Abstract
Acute exacerbations of Chronic Obstructive Pulmonary Disease (AECOPD) and community acquired pneumonia (CAP) are two common acute attacks in COPD patients and it is not always easy to determine whether a COPD patient at admission has parenchymal infection or bronchial infection. Comprehensive comparison between AECOPD patients and CAP patients with COPD (COPD + CAP) can help us understand them better. We retrospectively collected the medical records of AECOPD and COPD + CAP patients. Systemic inflammation, eosinophilic inflammation, damage to other organs, common chronic comorbidities, structural changes, phenotype and endotype distributions and coagulation functions between two groups were compared and correlations of these characteristics in total subjects, AECOPD patients and COPD + CAP patients were analyzed. Logistic regression analysis was performed to select helpful biomarkers for distinguishing between them. Receiver operator characteristic (ROC) curve was plotted to assess the diagnostic value of selected biomarkers and their combination. A nomogram was established for the differential diagnosis of AECOPD and COPD + CAP. A total of 206 patients were included into our analysis. In these subjects, 104 patients were classified as AECOPD group and 102 patients were considered to have COPD + CAP mainly based on their chest CT scan results. The counts of eosinophils (EOS), basophils (BAS) and lymphocytes (LYM) and percentage of total white blood cell count, hemoglobin and hematocrit were increased in AECOPD patients compared with COPD + CAP patients. The counts of neutrophils (NEU) and percentage of total white blood cell count, C-reactive protein (CRP), Erythrocyte sedimentation rate (ESR), fibrinogen, D-dimer and N-Terminal pro-brain natriuretic peptide (NT-proBNP) levels were increased in COPD + CAP patients. After logistic regression analysis, EOS < 0.5 × 109/L, ESR ≥ 8 mm/H and NT-proBNP ≥ 100 pg/mL were selected as helpful biomarkers for diagnosis of COPD + CAP instead of AECOPD. Area under the ROC curve (AUC) of the combination of selected biomarkers was 0.764(0.698-0.829). A nomogram was established and the calibration curve suggested that fitting efficiency of the nomogram was good. AECOPD and COPD + CAP are markedly different, mainly reflected in eosinophilic inflammation, systemic inflammation and coagulation function. Correlations between some common inflammatory biomarkers are also different in the two groups. A nomogram was established to offer help to clinicians for differential diagnosis of these two diseases.
Background
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease characterized by persistent respiratory symptoms and airflow limitation, with acute exacerbations which impair lung function [Citation1]. It is already the third leading cause of death which the World Health Organization predicted would happen by 2030 [Citation2, Citation3]. The social and economic burdens of COPD are projected to increase because of aging of population and continued exposure to noxious gases or particles such as cigarette smoke [Citation4].
An acute exacerbation of COPD (AECOPD) is defined as acute worsening of respiratory symptoms that results in additional therapy. The criteria is more than day-to-day variation in respiratory symptoms, including increased dyspnea, increased sputum volume and worsening in sputum purulence (generally green or yellow) [Citation5]. Exacerbation episodes are associated with declined lung function, increased mortality and poor quality of life [Citation3].
The risk of CAP is elevated in COPD patients [Citation6]. AECOPD and pneumonia are two common acute attacks in COPD patients and their symptoms are similar. As the clarity of CT increases, many asymptomatic nodules are detected, which increases the uncertainty of diagnosis to a certain extent. Finding biomarkers which are helpful to distinguish between them is necessary. In the past decades, several researches have studied the differences between AECOPD and COPD + CAP [Citation7, Citation8, Citation9, Citation10]. They compared the background, clinical and evolutive manifestations, disease course, infectious etiologies, and inflammatory response between them. To our knowledge, no research has compared the eosinophilic, structural characteristics, phenotypes or endotypes of AECOPD and COPD + CAP. Eosinophils are reported to play important roles in COPD in recent years [Citation11]. The aim of this study is to compare the characteristics of AECOPD and COPD + CAP, including systemic inflammation, eosinophilic inflammation, damage to other organs, common chronic comorbidities, structural changes, phenotype and endotype distributions and coagulation functions, find biomarkers which can better differentiate between them.
Methods
Study population
We retrospectively collected the medical records of AECOPD patients and COPD + CAP patients admitted to the department of respiratory and critical care medicine of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, from January 2015 to July 2020. COPD was diagnosed based on medical history, clinical manifestation, chest radiograph and spirometry in accordance with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) [Citation1]. Specifically, COPD should be considered in any patient with dyspnea, chronic cough or sputum production, and/or a history of exposure to risk factors for the disease. Spirometry is required to make the diagnosis in this clinical context; a post bronchodilator FEV1/FVC < 0.70 confirms the presence of persistent airflow limitation and identifies the presence of COPD in patients with appropriate symptoms and predisposing risks. Spirometry was conducted at least six months before admission to hospital due to AECOPD or CAP. AECOPD was defined as presentation of at least two of following manifestations: increased volume of sputum, change of sputum color and worsening of dyspnea [Citation12]. CAP was diagnosed according to symptoms, physical examination and chest CT scan (new pulmonary infiltrate) [Citation13]. Patients with uncertain CT diagnosis were excluded. The study was approved by the hospital ethics committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Inclusion and exclusion criteria
Patients were included if they met the following criteria: males aged between 40-85 years old with a smoking history more than 20 pack-years. Exclusion criteria were as follows: no spirometry report, admission to hospital in one month before this study, treatment with immunosuppressant, malignant disease. Patients with other chronic respiratory diseases such as asthma, interstitial lung disease, active tuberculosis and bronchiectasis were excluded.
Definition of phenotypes and endotypes of COPD
COPD is a heterogeneous disease. In order to better understand the characteristics of AECOPD and COPD + CAP patients, we compared the phenotype and endotype distributions between these two groups. Patients with chronic bronchitis or emphysema were confirmed by the report of chest CT. COPD with high eosinophils were diagnosed with circulating eosinophils ≥ 100/μL at admission [Citation14]. COPD with high neutrophils were diagnosed with circulating neutrophils > clinical reference threshold at admission. COPD with systemic inflammation was defined with 3 high biomarkers (CRP > 3 mg/L, Fibrinogen(Fbg)>14 μmol/L and leukocyte count > 9 × 109/L) [Citation15]. “Pink puffer” (PP) patients were defined as presence of emphysema on CT scan and normal pressure of arterial carbon dioxide (PaCO2 ≤ 45 mmHg) while “Blue bloater” (BB) patients were defined as absence of emphysema and hypercapnia (PaCO2 > 45 mmHg) derived from previous publications [Citation16, Citation17].
Data extraction
Medical records were screened and data were extracted on admission. From each patient, the following information was extracted: demographic characteristics (age, sex, height, weight, smoking history and comorbidities), clinical manifestation (cough, sputum and sputum color, dyspnea, fever, chill, heart rate and blood pressure), pulmonary function (forced expiratory volume in one second (FEV1), FEV1% pred, FEV1% change, forced vital capacity (FVC), FEV1/FVC, inspiratory capacity (IC), IC% pred), fractional exhaled nitric oxide (FENO), pulmonary radiograph (emphysema, chronic bronchitis), laboratory data (blood routine test, D-dimer, fibrinogen, cardiac enzymes, renal function, CD4+ and CD8+ T cell, ESR, ESR, blood gas analysis).
Statistical analysis
Continuous variables were expressed as mean ± SEM or median (interquartile range (IQR)) as appropriate. Categorical variables were expressed as frequencies and proportions. Comparisons between two groups were performed with Student’s t-test (normally distributed) or the Mann-Whitney U-test for continuous variables and χ2 for categorical variables. Correlation was analyzed with the Spearman Rank Order
Correlation. For most variables, the percentages of missing values were less than 5%. The proportions of missing data were <15% and were imputed with multiple imputation as reported [Citation18]. A multivariable logistic regression analysis was conducted to find helpful biomarkers which can provide better discrimination of AECOPD and COPD + CAP. In order to perform logistic regression analysis, continuous variables were converted into binary variables using a cutoff point. Cutoff point was determined by Yoden index. ROC curve and AUC were plotted and calculated to evaluate the diagnostic value of selected variables and their combination. To make the result of this research feasible to clinicians, a nomogram was established. Calibration curve was plotted to assess the fitting efficiency of the nomogram.
All statistical analyses were performed with R software 3.6.2, Statistical Package for Social Sciences 26.0 and GraphPad Prism 7. Two-sided P value <0.05 was considered statistically significant.
Results
Characteristics of subjects
After searching medical records for patients with AECOPD and COPD + CAP patients, 3306 patients were screened in total. 2654 patients were excluded because there were no specific lung function data. Because of the default of lung function test and medical records in our hospital, only lung function test results instead of specific data were recorded in many cases. Patients diagnosed in other hospitals also do not have specific lung function data. 253 patients were excluded because they received treatment in local hospitals during the past one month before admission to Tongji Hospital. 193 patients were adimited to Tongji Hospital more than once. For patients with repeated admissions, only one medical record was selected according inclusion and exclusion criteria. At last, a total of 206 patients were included into our analysis. General Characteristics of the 206 patients involved in this analysis were shown in . In these subjects, 104 patients were classified as AECOPD group and 102 patients were considered to have COPD + CAP. Basic characteristics between groups were not statistically different, including age, smoking index, body mass index (BMI), lung function and some chronic comorbidities common in COPD patients. The number of patients with fever in COPD + CAP group was greater than AECOPD group (23, 22.5% vs 7, 6.7%; p = 0.001).
Table 1. General characteristic of study cohorts.
Levels of laboratory measurements in two groups
Circulating neutrophils of COPD + CAP patients were more than AECOPD patients while lymphocytes of AECOPD patients were more than COPD + CAP patients. Interestingly, circulating eosinophils and basophils in AECOPD group were increased compared with COPD + CAP group. Coagulation biomarkers in COPD + CAP patients were also increased compared with AECOPD patients (shown in and ). COPD + CAP patients have significantly higher systemic inflammation and greater damage to myocardium than AECOPD patients, mainly manifested as higher CRP, ESR and NT-proBNP levels in COPD + CAP patients compared with AECOPD patients. (CRP: 15.70(4.00-51.72) vs 4.50(1.10-38.25); ESR: 17.00(7.00-35.25) vs 7.00(3.00-22.25); NT-proBNP: 130.5(64-365.5) vs 64.5(29-182.5)) ( and ). We did not find significant difference in blood gas analysis between two cohorts ().
Figure 1. Panel of representative laboratory measurements in AECOPD patients and COPD + CAP patients. The data are presented as median (P25quartile, P75 quartile). *p < 0.05; **p < 0.01.
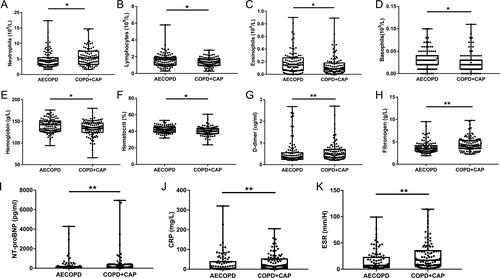
Table 2. Results of routine blood examination and coagulation function at admission.
Table 3. Results of T lymphocytes, inflammatory biomarkers and blood biochemistry examination at admission.
Table 4. Results of blood gas analysis at Admission.
Phenotypes and endotypes of COPD in two groups
COPD is a heterogeneous disease with many phenotypes and endotypes. With the aim of targeted therapy and furtherly demonstrating characteristics of the two groups of patients, we compared phenotype and endotype distribution in two groups. The result was shown in . We can see that the percentage of patients with systemic inflammation was higher in COPD + CAP group (15.69% vs 4.81%, p = 0.01) while percentage of patients with high eosinophils was higher in AECOPD group (65.38% vs 50%, p = 0.03).
Table 5. Phenotypes and endotypes of COPD in two groups.
Correlations between laboratory measurements in total subjects and two groups
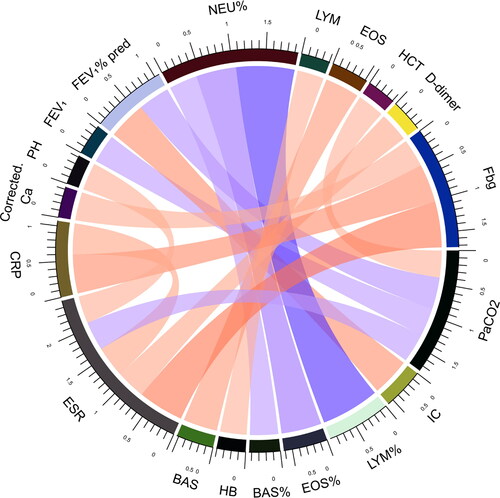
In order to furtherly investigate the pathophysiological mechanisms of differences between the two groups, correlations between laboratory measurements were analyzed in all subjects and the two groups respectively. Biomarkers with absolute value of correlation coefficient greater than 0.4 and P value less than 0.05 were shown (). The correlation between IC and FEV1% pred, Lymphocytes % of total white blood cell counts (LYM%) and Neutrophils % of total white blood cell counts (NEU%), Eosinophils % of total white blood cell counts (EOS%) and NEU%, Basophils % of total white blood cell counts (BAS%) and NEU%, BAS and EOS, CRP and Fbg, ESR and Fbg existed in total subjects, AECOPD group and COPD + CAP group. Interestingly, in total subjects and COPD + CAP group, FEV1 or FEV1% pred was inversely correlated with PaCO2 and D-dimer or CRP was positively correlated with ESR, but these correlations disappeared in AECOPD group. Instead, in total subjects and AECOPD group, EOS or BAS and LYM, Fbg and D-dimer was positively correlated, but theses correlations didn’t exist in COPD + CAP group. The correlation between platelet(PLT) and white blood count(WBC), MON% and NEU%, Glu and NEU%, NT-proBNP and eGFR only existed in AECOPD group. Correlation between Hemoglobin (Hb) and LYM, PaCO2 and Hematocrit (HCT), Corrected Ca and Fb, PH and ESR, PaCO2 and ESR only existed in COPD + CAP group. CRP was positively correlated with D-dimer in total subjects.
Figure 2. Correlations between laboratory measurements in total subjects. Biomarkers in total subjects with absolute value of correlation coefficient greater than 0.4 and P value less than 0.05 were shown. Each edge represents a correlation colored by positive correlation(red) and negative correlation(blue). Depth of the color represents the strength of correlation. The darker color represents stronger correlation.
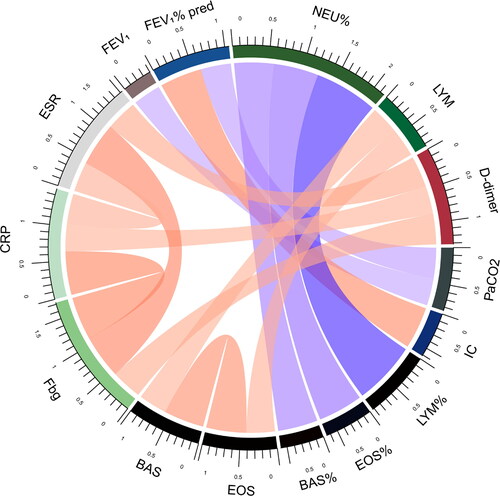
Figure 3. Correlations between laboratory measurements in AECOPD patients. Biomarkers in AECOPD patients with absolute value of correlation coefficient greater than 0.4 and P value less than 0.05 were shown. Each edge represents a correlation colored by positive correlation(red) and negative correlation(blue). Depth of the color represents the strength of correlation. The darker color represents stronger correlation.
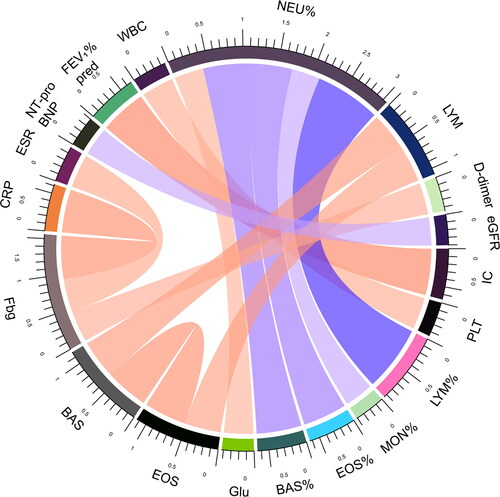
Figure 4. Correlations between laboratory measurements in COPD + CAP patients. Biomarkers in COPD + CAP patients with absolute value of correlation coefficient greater than 0.4 and P value less than 0.05 were shown. Each edge represents a correlation colored by positive correlation(red) and negative correlation(blue). Depth of the color represents the strength of correlation. The darker color represents stronger correlation.
Helpful biomarkers and their diagnostic value
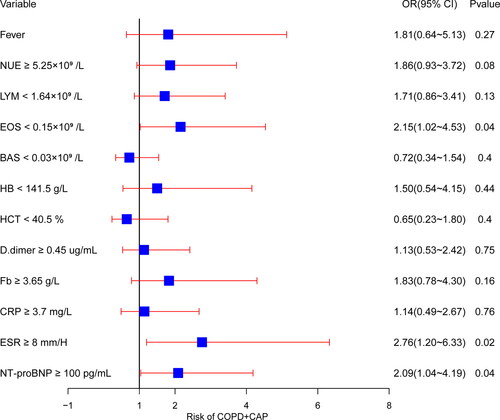
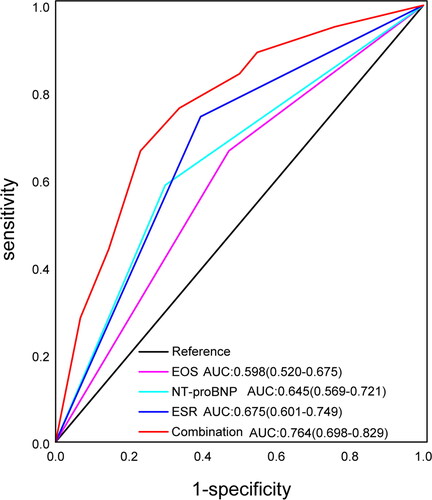
The logistic regression result was presented as forest plot (). P values of Omnibus Tests of Model Coefficients and Hosmer-Lemeshow goodness-of-fit test was less than 0.001 and 0.512 respectively. The analysis indicated that EOS, ESR and NT-proBNP were helpful biomarkers in distinguishing between COPD + CAP and AECOPD. In detail, for differential diagnosis of COPD + CAP versus AECOPD, patients with EOS < 0.5 × 109/L, ESR ≥ 8 mm/H and NT-proBNP ≥ 100 pg/mL are more likely to be diagnosed with COPD + CAP. In order to assess the diagnostic value of EOS < 0.5 × 109, ESR ≥ 8 mm/H, NT-proBNP ≥ 100 pg/mL and their combination, ROC curve and the AUC was calculated (). The AUC (95%CI, P value) were respectively 0.598(0.520-0.675, p = 0.013), 0.675(0.601-0.749, p < 0.001), 0.645(0.569-0.721, p < 0.001), 0.764(0.698-0.829, p < 0.001).
Nomogram and calibration curve
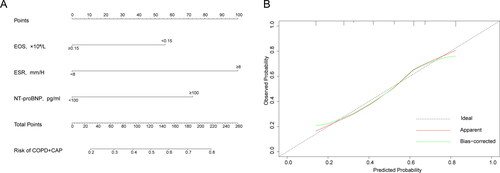
After logistic regression selection and AUC calculation, the combination of EOS < 0.5 × 109, ESR ≥ 8 mm/H and NT-proBNP ≥ 100 pg/mL was considered a good method to distinguish COPD + CAP from AECOPD. Therefore, we established a nomogram to incorporate selected biomarkers (). A calibration curve was plotted to assess the nomogram (). We can see that the predicted probability of COPD + CAP was greatly consistent with observed probability of COPD + CAP.
Figure 7. Nomogram for distinguishing COPD + CAP from AECOPD and the calibration curve. To use a nomogram(A), draw a vertical line to identify the corresponding points of each variable according to its status. Then sum the points of all variables to find the position on the total points axis. Draw a vertical line between the position and the lower line of the nomogram, you can determine the probability of COPD + CAP at the lower line of the nomogram. “Ideal” line in the calibration curve(B) represents the predicted probability is completely consistent with the observed probability. “Apparent” line represents the theoretical result. “Bias-corrected” line represents corrected result of actual phenomenon.
Discussion
The admission rate of COPD is very high because of AECOPD and COPD + CAP. As the commonest acute attacks of COPD, differential diagnosis of them has not been paid enough attention in clinic, even though GOLD has placed pneumonia as the first disease to distinguish when AECOPD is diagnosed [Citation1]. It has been reported that one in five AECOPD patients have concomitant undiagnosed CAP [Citation10, Citation19]. Unless a COPD patient with symptoms worsening is manifested as very characteristic pneumonic symptoms such as fever or significant infiltrate in chest CT, or he will be diagnosed as AECOPD. Chest CT plays an important role in differential diagnosis for COPD + CAP and AECOPD, but as the clarity of CT becomes greater, many “patients” without any symptoms are reported as mild lung inflammation in CT. Therefore, it makes the differential diagnosis of AECOPD and COPD + CAP become more difficult. With the aim of better differential diagnosis of COPD + CAP and AECOPD and understand them better, we compared the systemic inflammation, eosinophilic inflammation, damage to other organs, common comorbidities, structural changes, phenotype and endotype distributions and coagulation functions between two groups and established a nomogram to show helpful biomarkers.
Traditional view holds that COPD and asthma are markedly different. Neutrophils are important in COPD while eosinophils are important in asthma. Recently, eosinophils are reported to play an important role in COPD. High eosinophils in COPD patients predict good responsiveness to corticosteroids and severe exacerbations [Citation20, Citation21]. Eosinophils may prevent the microbiota of COPD patients from dysbiosis [Citation22] and low eosinophilic patients are more susceptible to pneumonia when treated with corticosteroids [Citation23]. Basophils are closely related to eosinophils. IL-5 is an important modulator for the development and maturation for eosinophils and basophils [Citation24–26]. Our result indicated that eosinophils were positively correlated with basophils in total subjects, AECOPD patients and COPD + CAP patients. Basophils are significantly increased in the lungs of advanced COPD patients and are considered to play an important role in emphysema [Citation27, Citation28]. In our research, circulating eosinophils and basophils counts and percentage of total white blood cell count were increased in AECOPD patients compared with COPD + CAP patients. Consistent with this, the percentage of high eosinophilic patients in AECOPD group is higher than that of COPD + CAP group. Therefore, our result shed light on eosinophilic difference between AECOPD and COPD + CAP.
It has been controversial for a long time whether AECOPD is caused by virus or bacterial infection [Citation29]. Our research found that circulating neutrophils counts and percentage of total white blood cell counts in COPD + CAP group were higher than that in AECOPD group. Lymphocytes counts and percentage of total white blood cell counts in AECOPD group were higher than that in COPD + CAP group. Our results may support the notion that AECOPD may be more likely caused by virus.
Our research indicated that the levels of hemoglobin and hematocrit of AECOPD patients were higher than COPD + CAP patients. AECOPD patients are more susceptible to dyspnea in previous study which compared the same cohort of patients in 8 years ago [Citation8]. In our research, the percentage of patients with dyspnea in AECOPD patients was higher than that in COPD + CAP patients, although did not achieve statistical significance. So higher levels of hemoglobin and hematocrit in AECOPD patients may be due to long-term hypoxia. On the other hand, hemoglobin and hematocrit are associated with oxidative stress, which plays an important role in COPD [Citation30, Citation31]. Chronic hypoxia leads to impaired calcium handling in the sarcoplasmic reticulum (SR), which may be mediated by oxidative stress [Citation32]. This result indicated that the levels of oxidative stress may be higher in AECOPD patients than COPD + CAP patients.
COPD + CAP patients have higher levels of systemic inflammation, mainly manifested as higher levels of CRP, ESR and fibrinogen. Besides, the percentage of patients with increased systemic inflammation in COPD + CAP group is larger than that of AECOPD group. Compared with AECOPD patients, the levels of NT-proBNP in COPD + CAP group is higher, it suggested that increased systemic inflammation in COPD + CAP patients may be more harmful to cardiac function than AECOPD patients. Moreover, levels of D-dimer in COPD + CAP patients was higher than AECOPD patients, which may implicate higher risk of pulmonary embolism and venous thromboemboli in COPD + CAP patients. To sum up, for COPD + CAP patients, clinicians should be more aware of the systemic inflammation, damage to other organs and risk of pulmonary embolism and venous thromboemboli.
Correlation analysis showed that fibrinogen and D-dimer were positively correlated in total subjects and AECOPD patients, but in COPD + CAP patients, this correlation disappeared. We speculate that it may be due to the increased systemic inflammation in COPD + CAP patients which destroyed the correlation. Similarly, in total subjects and COPD + CAP patients, ESR and D-dimer, ESR and CRP were respectively correlated, but in AECOPD patients, these correlations all disappeared.
After logistic regression selection, circulating EOS < 0.5 × 109/L, ESR ≥ 8 mm/H and NT-proBNP ≥ 100 pg/mL were found to be helpful biomarkers for differential diagnosis of COPD + CAP and AECOPD. AUC of the combination of these three biomarkers was 0.777. In a previous study performed in 8 years ago, CRP was selected as a helpful biomarker and the AUC of it was 0.71 [Citation8]. In our research, the levels of CRP in COPD + CAP patients were higher than AECOPD patients, but it is not a very helpful biomarker in logistic regression analysis. Maybe the difference is because AECOPD patients in previous study were more severe than COPD + CAP patients (FEV1% pred).
Nomogram is a good differential diagnostic method which can be conveniently adopted by clinicians. It has been widespreadly used in recent years [Citation33, Citation34]. We established a nomogram incorporating selected biomarkers. With this nomogram, clinicians can make a better diagnosis and treatment decision when encountering a COPD patient. The calibration curve showed that fitting efficiency of the nomogram was good.
In our study, we use chest CT scan as the gold standard of CAP although CT is not standard clinical practice in the majority of centers around the world. Compared with chest X-ray, CT scan is more expensive and delivers more radiation, thus to some extent restricting its applications. However, CT scan has superior resolution and is more helpful for the diagnosis and differential diagnosis of many respiratory diseases. Therefore, respiratory physicians tend to prediscribe CT scan for patients if possible, for better diagnosis and treatments. In our study, we think using CT as the gold standard for diagnosis of CAP is better than chest X-ray because CT can capture lung lesions which may be omitted by X-ray. Moreover, most patients in the respiraotry department of Tongji Hospital underwent chest CT scan. Hence the conclusions we draw in our study will be more accurate and can be widely used, especially helpful when some patients have negative results in chest X-ray.
Conclusions
In conclusion, our analysis indicated that AECOPD and COPD + CAP are markedly different, mainly reflected in eosinophilic inflammation, systemic inflammation and coagulation function. Correlations between some common inflammatory biomarkers were also different in two groups. A nomogram was established to offer help to clinicians for differential diagnosis of these two diseases.
Authors’ contributions
SG screened medical records, extracted information, analyzed data and drafted the manuscript. YD participated in subjects inclusion and exclusion. JC participated in data extraction. JW conceived of the study and its design, analyzed the data and revised the manuscript. All authors read and approved the final manuscript.
Declaration of interest
The authors report no conflict of interest.
Additional information
Funding
References
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. DOI:https://doi.org/10.1164/rccm.201701-0218PP
- Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet Respir Med. 2017;5(9):691–706. DOI:https://doi.org/10.1016/s2213-2600(17)30293-x
- Labaki WW, Rosenberg SR. Chronic obstructive pulmonary disease. Ann Intern Med. 2020;173(3):Itc17–itc32. DOI:https://doi.org/10.7326/aitc202008040
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. DOI:https://doi.org/10.1371/journal.pmed.0030442
- Wedzicha J-C, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49(3):1600791(1-16). DOI:https://doi.org/10.1183/13993003.00791-2016
- Suissa S. Inhaled corticosteroids preventing pneumonia mortality: Paradox or selection bias? Eur Respir J. 2019;53(2):1802112(1-5) DOI:https://doi.org/10.1183/13993003.02112-2018
- Lieberman D, Lieberman D, Gelfer Y, et al. Pneumonic vs nonpneumonic acute exacerbations of COPD. Chest. 2002;122(4):1264–1270. DOI:https://doi.org/10.1378/chest.122.4.1264
- Huerta A, Crisafulli E, Menéndez R, et al. Pneumonic and nonpneumonic exacerbations of COPD: inflammatory response and clinical characteristics. Chest. 2013;144(4):1134–1142. DOI:https://doi.org/10.1378/chest.13-0488
- Gómez-Junyent J, Garcia-Vidal C, Viasus D, et al. Clinical features, etiology and outcomes of community-acquired pneumonia in patients with chronic obstructive pulmonary disease. PLoS One. 2014;9(8):e105854. DOI:https://doi.org/10.1371/journal.pone.0105854
- Crisafulli E, Manco A, Ferrer M, et al. Pneumonic versus nonpneumonic exacerbations of chronic obstructive pulmonary disease. Semin Respir Crit Care Med. 2020;41(6):817–829. DOI:https://doi.org/10.1055/s-0040-1702196
- MacDonald MI, Osadnik CR, Bulfin L, et al. Low and high blood eosinophil counts as biomarkers in hospitalized acute exacerbations of COPD. Chest. 2019;156(1):92–100. DOI:https://doi.org/10.1016/j.chest.2019.02.406
- Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204. DOI:https://doi.org/10.7326/0003-4819-106-2-196
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2(Suppl 2):S27–S72. DOI:https://doi.org/10.1086/511159
- Martinez-Garcia MA, Faner R, Oscullo G, et al. Inhaled steroids, circulating eosinophils, chronic airway infection, and pneumonia risk in chronic obstructive pulmonary disease. A network analysis. Am J Respir Crit Care Med. 2020;201(9):1078–1085. DOI:https://doi.org/10.1164/rccm.201908-1550OC
- Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. Jama. 2013;309(22):2353–2361. DOI:https://doi.org/10.1001/jama.2013.5732
- Burrows B, Fletcher CM, Heard BE, et al. The emphysematous and bronchial types of chronic airways obstruction. A clinicopathological study of patients in London and Chicago. Lancet. 1966;1(7442):830–835. DOI:https://doi.org/10.1016/S0140-6736(66)90181-4
- Tirlapur VG, Mir MA. Nocturnal hypoxemia and associated electrocardiographic changes in patients with chronic obstructive airways disease. N Engl J Med. 1982;306(3):125–130. DOI:https://doi.org/10.1056/NEJM198201213060301
- Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. DOI:https://doi.org/10.1136/bmj.b2393
- Stefan MS, Shieh MS, Pekow PS, et al. Trends in mechanical ventilation among patients hospitalized with acute exacerbations of COPD in the United States, 2001 to 2011. Chest. 2015;147(4):959–968. DOI:https://doi.org/10.1378/chest.14-1216
- Segal LN, Martinez FJ. Chronic obstructive pulmonary disease subpopulations and phenotyping. J Allergy Clin Immunol. 2018;141(6):1961–1971. DOI:https://doi.org/10.1016/j.jaci.2018.02.035
- Vedel-Krogh S, Nielsen SF, Lange P, et al. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen general population STUDY. Am J Respir Crit Care Med. 2016;193(9):965–974. DOI:https://doi.org/10.1164/rccm.201509-1869OC
- Contoli M, Pauletti A, Rossi MR, et al. Long-term effects of inhaled corticosteroids on sputum bacterial and viral loads in COPD. Eur Respir J. 2017;50(4):1700451(1-10). DOI:https://doi.org/10.1183/13993003.00451-2017
- Pavord ID, Lettis S, Anzueto A, et al. Blood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient-level Meta-analysis. Lancet Respir Med. 2016;4(9):731–741. DOI:https://doi.org/10.1016/S2213-2600(16)30148-5
- Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15(5):271–282. DOI:https://doi.org/10.1038/nri3831
- Willebrand R, Voehringer D. Regulation of eosinophil development and survival. Curr Opin Hematol. 2017;24(1):9–15. DOI:https://doi.org/10.1097/moh.0000000000000293
- Sridhar S, Liu H, Pham TH, et al. Modulation of blood inflammatory markers by benralizumab in patients with eosinophilic airway diseases. Respir Res. 2019;20(1):14. DOI:https://doi.org/10.1186/s12931-018-0968-8
- Jogdand P, Siddhuraj P, Mori M, et al. Eosinophils, basophils and type 2 immune microenvironments in COPD-affected lung tissue. Eur Respir J. 2020;55(5):1900110(1-14). DOI:https://doi.org/10.1183/13993003.00110-2019
- Shibata S, Miyake K, Tateishi T, et al. Basophils trigger emphysema development in a murine model of COPD through IL-4-mediated generation of MMP-12-producing macrophages. Proc Natl Acad Sci U S A. 2018;115(51):13057–13062. DOI:https://doi.org/10.1073/pnas.1813927115
- Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005;26(6):1138–1180. DecDOI:https://doi.org/10.1183/09031936.05.00055705
- Suliman HB, Ali M, Piantadosi CA. Superoxide dismutase-3 promotes full expression of the EPO response to hypoxia. Blood. 2004;104(1):43–50. DOI:https://doi.org/10.1182/blood-2003-07-2240
- Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27. DOI:https://doi.org/10.1016/j.jaci.2016.05.011
- Yeung HM, Hung MW, Fung ML. Melatonin ameliorates calcium homeostasis in myocardial and ischemia-reperfusion injury in chronically hypoxic rats. J Pineal Res. 2008;45(4):373–382. DOI:https://doi.org/10.1111/j.1600-079X.2008.00601.x
- Wang S, Tian S, Li Y, et al. Development and validation of a novel scoring system developed from a nomogram to identify malignant pleural effusion. EBioMedicine. 2020;58:102924. DOI:https://doi.org/10.1016/j.ebiom.2020.102924
- Zheng YM, Li J, Liu S, et al. MRI-Based radiomics nomogram for differentiation of benign and malignant lesions of the parotid gland. Eur Radiol. 2021;31(6):4042–4052. DOI:https://doi.org/10.1007/s00330-020-07483-4