005
- Juthani-Mehta M, De Rekeneire N, Allore H, Health ABC Study, et al. Modifiable risk factors for pneumonia requiring hospitalization of community-dwelling older adults: the Health, Aging, and Body Composition Study. J Am Geriatr Soc. 2013;61(7):1111–1118. DOI:10.1111/jgs.12325
- Labaki WW, Xia M, Murray S, et al. NT-proBNP in Stable COPD and future exacerbation risk: analysis of the SPIROMICS cohort. Respir Med. 2018;140:87–93. DOI:10.1016/j.rmed.2018.06.005
- Stolz D, Breidthardt T, Christ-Crain M, et al. Use of B-type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest. 2008;133(5):1088–1094. DOI:10.1378/chest.07-1959
- Medina AM, Marteles MS, Saiz EB, et al. Prognostic utility of NT-proBNP in acute exacerbations of chronic pulmonary diseases. Eur J Intern Med. 2011;22(2):167–171. DOI:10.1016/j.ejim.2010.12.002
- Marcun R, Sustic A, Brguljan PM, et al. Cardiac biomarkers predict outcome after hospitalisation for an acute exacerbation of chronic obstructive pulmonary disease. Int J Cardiol. 2012;161(3):156–159. DOI:10.1016/j.ijcard.2012.05.044
- Hoiseth AD, Omland T, Hagve TA, et al. NT-proBNP independently predicts long term mortality after acute exacerbation of COPD – a prospective cohort study. Respir Res. 2012;13(1):97. DOI:10.1186/1465-9921-13-97
- Chang CL, Robinson SC, Mills GD, et al. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax. 2011;66(9):764–768. DOI:10.1136/thx.2010.155333
