Abstract
Patients with chronic obstructive pulmonary disease (COPD) experience high rates of hospital readmissions, placing substantial clinical and economic strain on the healthcare system. Therefore, it is essential to implement evidence-based strategies for preventing these readmissions. The primary objective of our systematic review was to identify and describe the domains of existing primary research on strategies aimed at reducing hospital readmissions among adult patients with COPD. We also aimed to identify existing gaps in the literature to facilitate future research efforts. A total of 843 studies were captured by the initial search and 96 were included in the final review (25 randomized controlled trials, 37 observational studies, and 34 non-randomized interventional studies). Of the included studies, 72% (n = 69) were considered low risk of bias. The majority of included studies (n = 76) evaluated patient-level readmission prevention strategies (medication and other treatments (n = 25), multi-modal (n = 19), follow-up (n = 16), telehealth (n = 8), education and coaching (n = 8)). Fewer assessed broader system- (n = 13) and policy-level (n = 7) strategies. We observed a trend toward reduced all-cause readmissions with the use of medication and other treatments, as well as a trend toward reduced COPD-related readmissions with the use of multi-modal and broader scale system-level interventions. Notably, much of this evidence supported shorter-term (30-day) readmission outcomes, while little evidence was available for longer-term outcomes. These findings should be interpreted with caution, as considerable between-study heterogeneity was also identified. Overall, this review identified several evidence-based interventions for reducing readmissions among patients with COPD that should be targeted for future research.
Supplemental data for this article is available online at https://doi.org/10.1080/15412555.2021.1955338 .
Introduction
Characterized by progressive airway flow limitation, chronic obstructive pulmonary disease (COPD) is a substantial cause of morbidity and mortality worldwide. Contributing to 3.2 million deaths in 2019, the disease was ranked as the third leading cause of death by the World Health Organization [Citation1]. COPD places a tremendous amount of strain on healthcare systems globally, driven largely by high rates of hospital readmissions. Common risk factors for all-cause readmission among patients with COPD include comorbidities, recent exacerbations and hospitalizations, and greater index length of stay [Citation2]. Globally, 30-day COPD-related readmission rates range from 2.6%–82.2% and 12-month rates from 25.0%–87.0% [Citation3]. In their analysis of Medicare claims data, Shah et al. [Citation4] found a 30-day all-cause readmission rate of 20.2% for patients with COPD, of which COPD was the most common cause (27.6% of all readmissions). Given the considerable burden COPD readmissions place on health systems, it is imperative to develop effective prevention strategies.
Existing COPD readmission prevention strategies vary in scope from the level of the individual patient (including education and self-management training, follow-up appointments, referrals to pulmonary rehabilitation (PR) programs, tele-healthcare, medications, medication reconciliation, or hospital-at-home care) to broader healthcare policy [Citation5]. To date, several systematic reviews and meta-analyses have evaluated an array of preventative strategies among patients with COPD [Citation6–13], generally observing a reduction in readmissions. Although these reviews are useful for assessing the effects of specific interventions, to our knowledge just one review has attempted to capture the extent of the literature on readmission prevention strategies. Prieto-Centurion et al. [Citation10] completed a systematic review of COPD readmission prevention strategies focused on RCTs only, not evaluating observational literature or quality improvement (QI) work. Without a complete picture of the literature evaluating outcomes in a real-world context, it becomes challenging to compare the effectiveness of interventions and to determine directions for future research. Thus, our primary objective was to identify and describe the domains of existing primary research on strategies aimed at reducing hospital readmissions among adult patients with COPD. We also aimed to identify existing gaps in the literature to facilitate future research efforts.
Methods
A systematic review was employed to achieve the study objectives. In conducting this review we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting checklist [Citation14]. There is no registered protocol associated with the review; however, all pertinent methodological details are available in the present manuscript and its supplement.
Eligibility criteria
We included English-language primary research studies (clinical trials, QI studies, and observational studies). Conference abstracts (i.e. no available full text), commentaries/opinions, case studies, case series, and review studies were excluded. The study population of interest was adult patients over 18 years of age with COPD. Patients with concomitant diagnoses (i.e. heart failure, asthma overlap) were included. The intervention of interest was any strategy used to prevent readmission to hospital, including patient-level, system-level, and policy-level interventions. Only those studies with a comparison group (e.g. co-intervention, standard of care) were included. The primary outcomes of interest were readmission-related (e.g. readmissions, emergency department (ED) visits, outpatient visits, exacerbations, length of stay (LOS). Secondary outcomes included mortality, resource utilization (e.g. costs, provider visits), and lifestyle factors (e.g. HRQoL, symptoms). Supplementary Table 1 contains a detailed summary of the inclusion and exclusion criteria used for this review.
Table 1. Summary of outcomes for all included studies (n = 96). Check marks indicate the number of studies with one or more positive outcomes in a particular category, blank boxes represent no positive outcomes in a category, and gray boxes represent no measured outcomes in a categorya.
Search strategy
Electronic searches were performed in EMBASE, MEDLINE, and the Cochrane Central Register of Controlled Trials (CENTRAL) from January 1, 2010, to March 11, 2021. The initial search was performed on April 23, 2020, and it was updated on March 11, 2021. The search included terms such as “hospital readmission” and “chronic obstructive lung disease”. To ensure that our search captured the full extent of the literature on this topic, we also cross-checked our included studies with those included in Prieto-Centurion et al. [Citation10]’s systematic review on readmission prevention strategies.
We chose to exclude studies published before 2010 as we were interested in identifying and describing readmission prevention strategies that would best inform current care, logistics, technology, and resources. We determined that older studies may differ in terms of their clinical context, patient demography (access to care, knowledge, engagement, etc.), and disease presentation and thus may not be as relevant to today’s care. Supplementary Table 2 presents the detailed search strategy and number of studies returned from each database.
Data extraction and synthesis
The search results from each of the three databases were consolidated and duplicates were removed. Title/abstract screening was performed by two independent reviewers (MB, IS) to identify relevant articles for full text screening. Any records that did not meet the pre-defined eligibility criteria (Supplementary Table 1) based on their title and abstract were excluded. Subsequently, the two reviewers screened the full text articles to confirm eligibility for the systematic review. For those studies excluded at the full text stage, a specific reason was identified based on the eligibility criteria. If more than one reason for exclusion applied, the following hierarchy was used to select a reason: (1) study design, (2) patient population, (3) intervention, and (4) outcome. Conflicts were resolved through consensus between the two reviewers and when required through a third arbitrator (DTW). All aspects of study consolidation and screening were managed using the Covidence platform [Citation15].
Detailed information on study design, population, country, setting, intervention and control groups, follow-up, and outcomes was extracted from each article. Data extraction was performed by two reviewers (MB, IS). One reviewer extracted the data and a second independently verified a random sample of 10% of the studies for accuracy. Any discrepancies were resolved through consensus between reviewers. Data extraction was managed using Microsoft Excel software.
The included studies were then grouped into three categories based on type of readmission prevention strategy: patient-level, system-level, and policy-level strategies. The patient-level category was further divided into five sub-groups: medication and other treatments, follow-up, multi-modal, telehealth, and education and coaching. The included studies were also grouped by study design (i.e. RCTs, observational studies, and non-randomized interventional studies) and synthesized in the form of qualitative descriptions, frequency counts, tables, and harvest plots.
Risk of bias assessment
Bias assessments were performed independently on each study by two reviewers (MB, IS). Conflicts were resolved through consensus between the two reviewers, and if an agreement could not be reached, through a third arbitrator (MJ, DTW). The Cochrane Risk of Bias tool [Citation16] was used to assess the RCTs, where a score of eight or higher was considered low risk of bias and a score below eight was considered high risk of bias. The Newcastle-Ottawa Scale [Citation17] was used to evaluate the observational studies, where a score of seven or higher was considered low risk of bias and a score below seven was considered high risk of bias. The Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool [Citation18] was used to assess the non-randomized interventional studies, where the bias of each study was rated as low (comparable to a well-performed RCT), moderate (sound evidence for a non-randomized study), severe (contains some important problems), critical (too problematic to provide useful evidence), or no information (not enough information to make a judgement). We considered the studies rated as low or moderate to be at a low risk of bias and studies rated as serious, critical, or no information to be at a high risk of bias.
Harvest plots
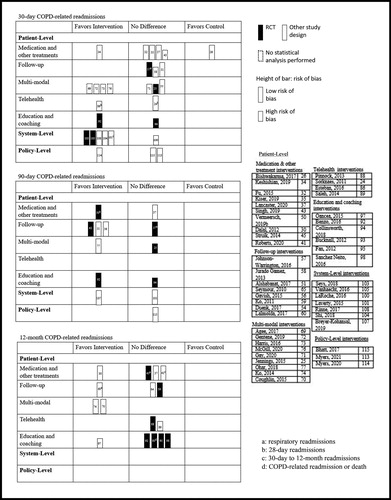
Given the heterogeneity of the included studies, we determined that it would not be appropriate to conduct a meta-analysis. Our review included both interventional and observational designs, which produced a variety of analysis techniques and effect estimates that could not be combined using traditional methods. Instead, we used harvest plots to visually display readmission outcomes. Harvest plots are a relatively novel synthesis tool used to illustrate the distribution of evidence by populating a matrix field based on statistical significance and direction of effect, thus allowing researchers to draw conclusions from heterogenous data [Citation19–21]. We created one harvest plot for all-cause readmissions and another for COPD-related readmissions, each presenting 30-day, 90-day, and 12-month time-points. Each row of the plots displayed outcomes specific to one of the seven intervention types and each column indicated the direction of effect based on statistical significance (favoring the intervention, favoring the control, or no statistically significant difference). Thus, each bar within the plot represented the results of a single study for a particular outcome of interest. Bars representing a RCT (highest quality evidence) were colored black, while bars representing the other study designs (lower quality evidence) were colored white. Further, the height of the bars indicated a study’s risk of bias assessment, where the shorter bars indicated a high risk and the taller bars low risk. While the majority of included studies performed a statistical analysis, some did not. A dotted outline around the bars was used to denote those studies where no statistical test was performed (in this case, direction of effect was determined by the absolute difference between the intervention and control groups).
Results
A total of 842 studies were returned by the database search and one study was identified through cross-checking a previous review [Citation10]. One hundred seventy-nine duplicates were identified and removed. A total of 664 unique studies were screened for eligibility at the title/abstract phase, 351 of which were deemed irrelevant. The remaining 313 studies were assessed for eligibility at the full text phase and 217 were excluded. The final qualitative synthesis included 96 studies ().
Figure 1. Study PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Flow Diagram.
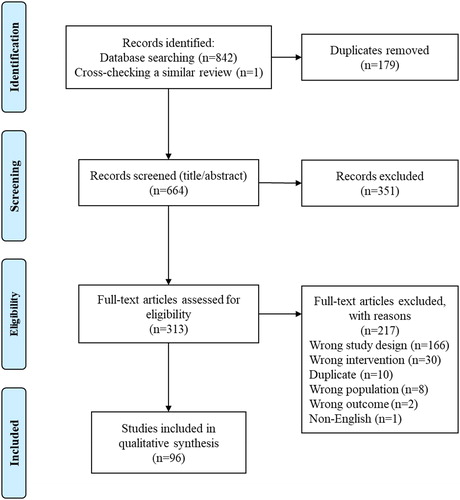
Supplementary Table 3 provides detailed information on the included studies, describing their study populations, sample characteristics, interventions, and primary and secondary outcomes. Supplementary Table 4 provides a summary of the main design characteristics and clinically relevant outcomes for each study. The 96 included studies ranged in date of publication from 2010 to 2021, with most studies (51%) published in 2017 or later. The sample sizes of the included studies ranged from n = 10 [Citation22] to n = 2,860,994 [Citation23]. There were 25 RCTs, 37 observational studies, and 34 non-randomized interventional studies (including six QI studies). A total of 57 studies took place in North America (six in Canada, 51 in the United States), 30 in Europe, and nine in Asia. Study follow-up periods ranged from 28 days [Citation24] to 36 months [Citation25] for readmission-related outcomes. and show the harvest plots for all-cause and COPD-related readmissions, respectively (at 30 days, 90 days, and 12 months).
Figure 2. Harvest plot for 30-day, 90-day, and 12-month all-cause readmissions by intervention type.
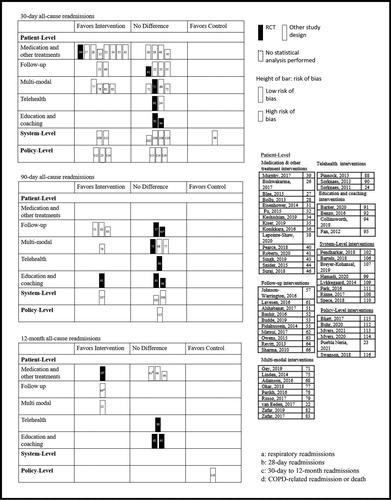
Figure 3. Harvest plot for 30-day, 90-day, and 12-month COPD-related readmissions by intervention type.
Patient-level strategies
Seventy-six studies (79%) evaluated patient-level readmission prevention strategies: 25 studied medication and other treatment-related interventions [Citation26–50], 16 studied follow-up interventions [Citation51–66], 19 studied multi-modal interventions [Citation22, Citation25, Citation67–83], eight studied telehealth interventions [Citation24, Citation84–90], and eight studied education and coaching interventions [Citation91–98].
There were 25 studies in the medication and other treatments sub-group (Supplementary Tables 3, 4). Fifteen studies focused on medications [Citation26, Citation28–30, Citation32–35, Citation37, Citation40–42, Citation47, Citation49, Citation50], three on noninvasive ventilation (NIV) [Citation39, Citation45, Citation46, Citation85], three on pharmacist medication reconciliation [Citation31, Citation38, Citation43], one on oral nutritional supplements [Citation44], one on a medication dispensing service [Citation27], one on long-term oxygen therapy [Citation48], and one on positive airway pressure therapy [Citation36]. Overall, results were varied for the readmission outcomes. For example, suggests that these interventions were relatively successful in reducing 30-day all-cause readmissions, while no notable differences were observed for the longer-term 90-day or 12-month readmissions. As such, Fu et al. [Citation32] observed a significant reduction in the odds of 30-day all-cause readmissions among those who received roflumilast within 14 days of discharge compared to those who did not (OR 0.59, 95% CI 0.37–0.93, p = 0.023). Conversely, Struik and colleagues [Citation45] found no significant difference in 12-month all-cause readmissions between those who received nocturnal NIV compared to standard treatment (NIV group median 1.0, range 0–9 vs. standard treatment group median 1.0, range 0–6; p = 0.23). shows that the effects of these interventions on COPD-related readmissions were largely insignificant. Seven of the 25 studies compared costs between groups. Three found lower costs in the intervention group compared to controls [Citation27, Citation30, Citation35], while two studies reported higher costs in the intervention group [Citation31, Citation44] and two reported no difference [Citation26, Citation47]. Four studies reported measures of HRQoL, two of which found a statistically significant improvement in the intervention group compared to controls (p < 0.05) [Citation39, Citation45, Citation46, Citation50]. Three studies recorded lung function, none of which saw a significant difference between groups for forced expiratory volume in one second (p > 0.05) [Citation45, Citation46, Citation50].
Sixteen studies evaluated follow-up interventions (Supplementary Tables 3, 4). Seven study interventions used telephone follow-ups [Citation51, Citation52, Citation54, Citation57, Citation60, Citation61, Citation63], five used in-person follow-ups [Citation53–56, Citation66], two used in-home follow-ups [Citation51, Citation58], and six used PR programs and/or at-home exercise programs [Citation57, Citation59, Citation60, Citation62, Citation64, Citation65]. Four of the studies were RCTs, just one of which reported a significant reduction in readmissions among the intervention group compared to controls (p < 0.05) [Citation65]. The harvest plots reflect a similar trend; most studies found no difference between intervention and control groups for both all-cause () and COPD-related () readmissions.
Nineteen studies focused on multi-modal interventions, all of which included some form of self-management education (e.g. disease education, action plan development) and follow-up (e.g. appointment set-up, nurse telephone follow-up) (Supplementary Tables 3, 4). In addition to the self-management and follow-up components, fourteen studies also included medication support (e.g. new medications, medication reconciliation) [Citation68, Citation70–72, Citation74–83] and five also included remote symptom monitoring (e.g. vital signs monitoring, symptom questionnaires) [Citation68, Citation69, Citation73, Citation75, Citation76]. Just three studies included aspects from all four groups [Citation68, Citation75, Citation76]. Most studies were observational [Citation77–79] or non-randomized interventional [Citation22, Citation68–74, Citation76, Citation81–83]. While these studies showed largely positive effects on readmission outcomes, the four included RCTs [Citation25, Citation67, Citation75, Citation80] showed either no significant difference (p > 0.05) or an increase in readmissions among the intervention group. Notably, suggests that while the majority of multi-modal interventions reduced 30-day COPD-related readmissions, a number of these studies were at a high risk of bias and/or did not perform a statistical analysis. Likewise, studies supporting a reduction in 30-day all-cause readmissions had similar methodological issues (). Lastly, the multi-modal interventions largely saw success in terms of patient adherence to their various intervention components. For example, Ohar et al. [Citation77] assessed a multi-modal intervention that resulted in significantly higher odds of 14-day outpatient follow-up attendance compared to usual care (OR 5.94, 95% CI 5.07–6.97). Findings on the mortality and lifestyle-related outcomes were mixed.
All interventions in the telehealth group (eight studies) included some form of symptom measurement (Supplementary Tables 3, 4). Most studies collected measurements once daily [Citation24, Citation85–90], while some collected measurements up to three times per day [Citation84]. Four studies involved video teleconsultations [Citation24, Citation85, Citation89, Citation90]. The harvest plots show that most studies found few to no differences in readmissions between the intervention and control groups (). However, in their secondary data analysis, Dyrvig et al. [Citation85] reported a significant increase in 42-day all-cause readmissions in the intervention group when compared to controls (OR 3.44, 95% CI 2.59–4.57). Of the four studies that reported mortality outcomes, only Dyrvig et al. [Citation85] reported a significant decrease in the intervention group compared to controls (hazard ratio 0.50, 95% CI 0.37–0.68 for 5-year mortality risk) while the others reported no significant difference (p > 0.05) [Citation86, Citation88, Citation90].
The education and coaching interventions (eight studies) typically included patient education and/or self-management sessions during hospital admissions with subsequent follow-up after discharge (Supplementary Tables 3, 4). Many studies included the development of a personalized action plan to implement in the event of symptom worsening. Seven of the eight studies in this group were RCTs [Citation91–96, Citation98] and one was a prospective cohort study [Citation97]. Overall, we observed largely mixed effects of the intervention on readmissions (). For example, Benzo et al. [Citation92] reported a significant reduction in COPD-related readmissions with a health coaching intervention at one (p = 0.017), three (p = 0.028), and six (p = 0.032) month intervals, but not at nine (p = 0.051) or 12 (p = 0.25) months. In terms of secondary outcomes, four studies reported on all-cause mortality; one found that mortality was significantly greater in the intervention group compared to controls (p = 0.003) [Citation95] and three found no significant difference between groups (p > 0.05) [Citation91, Citation93, Citation98].
System-level strategies
A total of 13 studies (14%) evaluated system-level interventions for readmission prevention, including standardized care pathways [Citation99–105], emergency department management [Citation106], care type (i.e. specialized respiratory care hospitals vs. general care hospitals) [Citation107], Veterans Affairs care [Citation108], GP visit tendency [Citation109], care quality [Citation110], and nurse staffing levels [Citation111] (Supplementary Tables 3, 4). Generally, we observed a positive effect of these system-level strategies on readmission outcomes. For instance, shows that all but one of the studies were successful in reducing 30-day COPD-related readmissions among the intervention group compared to controls. Vanhaecht et al.’s [Citation105] cluster RCT compared hospitals that had implemented a care pathway to hospitals with usual care. They found that COPD-related readmissions were significantly lower in the intervention group compared to controls at 30 days (OR 0.427, 95% CI 0.222–0.822), but not at six months (OR 0.642, 95% CI 0.347–1.188). Breyer-Kohansal et al.’s [Citation107] prospective cohort study found that patients admitted to specialized respiratory care hospitals had significantly lower 90-day COPD-related (p < 0.05) and all-cause (p < 0.01) readmissions compared to those admitted to general care hospitals.
Policy-level strategies
Seven studies (7%) focused on policy-level interventions for readmission prevention (Supplementary Tables 3, 4). Four evaluated the implementation of the Hospital Readmissions Reduction Program (HRRP) in the United States [Citation23, Citation112–114], two evaluated insurance policies [Citation115, Citation116], and one assessed smoking policies [Citation117]. and suggest that the effects of policy-level interventions on readmissions were varied. Bhatt et al. [Citation115]’s analysis of the Centers for Medicare & Medicaid Services Bundled Payments for Care Improvement initiative found no difference in readmissions between the intervention and control groups, and although the initiative resulted in a net savings of 4.3% ($45 165.03 USD), this figure did not include the extra $250 000 USD required for program implementation. Alternatively, Myers et al. [Citation114] reported a significant reduction in readmissions in a study comparing time periods before vs. after the implementation of the HRRP for COPD (interrupted time series analysis p = 0.009).
Bias assessment
Twenty-five RCTs were assessed for risk of bias using the Cochrane Risk of Bias tool, of which 19 were deemed low risk and six were deemed high risk (Supplementary Table 5). Thirty-seven observational studies were assessed using the Newcastle-Ottawa Scale, where 33 were deemed low risk and four were deemed high risk (Supplementary Table 6). Thirty-four non-randomized interventional studies were assessed for risk of bias using the ROBINS-I tool, where 17 were deemed low risk and 17 were deemed high risk (Supplementary Table 7). The results of those studies at a high risk of bias were considered with a level of caution, and our findings stayed largely the same upon removing these studies from our qualitative analysis. The only exception was for the multi-modal interventions where, as discussed above, many of the studies supporting a reduction in readmissions were at a high risk of bias.
Summary of results
summarizes the outcomes of interest by intervention type. Overall, the multi-modal interventions showed the most positive trends in terms of readmission-related outcomes (readmissions, emergency visits, outpatient visits, exacerbations, and LOS). Of the 19 studies in this group, 14 reported at least one positive readmission-related outcome. In addition, we observed that studies evaluating medications and other treatments and those evaluating broader system-level strategies showed largely positive readmission-related outcomes. The harvest plots highlight these same intervention types as positive, particularly in reducing 30-day readmissions ( and ). The plots also indicate that in comparison to the shorter-term 30-day outcomes, relatively less evidence is available for the longer-term 90-day and 12-month outcomes.
In terms of the secondary outcomes (mortality, resource utilization, and lifestyle factors), we found that reporting was inconsistent between studies (). Among the 96 included studies, 11 reported on cost-related outcomes with largely mixed findings. Five studies reported evidence for overall higher costs among the control group compared to the intervention group [Citation22, Citation27, Citation30, Citation35, Citation78], while two studies reported no cost difference between the treatment groups [Citation26, Citation47], and four studies reported evidence for overall higher costs among the intervention group [Citation31, Citation44, Citation104, Citation115].
Discussion
This systematic review identified 96 studies of readmission prevention strategies for adult patients with COPD. We observed varied results in terms of readmission outcomes. Notably, compared to controls, we observed a general decrease in readmissions for multi-modal, medication-related, and broader system-level strategies. Further, only 11% (n = 11) of the included studies reported cost-related outcomes, suggesting that additional research is needed to investigate the cost-effectiveness of these interventions prior to their widespread implementation.
We observed that medication and other treatment-related interventions tended to reduce hospital readmissions. Existing reviews support the utility of these types of interventions for improving patient outcomes [Citation118, Citation119]. We also observed trends toward the success of multi-modal interventions in reducing hospital readmissions, most of which included some type of medication or treatment supports. In line with this finding, Ospina et al.’s [Citation8] systematic review and meta-analysis of COPD discharge care also identified a positive effect on hospital readmissions for multimodal interventions (containing 2–12 interventions; RR 0.80, 95% CI 0.65–0.99, n = 4 studies). Multi-modal interventions are of particular interest to clinicians as they work to bridge the hospital and post-discharge periods and support the complex and heterogeneous nature of COPD [Citation5, Citation120]. Overall, these findings indicate that future research efforts should focus on medications and multi-modal interventions given that they each provide evidence supporting reduced hospital readmissions among patients with COPD.
Most studies in this review assessed the effectiveness of patient-level interventions on reducing hospital readmissions, while substantially fewer evaluated big-picture system- and policy-level interventions. This finding was expected as many of the existing evidence syntheses related to COPD readmission prevention also focus on patient-level interventions, such as early supported discharge/hospital-at home [Citation6], discharge care bundles [Citation8], and early pulmonary rehabilitation [Citation12]. Despite this discrepancy, system- and policy-level interventions may be valuable for reducing COPD readmissions on a larger scale. We observed a general trend toward a reduction in COPD readmissions among most of the included system-level studies, as well as some of the policy-level studies. System- and policy-level interventions affect change at a population level, and as a result have a larger magnitude of effect than individual-level studies. Furthermore, broader-level strategies, such as smoke free policies, have the potential to simultaneously improve outcomes across other chronic conditions including lung cancer and cardiovascular disease [Citation121]. This is especially important given that comorbidity is one of the major risk factors for both all-cause and COPD-related readmissions among patients with COPD [Citation2, Citation3].
While some interventions trended toward success in reducing short-term readmissions, we observed fewer positive effects on longer-term readmissions. We also observed a general lack of evidence exploring outcomes at these longer-term time points. It is known that an intervention is more likely to be successful over the shorter term, however its level of effectiveness may decline over the longer-term, particularly once the intervention itself ceases. These findings highlight the need to consider the sustainability of readmission prevention strategies when moving forward with future research. One useful intervention for this purpose may be self-management, which works to empower patients and achieve sustained behavior change [Citation122]. Another possible approach includes interventions that focus on continuity of care post-discharge. This may include not only self-management but also education, medication reconciliation, follow-up, and telemonitoring [Citation13]. As such, these types of interventions may be useful in addressing the modifiable risk factors for readmission, which include smoking status, alcohol use, and underweight (all-cause readmissions), as well as malnutrition (COPD-related readmissions) [Citation2, Citation3]. In summary, additional research is needed to examine and clarify the sustainability of interventions for readmission prevention using self-management techniques.
This review had several strengths. It was one of the first reviews to capture the full extent of the literature surrounding readmission prevention strategies for patients with COPD. Previous syntheses often focused on RCTs only [Citation6, Citation10, Citation11], whereas our review also included important evidence from observational and QI studies and has been updated to include the most recent research. Data from an observational study may be more useful for assessing the long-term effectiveness of an intervention compared to a RCT, as it evaluates the intervention within a real-world context. Another strength of this review was that it used validated scales to assess risk of bias among the individual studies. We used the Cochrane Risk of Bias tool [Citation16] to assess RCTs, the Newcastle-Ottawa Scale [Citation17] to assess observational studies, and the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool [Citation18] to assess non-randomized interventional studies. The majority (72%) of studies were deemed low risk of bias, indicating that the findings of our review were largely based on high quality research.
Despite its strengths, the review also had some limitations we would like to acknowledge. Firstly, only those studies written in the English language were included. This decision was made for feasibility purposes; however, it may have omitted otherwise eligible studies. Secondly, this review captured substantial heterogeneity between studies, and therefore its findings should be interpreted with a degree of caution. Prieto-Centurion and colleagues [Citation10] voiced similar concerns in their systematic review of RCTs for COPD readmission prevention, highlighting substantial variability in intervention design, measurement and reporting of outcomes, and reporting of patient baseline characteristics (which may be risk factors for readmission). In the absence of methodological standardization, the included studies produced a wide range of effects on readmission outcomes.
Conclusions
Our review observed a general decrease in all-cause readmissions for medication-related prevention strategies and in COPD-related readmissions for multi-modal and broader system-level prevention strategies, suggesting areas of future research focus. However, it is important to acknowledge that substantial methodological heterogeneity exists in this area of research. Researchers must develop standards for evaluating the effectiveness of readmission prevention strategies, including those for the measurement and reporting of baseline patient characteristics and study outcomes. Overall, this review provides a comprehensive summary of the trends in COPD readmission prevention literature over the last decade and highlights promising strategies for future research.
Supplemental Material
Download Zip (679.6 KB)Declaration of interest
The authors report no conflict of interest.
Additional information
Funding
References
- Global health estimates 2019: deaths by cause, age, sex, by country and region, 2000–2019. In: World Health Organization, editor. Geneva 2020.
- Alqahtani JS, Njoku CM, Bereznicki B, et al. Risk factors for all-cause hospital readmission following exacerbation of COPD: a systematic review and meta-analysis. Eur Respir Rev. 2020;29(156):190166. DOI:10.1183/16000617.0166-2019
- Njoku CM, Alqahtani JS, Wimmer BC, et al. Risk factors and associated outcomes of hospital readmission in COPD: a systematic review. Respir Med. 2020;173:105988. DOI:10.1016/j.rmed.2020.105988
- Shah T, Churpek MM, Coca Perraillon M, et al. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219–1226. DOI:10.1378/chest.14-2181
- Shah T, Press VG, Huisingh-Scheetz M, et al. COPD readmissions: addressing COPD in the era of value-based health care. Chest. 2016;150(4):916–926. DOI:10.1016/j.chest.2016.05.002
- Echevarria C, Brewin K, Horobin H, et al. Early supported discharge/hospital at home For acute exacerbation of chronic obstructive pulmonary disease: a review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2016;13(4):523–533. DOI:10.3109/15412555.2015.1067885
- Health Quality Ontario. Effect of early follow-up after hospital discharge on outcomes in patients with heart failure or chronic obstructive pulmonary disease: a systematic review. Ontario Health Technol Assess Ser. 2017;17(8):1–37.
- Ospina MB, Mrklas K, Deuchar L, et al. A systematic review of the effectiveness of discharge care bundles for patients with COPD. Thorax. 2017;72(1):31–39. DOI:10.1136/thoraxjnl-2016-208820
- Pedersen PU, Ersgard KB, Soerensen TB, et al. Effectiveness of structured planned post discharge support to patients with chronic obstructive pulmonary disease for reducing readmission rates: a systematic review. JBI Database Syst Rev Implement Rep. 2017;15(8):2060–2086. DOI:10.11124/JBISRIR-2016-003045
- Prieto-Centurion V, Markos MA, Ramey NI, et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations. A systematic review. Ann Am Thorac Soc. 2014;11(3):417–424. DOI:10.1513/AnnalsATS.201308-254OC
- Ridwan ES, Hadi H, Wu YL, et al. Effects of transitional care on hospital readmission and mortality rate in subjects with COPD: a systematic review and meta-analysis. Respir Care. 2019;64(9):1146–1156. DOI:10.4187/respcare.06959
- Ryrso CK, Godtfredsen NS, Kofod LM, et al. Lower mortality after early supervised pulmonary rehabilitation following COPD-exacerbations: a systematic review and meta-analysis. BMC Pulm Med. 2018;18(1):154. DOI:10.1186/s12890-018-0718-1
- Yang F, Xiong ZF, Yang C, et al. Continuity of care to prevent readmissions for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;14(2):251–261. DOI:10.1080/15412555.2016.1256384
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. DOI:10.1371/journal.pmed.1000097
- Veritas Health Innovation. Covidence Melbourne, Australia 2020. [cited 2021 Jan 15]. Available from: https://www.covidence.org/.
- Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. DOI:10.1136/bmj.d5928
- Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses: Ottawa Hospital Research Institute; n.d. [cited 2021 April 9]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. DOI:10.1136/bmj.i4919
- Burns J, Polus S, Brereton L, et al. Looking beyond the forest: Using harvest plots, gap analysis, and expert consultations to assess effectiveness, engage stakeholders, and inform policy. Res Synth Methods. 2018;9(1):132–140. DOI:10.1002/jrsm.1284
- Crowther M, Avenell A, MacLennan G, et al. A further use for the harvest plot: a novel method for the presentation of data synthesis. Res Synth Methods. 2011;2(2):79–83. DOI:10.1002/jrsm.37
- Ogilvie D, Fayter D, Petticrew M, et al. The harvest plot: a method for synthesising evidence about the differential effects of interventions. BMC Med Res Methodol. 2008;8(1):8. DOI:10.1186/1471-2288-8-8
- van Eeden AE, van de Poll I, van Vulpen G, et al. Effectiveness of case management in the prevention of COPD re-admissions: a pilot study. BMC Res Notes. 2017;10(1):621. DOI:10.1186/s13104-017-2946-5
- Puebla Neira DA, Hsu ES, Kuo YF, et al. Readmissions reduction program: mortality and readmissions for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(4):437–446. DOI:10.1164/rccm.202002-0310OC
- Sorknaes AD, Madsen H, Hallas J, et al. Nurse tele-consultations with discharged COPD patients reduce early readmissions-an interventional study. Clin Respir J. 2011;5(1):26–34. DOI:10.1111/j.1752-699X.2010.00187.x
- Jennings JH, Thavarajah K, Mendez MP, et al. Predischarge bundle for patients with acute exacerbations of COPD to reduce readmissions and ED visits: a randomized controlled trial. Chest. 2015;147(5):1227–1234. DOI:10.1378/chest.14-1123
- Bishwakarma R, Zhang W, Kuo YF, et al. Long-acting bronchodilators with or without inhaled corticosteroids and 30-day readmission in patients hospitalized for COPD. Int J Chron Obstruct Pulmon Dis. 2017; 12:477–486. DOI:10.2147/COPD.S122354
- Blee J, Roux RK, Gautreaux S, et al. Dispensing inhalers to patients with chronic obstructive pulmonary disease on hospital discharge: effects on prescription filling and readmission. Am J Health-Syst Pharm. 2015;72(14):1204–1208. DOI:10.2146/ajhp140621
- Bollu V, Ernst FR, Karafilidis J, et al. Hospital readmissions following initiation of nebulized arformoterol tartrate or nebulized short-acting beta-agonists among inpatients treated for COPD. Int J Chron Obstruct Pulmon Dis. 2013;8:631–639.
- Bollu V, Guerin A, Gauthier G, et al. Readmission risk in chronic obstructive pulmonary disease patients: comparative study of nebulized β2-Agonists. Drugs Real World Outcomes. 2017;4(1):33–41. DOI:10.1007/s40801-016-0097-y
- Dalal AA, Shah M, D’Souza AO, et al. Rehospitalization risks and outcomes in COPD patients receiving maintenance pharmacotherapy. Respir Med. 2012;106(6):829–837. DOI:10.1016/j.rmed.2011.11.012
- Eisenhower C. Impact of pharmacist-conducted medication reconciliation at discharge on readmissions of elderly patients with COPD. Ann Pharmacother. 2014;48(2):203–208. DOI:10.1177/1060028013512277
- Fu AZ, Sun SX, Huang X, et al. Lower 30-day readmission rates with roflumilast treatment among patients hospitalized for chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:909–915. DOI:10.2147/COPD.S83082
- Kawasumi Y, Paterson MJ, Morrow RL, et al. Comparative effectiveness of tiotropium and ipratropium in prevention of hospital readmission for COPD: a population-based cohort study. Clin Ther. 2013;35(4):523–531.e1. DOI:10.1016/j.clinthera.2012.10.007
- Keshishian A, Xie L, Dembek C, et al. Reduction in hospital readmission rates among Medicare beneficiaries with chronic obstructive pulmonary disease: a real-world outcomes study of nebulized bronchodilators. Clin Ther. 2019;41(11):2283–2296. DOI:10.1016/j.clinthera.2019.09.001
- Kiser TH, Reynolds PM, Moss M, et al. Impact of macrolide antibiotics on hospital readmissions and other clinically important outcomes in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease: a propensity score-matched cohort study. Pharmacotherapy. 2019;39(3):242–252. DOI:10.1002/phar.2221
- Konikkara J, Tavella R, Willes L, et al. Early recognition of obstructive sleep apnea in patients hospitalized with COPD exacerbation is associated with reduced readmission. Hosp Pract (1995). 2016;44(1):41–47. DOI:10.1080/21548331.2016.1134268
- Lancaster JW, McAuliffe L, O’Gara E, et al. Impact of antibiotic choice on readmission in adults experiencing an acute COPD exacerbation. Am J Health Syst Pharm. 2021;78(Suppl 1):S26–S32. DOI:10.1093/ajhp/zxaa317
- Lapointe-Shaw L, Bell CM, Austin PC, et al. Community pharmacy medication review, death and re-admission after hospital discharge: a propensity score-matched cohort study. BMJ Qual Saf. 2020;29(1):41–51. DOI:10.1136/bmjqs-2019-009545
- Murphy PB, Rehal S, Arbane G, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA. 2017;317(21):2177–2186. DOI:10.1001/jama.2017.4451
- Pearce JA, Shiltz DL, Ding Q. Effectiveness and safety comparison for systemic corticosteroid therapy with and without inhaled corticosteroids for COPD exacerbation management. Ann Pharmacother. 2018;52(11):1070–1077. DOI:10.1177/1060028018777769
- Roberts MH, Mapel DW, Petersen H. Comparative causal analysis of the effects of long-acting muscarinic antagonist versus no long-acting bronchodilator use on readmission or mortality after hospitalization for chronic obstructive pulmonary disease. Drugs Real World Outcomes. 2020;7(1):1–17. DOI:10.1007/s40801-019-00171-w
- Singer D, Bengtson LGS, Elliott C, et al. Healthcare resource utilization, exacerbations, and readmissions among Medicare patients with chronic obstructive pulmonary disease after long-active Muscarinic antagonist therapy initiation with soft mist versus dry powder inhalers. Int J Chron Obstruct Pulmon Dis. 2020;15:3239–3250. DOI:10.2147/COPD.S284678
- Singh D, Fahim G, Ghin HL, et al. Effects of pharmacist-conducted medication reconciliation at discharge on 30-day readmission rates of patients with chronic obstructive pulmonary disease. J Pharm Pract. 2019;48(2):203–208.
- Snider JT, Jena AB, Linthicum MT, et al. Effect of hospital use of oral nutritional supplementation on length of stay, hospital cost, and 30-day readmissions among medicare patients with COPD. Chest. 2015;147(6):1477–1484. DOI:10.1378/chest.14-1368
- Struik FM, Sprooten RTM, Kerstjens HAM, et al. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax. 2014;69(9):826–834. DOI:10.1136/thoraxjnl-2014-205126
- Suraj KP, Jyothi E, Rakhi R. Role of domiciliary noninvasive ventilation in chronic obstructive pulmonary disease patients requiring repeated admissions with acute Type II respiratory failure: a prospective cohort study. Indian J Crit Care Med. 2018;22(6):397–401. DOI:10.4103/ijccm.IJCCM_61_18
- Tran M, Xiang P, Rascati KL, et al. Predictors of appropriate pharmacotherapy management of COPD exacerbations and impact on 6-month readmission. J Manag Care Spec Pharm. 2016;22(10):1186–1193. DOI:10.18553/jmcp.2016.22.10.1186
- Turner AM, Sen S, Steeley C, et al. Evaluation of oxygen prescription in relation to hospital admission rate in patients with chronic obstructive pulmonary disease. BMC Pulm Med. (1)2014;14:127. DOI:10.1186/1471-2466-14-127
- Vermeersch K, Belmans A, Bogaerts K, et al. Treatment failure and hospital readmissions in severe COPD exacerbations treated with azithromycin versus placebo - a post-hoc analysis of the BACE randomized controlled trial. Respir Res. 2019;20(1):237. DOI:10.1186/s12931-019-1208-6
- Vermeersch K, Gabrovska M, Aumann J, et al. Azithromycin during acute chronic obstructive pulmonary disease exacerbations requiring hospitalization (BACE). A multicenter, Randomized, Double-Blind, Placebo-controlled Trial. Am J Respir Crit Care Med. 2019;200(7):857–868. DOI:10.1164/rccm.201901-0094OC
- Alshabanat A, Otterstatter MC, Sin DD, et al. Impact of a COPD comprehensive case management program on hospital length of stay and readmission rates. Int J Chron Obstruct Pulmon Dis. 2017;12:961–971. DOI:10.2147/COPD.S124385
- Bashir B, Schneider D, Naglak MC, et al. Evaluation of prediction strategy and care coordination for COPD readmissions. Hosp Pract (1995). 2016;44(3):123–128. DOI:10.1080/21548331.2016.1210472
- Budde J, Agarwal P, Mazumdar M, et al. Follow-up soon after discharge may not reduce COPD readmissions. Chronic Obstr Pulm Dis. 2019;6(2):129–131. DOI:10.15326/jcopdf.6.2.2018.0149
- Duenk RG, Verhagen C, Bronkhorst EM, et al. Proactive palliative care for patients with COPD (PROLONG): a pragmatic cluster controlled trial. Int J Chron Obstruct Pulmon Dis. 2017;12:2795–2806. DOI:10.2147/COPD.S141974
- Fidahussein SS, Croghan IT, Cha SS, et al. Posthospital follow-up visits and 30-day readmission rates in chronic obstructive pulmonary disease. Risk Manag Healthc Policy. 2014;7:105–112.
- Gavish R, Levy A, Dekel OK, et al. The association between hospital readmission and pulmonologist follow-up visits in patients with COPD. Chest. 2015;148(2):375–381. DOI:10.1378/chest.14-1453
- Johnson-Warrington V, Rees K, Gelder C, et al. Can a supported self-management program for COPD upon hospital discharge reduce readmissions? A randomized controlled trial. Int J Chron Obstruct Pulmon Dis. 2016;11:1161–1169.
- Jurado Gamez B, Feu Collado N, Jurado Garcia JC, et al. Home intervention and predictor variables for rehospitalization in chronic obstructive pulmonary disease exacerbations. Arch Bronconeumol. 2013;49(1):10–14. DOI:10.1016/j.arbr.2012.11.002
- Ko FW, Dai DL, Ngai J, et al. Effect of early pulmonary rehabilitation on health care utilization and health status in patients hospitalized with acute exacerbations of COPD. Respirology. 2011;16(4):617–624. DOI:10.1111/j.1440-1843.2010.01921.x
- Lalmolda C, Coll-Fernandez R, Martinez N, et al. Effect of a rehabilitation-based chronic disease management program targeting severe COPD exacerbations on readmission patterns. Int J Chron Obstruct Pulmon Dis. 2017;12:2531–2538. DOI:10.2147/COPD.S138451
- Lavesen M, Ladelund S, Frederiksen AJ, et al. Nurse-initiated telephone follow-up on patients with chronic obstructive pulmonary disease improves patient empowerment, but cannot prevent readmissions. Dan Med J. 2016;63(10):A5276.
- Matsui H, Jo T, Fushimi K, et al. Outcomes after early and delayed rehabilitation for exacerbation of chronic obstructive pulmonary disease: a nationwide retrospective cohort study in Japan. Respir Res. 2017;18(1):68. DOI:10.1186/s12931-017-0552-7
- Owens JM, Garbe RA. Effect of enhanced psychosocial assessment on readmissions of patients with chronic obstructive pulmonary disease. Soc Work Health Care. 2015;54(3):234–251. DOI:10.1080/00981389.2015.1005269
- Revitt O, Sewell L, Morgan MDL, et al. Short outpatient pulmonary rehabilitation programme reduces readmission following a hospitalization for an exacerbation of chronic obstructive pulmonary disease. Respirology. 2013;18(7):1063–1068.
- Seymour JM, Moore L, Jolley CJ, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax. 2010;65(5):423–428. DOI:10.1136/thx.2009.124164
- Sharma G, Kuo Y-F, Freeman JL, et al. Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010;170(18):1664–1670. DOI:10.1001/archinternmed.2010.345
- Aboumatar H, Naqibuddin M, Chung S, et al. Effect of a hospital-initiated program combining transitional care and long-term self-management support on outcomes of patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. JAMA. 2019;322(14):1371–1380. DOI:10.1001/jama.2019.11982
- Adamson SL, Burns J, Camp PG, et al. Impact of individualized care on readmissions after a hospitalization for acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:61–71.
- Agee J. Reducing chronic obstructive pulmonary disease 30-day readmissions: a nurse-led evidence-based quality improvement project. J Nurs Adm. 2017;47(1):35–40. DOI:10.1097/NNA.0000000000000434
- Coughlin S, Liang WE, Parthasarathy S. Retrospective assessment of home ventilation to reduce rehospitalization in chronic obstructive pulmonary disease. J Clin Sleep Med. 2015;11(06):663–670. DOI:10.5664/jcsm.4780
- Gay E, Desai S, McNeil D. A multidisciplinary intervention to improve care for high-risk COPD patients. Am J Med Qual. 2020;35(3):231–235. DOI:10.1177/1062860619865329
- Gentene AJ, Guido MR, Woolf B, et al. Multidisciplinary team utilizing pharmacists in multimodal, bundled care reduce chronic obstructive pulmonary disease hospital readmission rates. J Pharm Pract. 2021;34(1):110–116.
- Harris S, Lang B, Percy RE, et al. Reducing 30-day readmissions for chronic obstructive pulmonary disease. Medsurg Nurs. 2016;25(6):403–422.
- Ko FWS, Ngai JCN, Ng SSS, et al. COPD care programme can reduce readmissions and in-patient bed days. Respir Med. 2014;108(12):1771–1778. DOI:10.1016/j.rmed.2014.09.019
- Linden A, Butterworth S. A comprehensive hospital-based intervention to reduce readmissions for chronically ill patients: a randomized controlled trial. Am J Manag Care. 2014;20(10):783–792.
- McGill C. Reducing COPD rehospitalizations. Home Healthc Now. 2020;38(2):80–85. DOI:10.1097/NHH.0000000000000839
- Ohar JA, Loh CH, Lenoir KM, et al. A comprehensive care plan that reduces readmissions after acute exacerbations of COPD. Respir Med. 2018;141:20–25. DOI:10.1016/j.rmed.2018.06.014
- Parikh R, Shah TG, Tandon R. COPD exacerbation care bundle improves standard of care, length of stay, and readmission rates. Int J Chron Obstruct Pulmon Dis. 2016;11(1):577–583.
- Russo AN, Sathiyamoorthy G, Lau C, et al. Impact of a post-discharge integrated disease management program on COPD hospital readmissions. Respir Care. 2017;62(11):1396–1402. DOI:10.4187/respcare.05547
- Silver PC, Kollef MH, Clinkscale D, et al. A respiratory therapist disease management program for subjects hospitalized with COPD. Respir Care. 2017;62(1):1–9. DOI:10.4187/respcare.05030
- Zafar MA, Loftus TM, Palmer JP, et al. COPD care bundle in emergency department observation unit reduces emergency department revisits. Respir Care. 2020;65(1):1–10. DOI:10.4187/respcare.07088
- Zafar MA, Nguyen B, Gentene A, et al. Pragmatic challenge of sustainability: long-term adherence to COPD care bundle maintains lower readmission rate. Jt Comm J Qual Patient Saf. 2019;45(9):639–645. DOI:10.1016/j.jcjq.2019.05.011
- Zafar MA, Panos RJ, Ko J, et al. Reliable adherence to a COPD care bundle mitigates system-level failures and reduces COPD readmissions: a system redesign using improvement science. BMJ Qual Saf. 2017;26(11):908–918. DOI:10.1136/bmjqs-2017-006529
- Chau J, Lee D, Yu D, et al. A feasibility study to investigate the acceptability and potential effectiveness of a telecare service for older people with chronic obstructive pulmonary disease. Int J Med Inform. 2012;81(10):674–682. DOI:10.1016/j.ijmedinf.2012.06.003
- Dyrvig A, Gerke O, Kidholm K, et al. A cohort study following up on a randomised controlled trial of a telemedicine application in COPD patients. J Telemed Telecare. 2015;21(7):377–384. DOI:10.1177/1357633X15572202
- Esteban C, Moraza J, Iriberri M, et al. Outcomes of a telemonitoring-based program (telEPOC) in frequently hospitalized COPD patients. Int J Chron Obstruct Pulmon Dis. 2016;11:2919–2930. DOI:10.2147/COPD.S115350
- Ho T-W, Huang C-T, Chiu H-C, et al. Effectiveness of telemonitoring in patients with chronic obstructive pulmonary disease in Taiwan-a randomized controlled trial. Sci Rep. 2016;6:23797. DOI:10.1038/srep23797
- Pinnock H, Hanley J, McCloughan L, et al. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: researcher blind, multicentre, randomised controlled trial. BMJ. 2013;347:f6070. DOI:10.1136/bmj.f6070
- Saleh S, Larsen JP, Bergsaker-Aspoy J, et al. Re-admissions to hospital and patient satisfaction among patients with chronic obstructive pulmonary disease after telemedicine video consultation - a retrospective pilot study. Multidiscip Respir Med. 2014;9(1):6. DOI:10.1186/2049-6958-9-6
- Sorknaes A, Bech M, Madsen H, et al. The effect of real-time teleconsultations between hospital-based nurses and patients with severe COPD discharged after an exacerbation. J Telemed Telecare. 2013;19(8):466–474. DOI:10.1177/1357633X13512067
- Barker RE, Jones SE, Banya W, et al. The effects of a video intervention on posthospitalization pulmonary rehabilitation uptake. A randomized controlled trial. Am J Respir Crit Care Med. 2020;201(12):1517–1524. DOI:10.1164/rccm.201909-1878OC
- Benzo R, Vickers K, Novotny PJ, et al. Health coaching and chronic obstructive pulmonary disease rehospitalization: a randomized study. Am J Respir Crit Care Med. 2016;194(6):672–680. DOI:10.1164/rccm.201512-2503OC
- Bucknall CE, Miller G, Lloyd SM, et al. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ. 2012;344:e1060. DOI:10.1136/bmj.e1060
- Collinsworth AW, Brown RM, James CS, et al. The impact of patient education and shared decision making on hospital readmissions for COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:1325–1332. DOI:10.2147/COPD.S154414
- Fan VS, Gaziano JM, Lew R, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med. 2012;156(10):673–683. DOI:10.7326/0003-4819-156-10-201205150-00003
- Hegelund A, Andersen IC, Andersen MN, et al. The impact of a personalised action plan delivered at discharge to patients with COPD on readmissions: a pilot study. Scand J Caring Sci. 2020;34(4):909–918. DOI:10.1111/scs.12798
- Oancea C, Fira-Mladinescu O, Timar B, et al. Impact of medical education program on COPD patients: a cohort prospective study. Wien Klin Wochenschr. 2015;127(9–10):388–393. DOI:10.1007/s00508-015-0712-z
- Sanchez-Nieto J, Andujar-Espinosa R, Bernabeu-Mora R, et al. Efficacy of a self-management plan in exacerbations for patients with advanced COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1939–1947. DOI:10.2147/COPD.S104728
- Hamadi HY, Martinez D, Xu J, et al. Effects of post-discharge telemonitoring on 30-day chronic obstructive pulmonary disease readmissions and mortality. J Telemed Telecare. 2020. Online ahead of print. DOI:10.1177/1357633X20970402
- LaRoche KD, Hinkson CR, Thomazin BA, et al. Impact of an electronic medical record screening tool and therapist-driven protocol on length of stay and hospital readmission for COPD. Respir Care. 2016;61(9):1137–1143. DOI:10.4187/respcare.04588
- Laverty AA, Elkin SL, Watt HC, et al. Impact of a COPD discharge care bundle on readmissions following admission with acute exacerbation: interrupted time series analysis. PLoS One. 2015;10(2):e0116187. DOI:10.1371/journal.pone.0116187
- Pendharkar SR, Ospina MB, Southern DA, et al. Effectiveness of a standardized electronic admission order set for acute exacerbation of chronic obstructive pulmonary disease. BMC Pulm Med. 2018;18(1):93. DOI:10.1186/s12890-018-0657-x
- Seys D, Bruyneel L, Sermeus W, et al. Teamwork and adherence to recommendations explain the effect of a care Pathway on Reduced 30-day Readmission for Patients with a COPD Exacerbation . Int J Chron Obstruct Pulmon Dis. 2018;15(2):157–164. DOI:10.1080/15412555.2018.1434137
- Shi M, Wang J, Zhang L, et al. Effects of Integrated Case Payment on medical expenditure and readmission of inpatients with chronic obstructive pulmonary disease: a nonrandomized, comparative study in Xi County, China. Curr Med Sci 2018;38(3):558–566. DOI:10.1007/s11596-018-1914-1
- Vanhaecht K, Lodewijckx C, Sermeus W, et al. Impact of a care pathway for COPD on adherence to guidelines and hospital readmission: a cluster randomized trial. Int J Chron Obstruct Pulmon Dis. 2016;11:2897–2908. DOI:10.2147/COPD.S119849
- Bartels W, Adamson S, Leung L, et al. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease: factors predicting readmission. Int J Chron Obstruct Pulmon Dis . 2018;13:1647–1654. DOI:10.2147/COPD.S163250
- Breyer-Kohansal R, Hartl S, Breyer M-K, et al. The European COPD audit: adherence to guidelines, readmission risk and hospital care for acute exacerbations in Austria. Wien Klin Wochenschr. 2019;131(5–6):97–103. DOI:10.1007/s00508-019-1441-5
- Rinne ST, Elwy AR, Bastian LA, et al. Impact of multisystem health care on readmission and follow-up among Veterans hospitalized for chronic obstructive pulmonary disease. Med Care. 2017(Suppl 1);55:S20–S25. DOI:10.1097/MLR.0000000000000708
- Lykkegaard J, Larsen PV, Paulsen MS, et al. General practitioners’ home visit tendency and readmission-free survival after COPD hospitalisation: a Danish nationwide cohort study. NPJ Prim Care Respir Med. 2014;24:14100. DOI:10.1038/npjpcrm.2014.100
- Spece LJ, Donovan LM, Griffith MF, et al. Quality of care delivered to Veterans with COPD exacerbation and the association with 30-day readmission and death. Int J Chron Obstruct Pulmon Dis. 2018;15(5):489–495. DOI:10.1080/15412555.2018.1543390
- Park EC, Han KT, Kim SJ, et al. Nurse staffing and 30-day readmission of chronic obstructive pulmonary Disease Patients: A 10-year Retrospective Study of Patient Hospitalization . Asian Nurs Res (Korean Soc Nurs Sci). 2016;10(4):283–288. DOI:10.1016/j.anr.2016.09.003
- Buhr RG, Jackson NJ, Kominski GF, et al. Readmission rates for chronic obstructive pulmonary disease under the Hospital Readmissions Reduction Program: an interrupted time series analysis. J Gen Intern Med. 2020;35(12):3581–3590. DOI:10.1007/s11606-020-05958-0
- Myers LC, Cash R, Liu VX, et al. Reducing readmissions for chronic obstructive pulmonary disease in response to the Hospital Readmissions Reduction Program. Ann Am Thorac Soc. 2021. Online ahead of print. DOI:10.1513/AnnalsATS.202007-786OC
- Myers LC, Faridi MK, Hasegawa K, et al. The hospital readmissions reduction program and readmissions for chronic obstructive pulmonary disease, 2006-2015. Ann Am Thorac Soc. 2020;17(4):450–456. DOI:10.1513/AnnalsATS.201909-672OC
- Bhatt SP, Wells JM, Iyer AS, et al. Results of a Medicare Bundled Payments for Care Improvement initiative for chronic obstructive pulmonary disease readmissions. Ann Am Thorac Soc. 2017;14(5):643–648. DOI:10.1513/AnnalsATS.201610-775BC
- Swanson JO, Vogt V, Sundmacher L, et al. Continuity of care and its effect on readmissions for COPD patients: a comparative study of Norway and Germany. Health Policy. 2018;122(7):737–745. DOI:10.1016/j.healthpol.2018.05.013
- Stallings-Smith S, Hamadi HY, Peterson BN, et al. Smoke-free policies and 30-day readmission rates for chronic obstructive pulmonary disease. Am J Prev Med. 2019;57(5):621–628. DOI:10.1016/j.amepre.2019.06.008
- Jia X, Zhou S, Luo D, et al. Effect of pharmacist-led interventions on medication adherence and inhalation technique in adult patients with asthma or COPD: a systematic review and meta-analysis. J Clin Pharm Ther. 2020;45(5):904–917. DOI:10.1111/jcpt.13126
- Miravitlles M, D’Urzo A, Singh D, et al. Pharmacological strategies to reduce exacerbation risk in COPD: a narrative review. Respir Res. 2016;17(1):112. DOI:10.1186/s12931-016-0425-5
- Kripalani S, Theobald CN, Anctil B, et al. Reducing hospital readmission rates: current strategies and future directions. Annu Rev Med. 2014;65(1):471–485. DOI:10.1146/annurev-med-022613-090415
- Tan CE, Glantz SA. Association between smoke-free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: a meta-analysis. Circulation. 2012;126(18):2177–2183. DOI:10.1161/CIRCULATIONAHA.112.121301
- Zwerink M, Brusse-Keizer M, van der Valk P, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;3:CD002990.
