Abstract
Randomized trials of triple therapy including an inhaled corticosteroid (ICS) for chronic obstructive pulmonary disease (COPD) reported remarkable benefits on mortality compared with dual bronchodilators, likely resulting from ICS withdrawal at randomization. We compared triple therapy with dual bronchodilator combinations on major COPD outcomes in a real-world clinical practice setting. We identified a cohort of COPD patients, age 50 or older, treated during 2002-2018, from the United Kingdom’s Clinical Practice Research Datalink. Patients initiating treatment with a long-acting muscarinic antagonist (LAMA), a long-acting beta2-agonist (LABA) and an ICS on the same day, were compared with patients initiating a LAMA and LABA, weighted by fine stratification of propensity scores. Subjects were followed-up one year for all-cause mortality, severe exacerbation and pneumonia. The cohort included 117,729 new-users of LAMA-LABA-ICS and 26,666 of LAMA-LABA. The adjusted hazard ratio (HR) of all-cause mortality with LAMA-LABA-ICS compared with LAMA-LABA was 1.17 (95% CI: 1.04-1.31) while for severe exacerbation and pneumonia it was 1.19 (1.08-1.32) and 1.29 (1.16-1.45) respectively. However, mortality was not elevated with triple therapy among patients with asthma diagnosis (HR 0.99; 95% CI: 0.74-1.34), with two or more prior exacerbations (HR 0.88; 95% CI: 0.70-1.11), and with FEV1 percent predicted >30%. In a real-world setting of COPD treatment, triple therapy initiation was not more effective than dual bronchodilators at preventing all-cause mortality and severe COPD exacerbations. Triple therapy may be unsafe among patients without prior exacerbations, in whom ICS are not recommended, with no asthma diagnosis and with very severe airflow obstruction.
Supplemental data for this article is available online at https://doi.org/10.1080/15412555.2021.1977789 .
Introduction
The pharmacological treatment of chronic obstructive pulmonary disease (COPD) includes long-acting bronchodilators, namely long-acting muscarinic antagonists (LAMAs), long-acting beta2-agonists (LABAs) and inhaled corticosteroids (ICS), with the latter recommended to be added with increasing disease severity [Citation1] Triple therapy combining the three inhaler classes is now prevalent in the management of COPD, including its use as the initial treatment [Citation2]. With such widespread use, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) group emphasized the need for evidence on the escalation from dual bronchodilator to triple therapy, also stressing the need for effectiveness data on major outcomes of COPD, including mortality [Citation1,Citation3].
Recently, several large trials addressed the intensification from dual bronchodilator (LAMA-LABA) to triple therapy (LAMA-LABA-ICS) in COPD, with two of these reporting data on mortality [Citation4,Citation5]. The Informing the Pathway of COPD Treatment (IMPACT) and the Efficacy and Safety of Triple Therapy in Obstructive Lung Disease (ETHOS) trials reported significant hazard ratios of all-cause mortality over one year of 0.72 (95% confidence interval (CI) 0.53–0.99) and 0.51 (95% CI 0.33–0.80), respectively, comparing triple therapy with its dual bronchodilator formulation [Citation4,Citation5]. Related editorials endorsed the claim of major benefits on all-cause mortality with triple therapy [Citation6,Citation7]. However, these effects of triple therapy on mortality were shown to be limited exclusively to the first three months of follow-up time, with no benefit in the subsequent nine months [Citation8]. Such an early benefit is more compatible with an effect of abrupt ICS withdrawal at randomization rather than a direct effect of triple therapy [Citation9–11]. Indeed, among non-users of ICS prior to randomization, the HRs of mortality comparing triple therapy with dual bronchodilators were 1.25 (95% CI:0.60-2.59) and 1.49 (0.49-4.55) in IMPACT and ETHOS, respectively.
We assessed the effectiveness of triple combinations compared with dual bronchodilators on mortality and severe exacerbation, as well as severe pneumonia, using an observational study approach in a real world setting of clinical practice [Citation12,Citation13]. We also examined the comparative effectiveness according to history of asthma, prior exacerbations, lung function and blood eosinophil levels.
Methods
Data source
The base cohort of patients was previously employed for a study of long-acting bronchodilators without ICS [Citation14]. It was identified from the Clinical Practice Research Datalink (CPRD), a primary care database from the United Kingdom (UK) that contains primary care medical records for over 50 million people enrolled in more than 1800 general practices. Participating general practitioners in the CPRD-GOLD and Aurum networks record medical information as part of the routine care of patients, including demographic data, lifestyle factors, medical diagnoses recorded using Read and SNOMED codes, and prescriptions. Over 85% of the CPRD practices can be linked to the Hospital Episodes Statistics (HES) database. The CPRD population is representative of the overall population and these data sources have been validated [Citation15–17]. The information on medications and diagnoses has been validated and shown to be of high quality, particularly for studies of COPD [Citation16–20]. Our previous study of triple therapy with this database used only the smaller CPRD-GOLD network database [Citation21].
Study design
The source population included all patients with a diagnosis of COPD from January 1995 to November 2018 who subsequently received at least one prescription for a LAMA or a LABA after January 2002 when LAMAs became available. To increase the likelihood of a diagnosis of COPD, we excluded all patients less than 50 years of age on the date of their initial prescription for these long-acting bronchodilators. The source population was restricted to patients linkable to the Hospital Episodes Statistics (HES) database.
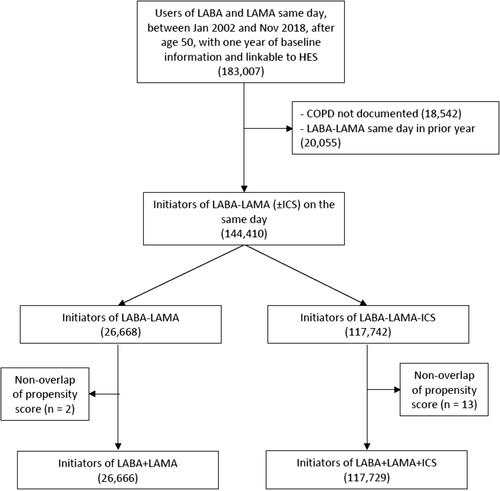
We identified the base cohort of all subjects who, during follow-up, received for the first time, prescriptions for a LAMA and a LABA on the same day as different prescriptions or in combined inhalers, either as initial treatment or as an addition of the other bronchodilator. We classified these subjects according to whether they also received an ICS on that same day (triple therapy LAMA-LABA-ICS), as different or combined inhalers, or not (dual bronchodilator therapy LAMA-LABA). Cohort entry was taken as the date of the first LAMA-LABA or LAMA-LABA-ICS. The study cohort included all subjects with at least one year of medical history prior to cohort entry to allow a baseline period to measure the covariates and to identify new use of these study combinations.
We used an incident new-user cohort design to compare the initiators of triple therapy with the initiators of dual bronchodilators, weighted by fine stratification weights computed using the probability of treatment propensity scores [Citation22–24]. Subjects in the study cohort were followed for up to one year from cohort entry, with follow-up ending at death, 30 November 2018, or the end of the patient’s registration in the practice, whichever occurred first.
Outcome events
The main outcome was death during the one-year follow-up. The first severe exacerbation was defined as a hospitalization for COPD (ICD-10: J41, J42, J43, J44) and the first severe pneumonia as hospitalization for community-acquired pneumonia (ICD10: J10.0; J11.0; J12-J18; J22; J69; J85.0; J85.1; J86). Hospitalizations for pneumonias that resulted in death were also considered as outcome events. These diagnostic codes have been shown to have good accuracy and were used in several studies of COPD using the CPRD [Citation14,Citation19,Citation20,Citation25,Citation26].
Covariates
The covariates used to balance the two treatment groups included several factors identified using lifestyle, diagnoses and prescriptions from CPRD and HES data. Age, sex, body mass index (BMI), smoking status and alcohol abuse were measured at or prior to cohort entry. The severity of COPD at treatment initiation was measured in different ways, including by the number of prior moderate and severe COPD exacerbations, and the use of other respiratory drugs, during the one-year baseline period, as well as by the percent predicted FEV1. A moderate exacerbation was defined by a new prescription for prednisolone. Other respiratory drugs included the number of prescriptions for short-acting inhaled beta-agonists and anticholinergics, methylxanthines, and antibiotics used for respiratory conditions. Prior use of LABA, LAMA or ICS was not included in the propensity score as these are potentially instrumental variables, associated with triple or dual bronchodilator initiation but not directly with the outcome, so that adjustment would introduce bias [Citation27]. The most recent measures of dyspnea, FEV1 and blood eosinophil count prior to cohort entry, were identified. Dyspnea was measured by mMRC, CAT score or the presence of dyspnea symptoms [Citation28]. The percent predicted FEV1 measurement was calculated from the absolute FEV1 value using age, sex and height, with race imputed as “Caucasian” for all [Citation29]. Baseline co-morbidity in the year prior to cohort entry was measured using clinical diagnoses, hospitalizations and prescriptions ().
Table 1. Baseline characteristics of the study cohort of 117,729 initiators of LAMA-LABA-ICS and 26,666 initiators of LAMA-LABA, after fine stratification weighting from probability of treatment propensity scores, with corresponding standardized mean differences.
Data analysis
The propensity score of treatment initiation was estimated by logistic regression using all covariates. Fine stratification weights were computed using 100 strata defined by the propensity score to create comparable arms of LAMA-LABA-ICS and LAMA-LABA initiators with the same covariate distribution as the study cohort [Citation24]. Standardized mean differences of covariates were computed to assess the comparability of the two treatment groups.
Rates of outcomes were computed crudely and weighted using fine stratification of propensity score weights. A weighted Cox proportional hazard regression with a robust variance estimator was used in an as-treated analysis to compare current use of LAMA-LABA-ICS and LAMA-LABA on the risks of death, severe exacerbation and severe pneumonia during the first year after treatment initiation. Accordingly, continuing exposure was measured by successive prescriptions of the initial treatment with a maximal 60-day gap between the prescription dates, with current use defined by the 60-day period after the last such prescription date. This as-treated time ended with a discontinuation of one component of the combination or the addition of an ICS in the LAMA-LABA arm.
The comparative analysis was stratified by prior asthma diagnosis, by the number of exacerbations during the baseline year, by baseline blood eosinophil counts as <150, 150–300, and >300 cells/µL, and by the baseline FEV1 percent predicted, using the regression model with an interaction term between these factors and treatment. In addition, the adjusted hazard ratio of mortality was fitted by continuous values of blood eosinophils counts using cubic splines.
Sensitivity analyses included an intention-to-treat analysis over the one-year follow-up. The continuous use definition for the as-treated analyses varied the prescription gap to 30 and 90 days. The analysis was stratified by whether the combination treatments were initiated, with no prior LAMA, LABA or ICS use, or were stepped up or stepped down, with some prior LAMA, LABA or ICS use. All analyses were conducted using SAS version 9.4. The study protocol was approved by the Independent Scientific Advisory Committee of the CPRD (Protocol 21_000491) and the Ethics Committee of the Jewish General Hospital (JGH Protocol #17-137), Montreal, Quebec, Canada.
Results
The study cohort included 117,729 new users of LAMA-LABA-ICS and 26,666 new users of LAMA-LABA (). The baseline characteristics of the patients, comparing initiators of triple therapy with initiators of dual bronchodilators after weighting by fine stratification of propensity scores, indicate that the two groups are well balanced except for the prior use of LAMA, LABA an ICS in the year before cohort entry, as expected from their exclusion from the propensity score because of their role as instrumental variables (). The unweighted baseline characteristics are provided in .
The mean treatment duration of the LAMA-LABA-ICS group was 5.5 months, primarily due to discontinuing at least one of the three components during the one-year follow-up (72%). For the LAMA-LABA initiators, the mean treatment duration was 4.8 months, truncated for adding ICS (19%) or discontinuing one of the two components (51%) during the one-year follow-up. The LABAs included salmeterol (59%), formoterol (30%), vilanterol (8%), and indacaterol or olodaterol (3%). The LAMAs included tiotropium (83%), umeclidinium (8%), glycopyrronium (5%) and aclidinium (4%), while the ICS included fluticasone (66%, of which 94% were propionate and 6% furoate), budesonide (23%) and beclomethasone (11%).
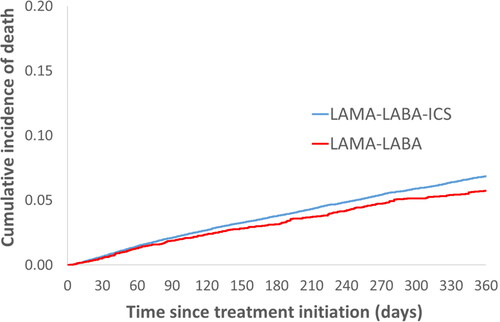
There were 4,277 deaths under LAMA-LABA-ICS treatment and 591 under LAMA-LABA during the one-year follow-up. The weighted cumulative incidence of death on triple therapy over 1 year was 7.0% compared with 5.8% on dual bronchodilators ().
Figure 2. One-year cumulative incidence of all-cause death comparing LAMA-LABA-ICS versus LAMA-LABA initiation, estimated using the Kaplan-Meier method, after adjustment by fine stratification weights from the probability of treatment propensity scores.
The adjusted hazard ratio (HR) of mortality associated with LAMA-LABA-ICS was 1.17 (95% CI: 1.04-1.31), relative to LAMA-LABA, while the HR of severe exacerbation with triple therapy was 1.19 (95% CI: 1.08-1.32), compared with LAMA-LABA (). The incidence of a severe pneumonia requiring hospitalization was increased with triple therapy (HR 1.29; 95% CI: 1.16-1.45), as were those whose hospitalization ended in death (HR 1.48; 95% CI: 1.16-1.89), relative to dual bronchodilators ().
Table 2. Adjusted hazard ratios of death, severe COPD exacerbation and severe pneumonia requiring hospitalization comparing LAMA-LABA-ICS initiation with LAMA-LABA initiation in patients with COPD in the first year after treatment initiation, from the as-treated analysis.
The stratification by asthma diagnosis found mortality elevated with triple therapy among patients with no prior asthma (HR 1.25; 95% CI: 1.11-1.41) but not among patients with prior asthma (HR 0.99; 95% CI: 0.74-1.34), compared with dual bronchodilators (). Mortality was also elevated with triple therapy among patients with no exacerbations in the year prior to cohort entry asthma (HR 1.44; 95% CI: 1.22-1.69), but not among patients with two or more prior exacerbations (HR 0.88; 95% CI: 0.70-1.11). Stratification by blood eosinophil count found no specific trend in mortality (). However, the corresponding spline analysis suggests a lower mortality with triple therapy in patients with very low eosinophil count (<50 cells/µL) and an increased mortality for those with 100-300 cell count (). Stratification by FEV1, conducted among the 70% of patients for whom this was available, shows that the elevated mortality with triple therapy is mainly among the patients with the baseline FEV1 percent predicted <30% (HR 1.66; 95% CI: 1.08-2.55), with no increase in mortality for patients above 30%. The stratified analyses for the severe exacerbation outcome show similar patterns ().
Figure 3. Adjusted hazard ratio of all-cause death (solid line) comparing LAMA-LABA-ICS with LAMA-LAMA initiation and 95% confidence intervals (dashed lines) according to blood eosinophil count (cells/µL) prior to treatment initiation, from the as-treated analysis fit by cubic splines.
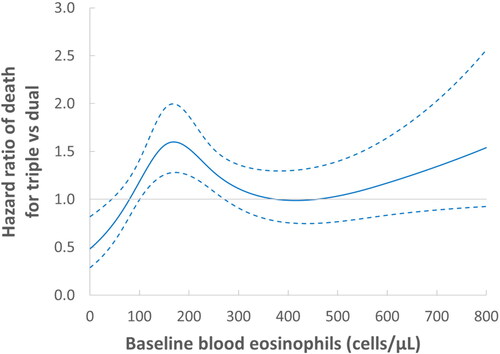
Table 3. Adjusted hazard ratios of mortality comparing LAMA-LABA-ICS with LAMA-LABA initiation in patients with COPD, from the as-treated analyses over one-year follow-up, stratified by prior asthma, number of exacerbations in the prior year, baseline peripheral blood eosinophil count and baseline FEV1 percent predicted.
Sensitivity analyses mainly confirmed the robustness of the results ( and ). In particular, as many patients were using some components of the treatments before initiating the triple and dual therapies, the stratified analysis by whether the combination treatments were used de novo or stepped up or down () shows that among the de novo initiators, the HR of mortality with triple therapy is equally elevated at 1.30 (95% CI: 1.06-1.60) as for those who used some of these drugs previously (HR 1.23; 95% CI: 1.07-1.41). As expected, the HRs were attenuated in the intention-to-treat analyses over the one-year follow-up ().
Discussion
This large observational study in the real-world setting of clinical practice found that patients with COPD newly prescribed a triple combination of LAMA, LABA and ICS, as initial or step up from existing treatment, had a modest increase in all-cause mortality and severe exacerbation over the first year of use, compared with those initiating treatment with LAMA and LABA. However, there was no such increase among the subgroup of patients with a prior asthma diagnosis, those with two or more exacerbations in the prior year, as well as those with moderate or severe airflow limitation (FEV1 percent predicted >30%).
Two main reasons can explain the differences between these findings and those of the recent IMPACT and ETHOS trials that reported significant reductions in all-cause death with triple therapy compared with dual bronchodilators [Citation4,Citation5]. First, the trials enrolled patients already treated, including with triple therapy and ICS, which had to be abruptly withdrawn before randomization and can introduce bias [Citation30]. Second, the trials allowed patients with a history of asthma and multiple COPD exacerbations, patients in whom ICS are indicated and should not be withdrawn. Together, these two issues will bias the results [Citation9–11].
The role of ICS withdrawal in the triple therapy randomized trials is important to examine when interpreting the results. First, ICS were discontinued in over 70% of the patients in the IMPACT and ETHOS trials, including around 40% already using triple therapy. This abrupt withdrawal of ICS among patients who responded to ICS and the imposed switch to a LAMA-LABA with no ICS by randomization can produce an early effect on exacerbations and major adverse outcomes, including mortality [Citation9–11]. Indeed, the reductions in mortality with triple therapy in IMPACT and ETHOS were limited to the first three months of follow-up time, with no or little reduction in the subsequent nine months [Citation8]. Such a pattern is consistent with the design’s abrupt ICS withdrawal at randomization, particularly in patients with a history of asthma [Citation9–11]. This was also the opinion of an FDA advisory committee on the IMPACT trial, that concluded the findings on mortality were the result of an “adverse impact of ICS withdrawal rather than the addition” of the triple therapy [Citation31].
Second, the effect of ICS withdrawal is also supported by the stratified analyses by prior ICS use. Among non-users of ICS prior to randomization, the HRs of mortality comparing triple therapy with dual bronchodilators were 1.25 (95% CI:0.60-2.59) and 1.49 (0.49-4.55) in IMPACT and ETHOS, respectively. The findings of our observational study are more in line with this subgroup analysis which is inherently unaffected by sudden ICS withdrawal. Our study included only new dual bronchodilator users who did not have to abruptly discontinue ICS or triple therapy prior to initiating the dual therapy. Morover, our sensitivity analysis stratified by prior treatment shows that the HR of mortality with triple therapy is equally elevated at 1.30 (95% CI: 1.06-1.60) among the de novo initiators of the two treatments as for those who used some of these drugs previously (HR 1.23; 95% CI: 1.07-1.41).
Finally, it is also informative to examine other large trials that studied the effectiveness of ICS-containing dual inhalers on reducing mortality in COPD. The TORCH and SUMMIT trials found that the ICS-based combined inhaler was associated with either a numerically lower or similar mortality when compared with placebo or with a long-acting bronchodilator [Citation32,Citation33]. These trials also imposed a withdrawal of ICS before randomization, involving up to 50% of the study patients. On the other hand, the Salford trial that compared the fluticasone furoate–vilanterol combination with “usual care” in COPD, did not impose such a withdrawal [Citation34]. Indeed, the comparator “usual care” patients were allowed to continue their regular treatment, including ICS. This trial resulted in 45 deaths in the fluticasone furoate–vilanterol group compared with 30 in the usual care group (relative risk 1.51; 95% confidence interval 0.96-2.38). The findings of our observational study are consistent with this trial, also unaffected by ICS withdrawal.
It is noteworthy that 44% of the patients treated with triple therapy did not have an exacerbation in the year prior to initiating this treatment and thus not recommended to receive this treatment. These patients would not have been eligible for the IMPACT and ETHOS trials. Our stratified analyses by this and other factors are quite revealing. First, mortality was not increased with triple therapy among patients with prior exacerbations, and particularly those with two or more, with the excess overall mortality exclusively among those 44% with no prior exacerbations. Second, while IMPACT and ETHOS also included patients with a history of asthma diagnosis, they did not provide stratified analyses by this central cofactor. Our study showed that the overall excess mortality with triple therapy occurred exclusively among those with no prior asthma, with no such increase among patients with a prior asthma diagnosis.
The analysis according to peripheral blood eosinophilia suggested a lower mortality with triple therapy in patients with very low eosinophil count (<50 cells/µL) and an increased mortality for those with counts between 100 and 300. Eosinopenia has long been recognized as a marker of acute infection [Citation35]. It might thus indirectly reflect prior exacerbations that did not lead to the prescription of prednisolone, but which may identify a further group of patients who benefit from ICS. However, our ability to link mortality with eosinophil counts is surely limited by the lack of specificity of all-cause mortality, the absence of eosinophil counts in more than 20% of patients and the variability of eosinophil counts over time [Citation36].
The new-user design is a major strength of our study. By identifying initiators of the triple and double combinations, including those adding to prior inhaler treatment and those stepping down, the design is not subject to the effects of abrupt treatment withdrawal imposed by the randomized trials of triple therapy, though patients previously used many of the components of these combinations. However, most of the 85% of prior ICS users among the triple therapy initiators represents patients who were treated with LABA-ICS inhalers, for whom a LAMA was added, making them new-users. Alternatively, the 27% of prior ICS users among the LAMA-LABA therapy initiators represents an ICS withdrawal, with a switch to LAMA-LABA. The stratified analysis by prior use of any of the components of the combined therapies found similar HRs of mortality with triple therapy for those who used and those who did not use some of these component drugs previously. Moreover, our new-user design inherently does not include subjects who previously used triple therapy, such as the 40% of patients in IMPACT and ETHOS who were already using triple therapy prior to randomization. By selecting new users, our observational study emulates clinical practice that does not force treatment withdrawal, while avoiding prevalent user bias [Citation22]. Second, the primary outcome of all-cause mortality is highly valid in the CPRD and the severe exacerbation outcome was based on hospitalization records and less subject to misclassification [Citation18,Citation19]. Finally, the size of this study is a major strength. Indeed, a previous observational study of triple therapy in COPD on the incidence of moderate or severe exacerbations, was too small to examine the outcome of mortality and had insufficient power to assess the outcome of severe exacerbation with precision [Citation21]. Limitations of the study include the measures of exposure which were based on prescriptions, not necessarily dispensed, which could introduce misclassification. This can also be compounded by the variable and potentially less than optimal inhaler technique in the real-world setting. The continuous treatment duration was relatively short because patients either discontinued or switched early, a reflection of the real-world clinical setting. The use of physician diagnoses to identify the cohort may have misclassified some patients with asthma as COPD, though the 50 years of age cutoff likely reduced this potential misclassification. Finally, residual confounding cannot be ruled out despite the use of propensity score weighting which created groups highly comparable on COPD severity.
Conclusion
This real-world clinical setting study of triple therapy treatment for COPD suggests that initiation of treatment with a LAMA-LABA-ICS triple combination is associated with an overall modest increase in mortality compared with a LAMA-LABA combination. However, the risk increase disappears in patients in whom ICS are recommended, namely with a history of asthma and those with two or more exacerbations in the prior year, as well as those with less than very severe airflow limitation (FEV1 percent predicted >30%). The risk was particularly elevated among the almost 50% of the patients who were treated with triple therapy despite having no COPD exacerbation prior to initiating this treatment, thus against current treatment recommendations. Treatment with triple therapy should be reassessed for many patients with COPD where a LAMA-LABA should be sufficient, particularly in view of the increased risk of hospitalization for pneumonia with the inhaled corticosteroid included in the triple combination.
Author contributions
Dr Ernst participated in study design, data interpretation, and writing of the manuscript. Ms Dell’Aniello participated in data analysis and writing of the manuscript. Pr. Suissa participated in data acquisition, study design, data interpretation, writing of the manuscript, and acts as guarantor of this entire manuscript.
Declaration of interest
Pr. Suissa has previously received research grants from Boehringer Ingelheim and Novartis and has participated in advisory board meetings or as speaker for AstraZeneca, Boehringer‐Ingelheim, and Novartis. Dr. Ernst and Ms Dell’Aniello have no conflicts of interest.
Supplemental Material
Download ()Acknowledgments
This study was conducted thanks to infrastructure funding from the Canadian Institutes of Health Research (CIHR) and the Canadian Foundation for Innovation (CFI). Pr. Suissa is the recipient of the Distinguished James McGill Professorship award. These sponsors had no input in the study.
References
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3):1700214. DOI:10.1183/13993003.00214-2017
- Quint JK, O’Leary C, Venerus A, et al. Prescribing pathways to triple therapy: a Multi-Country, retrospective observational study of adult patients with chronic obstructive pulmonary disease. Pulm Ther. 2020;6(2):333–350.
- Rodriguez-Roisin R, Rabe KF, Vestbo J, et al. Global initiative for chronic obstructive lung disease (GOLD) 20th anniversary: a brief history of time. Eur Respir J. 2017;50(1):1700671.
- Lipson DA, Crim C, Criner GJ, et al. Reduction in All-Cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201(12):1508–1516.
- Martinez FJ, Rabe KF, Ferguson GT, et al. Reduced All-Cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. A randomized, Double-Blind, multicenter, Parallel-Group study. Am J Respir Crit Care Med. 2021;203(5):553–564.
- Vestbo J. Fixed triple therapy in chronic obstructive pulmonary disease and survival. Living better, longer, or both?Am J Respir Crit Care Med. 2020;201(12):1463–1464.
- Calverley P. Reigniting the TORCH: chronic obstructive pulmonary disease mortality and inhaled corticosteroids revisited. Am J Respir Crit Care Med. 2021;203(5):531–532.
- Suissa S. Perplexing mortality data from triple therapy trials in COPD. Lancet Respir Med. 2021 Jul;9(7):684–685.
- Suissa S, Drazen JM. Making sense of triple inhaled therapy for COPD. N Engl J Med. 2018;378(18):1723–1724.
- Suissa S, Ariel A. Triple therapy trials in COPD: a precision medicine opportunity. Eur Respir J. 2018;52(6):1801848.
- Wedzicha JA, Banerji D, Kostikas K. Single-Inhaler triple versus dual therapy in patients with COPD. New Engl J Med. 2018;379(6):590–593.
- Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence - what is it and what can it tell us?N Engl J Med. 2016;375(23):2293–2297.
- Frieden TR. Evidence for health decision making - beyond randomized, controlled trials. N Engl J Med. 2017;377(5):465–475.
- Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness of initial LAMA versus LABA in COPD: Real-World cohort study. COPD. 2021;18(1):1–8.
- Wolf A, Dedman D, Campbell J, et al. Data resource profile: clinical practice research datalink (CPRD) aurum. Int J Epidemiol. 2019;48(6):1740–1740g.
- Herrett E, Thomas SL, Schoonen WM, et al. Validation and validity of diagnoses in the general practice research database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4–14.
- Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836.
- Quint JK, Mullerova H, DiSantostefano RL, et al. Validation of chronic obstructive pulmonary disease recording in the clinical practice research datalink (CPRD-GOLD). BMJ Open. 2014;4(7):e005540.
- Rothnie KJ, Müllerová H, Thomas SL, et al. Recording of hospitalizations for acute exacerbations of COPD in UK electronic health care records. Clin Epidemiol. 2016;8:771–782.
- Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness of LABA-ICS versus LAMA as initial treatment in COPD targeted by blood eosinophils: a population-based cohort study. Lancet Respir Med. 2018;6(11):855–862. DOI:10.1016/S2213-2600(18)30368-0
- Suissa S, Dell’Aniello S, Ernst P. Comparative effects of LAMA-LABA-ICS vs LAMA-LABA for COPD: Cohort study in Real-World clinical practice. CHEST. 2020;157(4):846–855.
- Johnson ES, Bartman BA, Briesacher BA, et al. The incident user design in comparative effectiveness research. Pharmacoepidemiol Drug Saf. 2013;22(1):1–6.
- Suissa S, Moodie EE, Dell’Aniello S. Prevalent new-user cohort designs for comparative drug effect studies by time-conditional propensity scores. Pharmacoepidemiol Drug Saf. 2017;26(4):459–468.
- Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ. 2019;367:l5657.
- Meropol SB, Metlay JP. Accuracy of pneumonia hospital admissions in a primary care electronic medical record database. Pharmacoepidemiol Drug Saf. 2012;21(6):659–665.
- Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness and safety of LABA-LAMA vs LABA-ICS Treatment of COPD in Real-World Clinical Practice . CHEST. 2019;155(6):1158–1165.
- Myers JA, Rassen JA, Gagne JJ, et al. Effects of adjusting for instrumental variables on bias and precision of effect estimates. Am J Epidemiol. 2011;174(11):1213–1222.
- Rebordosa C, Plana E, Aguado J, et al. GOLD assessment of COPD severity in the clinical practice research datalink (CPRD). Pharmacoepidemiol Drug Saf. 2019;28(2):126–133.
- Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations . Eur Respir J. 2012;40(6):1324–1343.
- Suissa S. Run-in bias in randomised trials: the case of COPD medications. Eur Respir J. 2017;49(6):1700361.
- FDA. Food and Drug Aministration; 2020. Available from: https://www.fda.gov/media/143921/download.
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. DOI:10.1056/NEJMoa063070
- Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387(10030):1817–1826.
- Vestbo J, Leather D, Diar BN, et al. Effectiveness of fluticasone Furoate-Vilanterol for COPD in clinical practice. N Engl J Med. 2016;375(13):1253–1260.
- Bass DA, Gonwa TA, Szejda P, et al. Eosinopenia of acute infection: production of eosinopenia by chemotactic factors of acute inflammation. J Clin Invest. 1980;65(6):1265–1271.
- Bafadhel M, Pavord ID, Russell REK. Eosinophils in COPD: just another biomarker?Lancet Respir Med. 2017;5(9):747–759. DOI:10.1016/S2213-2600(17)30217-5