Abstract
Patients with acute hypercapnic respiratory failure (AHRF) often require hospitalization and respiratory support. Early identification of patients at risk of readmission would be helpful. We evaluated 1-y readmission and mortality rates of patients admitted for undifferentiated AHRF and identified the impact of initial severity on clinically important outcomes. We retrospectively analyzed patients who presented with AHRF to the emergency department of St Michael’s Hospital in 2017. We collected data about patients’ characteristics, hospital admission, readmission and mortality one year after the index admission. We analyzed predictors of readmission and mortality and conducted a survival analysis comparing patients who did and did not receive ventilatory support. A cohort of 212 patients with AHRF who survived their hospital admission were analyzed. At one year, 150 patients (70.8%) were readmitted and 19 (9%) had died. Main diagnoses included chronic obstructive pulmonary disease (60%), congestive heart failure (36%), asthma (22%) and obesity (19%), and these categories of patients had similar 1 y readmission rates. One third had more than one coexisting chronic illness. Although comorbidities were more frequent in readmitted patients, only a history of previous hospital admissions remained associated with 1 y readmission and mortality in multivariate analysis. Need for ventilatory support at admission was not associated with higher 1 y probability of readmission or death. Undifferentiated AHRF is the presentation of multiple chronic illnesses. Patients who survive one episode of AHRF and with previous history of admission have the highest risk of readmission and death regardless of whether they receive ventilatory support during index admission.
Introduction
Acute on chronic hypercapnic respiratory failure (AHRF) is characterized by acutely elevated blood levels of carbon dioxide (pCO2) and acute or acute on chronic respiratory acidosis [Citation1, Citation2]. AHRF often necessitates hospitalization and the use of respiratory support strategies [Citation3]. By reducing intubation rate and hospital mortality, noninvasive ventilation (NIV) has been shown to be an effective treatment in AHRF due to exacerbations of chronic obstructive pulmonary disease (COPD) [Citation4–9]. Studies focusing on long term outcomes of COPD patients treated with NIV showed that despite a better hospital survival, they are at high risk (70–80%) of readmission to hospital within one year [Citation10]. Several studies showed these high hospital readmission rates in COPD patients with an hypercapnic exacerbation [Citation11–14]. However, other respiratory and cardiac conditions may also result in AHRF. For example, an increasing number of patients have been identified with obesity related hypercapnic respiratory failure or mixed conditions, due to the increased prevalence of obesity worldwide [Citation15]. Moreover, COPD patients are often underdiagnosed or misclassified [Citation16, Citation17].
Patients with AHRF in general have a high prevalence of comorbidities that can also contribute to respiratory failure. Recent studies in patients admitted to the Intensive Care Unit with AHRF have shown a high prevalence of sleep-related breathing disorders (50 to 80%) and hypertension or diastolic cardiac dysfunction (>40%) [Citation18, Citation19]. These comorbidities are often under-recognized or poorly investigated. Indeed, patients are sent home in prevision of a later complete work-up once they have stabilized; unfortunately, once they have entered the cycle of exacerbations, they are most frequently readmitted without returning to their baseline. Consequently, these patients are not optimally managed with regard to baseline investigations and subsequent diagnoses. Only a management strategy that addresses the potential for comorbid conditions (e.g. sleep-related disorders, CHF) during the index admission could potentially interrupt the cycle of readmissions and reduce readmission rates in this patient population [Citation20]. Patients with sleep disorders might especially benefit from a better management [Citation21]. These patients should be identified early during the index admission to try to break the vicious circle of multiple readmissions.
Although earlier studies have suggested that a higher risk of readmission exists in the most severe patients with AHRF, a study evaluating the impact of different severity levels is needed. We sought to retrospectively analyze the characteristics and outcomes of the overall population of patients presenting to the emergency department (ED) of an inner-city academic hospital with AHRF over a one-year period, assessed readmission and mortality at one year and the impact of the initial severity on presentation on 1 year outcomes.
Methods
Setting
We conducted a retrospective cohort study at St Michael’s Hospital, a 463-bedded teaching inner-city hospital in downtown Toronto (Canada) that evaluates approximately 70,000 patients per year. St. Michael’s Hospital includes a respirology department and a total of 65 Intensive Care Unit (ICU) beds. NIV treatment can be provided in the ED, ICUs or step-up unit (a 5-bed monitored unit supervised by general internal medicine physicians). The protocol was approved by the hospital research ethic board of the Hospital (n# 19-014C). A waiver of consent was obtained to conduct this study, due to its retrospective and observational nature.
Patients
We evaluated all patients who presented to the ED of St Michael’s hospital with AHRF during 2017. To identify our patient cohort, we searched in the National Ambulatory Care Reporting System (NACRS), a national database of the Canadian Institute for Health Information (CIHI) that captures information on client visits to facility- and community-based ambulatory care. We included adults ≥ 18 years of age who presented with AHRF that was defined as per blood gas analysis criteria (pH arterial ≤7.35 and PaCO2 ≥ 45 mmHg on arterial blood gas or pH venous ≤ 7.34 and PvCO2 ≥ 50 mmHg on venous blood gas) together with the presence of respiratory symptoms (codes “651–655” and “659” from the Canadian Emergency Department Information System (CEDIS)) as presenting complaint at ED admission We excluded patients with known diagnoses including cystic fibrosis, neuromuscular disease, interstitial lung disease, major thoracic cage disease, active lung cancer, central nervous system disease or drug overdose as reasons for admission, and presence of a tracheostomy. Each patient was followed for 12 months after discharge from his/her index admission.
Data collection
We reviewed medical charts to gather baseline data including demographic and anthropometric data, body mass index (BMI), risk factors, insurance data, being followed by a respirologist, relevant comorbidities, Charlson comorbidity index [Citation22], home medications, oxygen therapy and use of noninvasive ventilation devices, history of use of NIV or of intubation in the year prior to index admission, and number of hospital admissions and ICU admissions in the year prior to index admission. We also collected spirometry and echocardiograms conducted before the index admission. In the absence of this information, we collected spirometry and echocardiograms performed during index admission. We assumed that these investigations were conducted during a period of clinical stability.
Data on chronic underlying cardio-respiratory diseases that could lead to an hypercapnic exacerbation were collected. These chronic diseases included: COPD, asthma, obstructive sleep apnea (OSA) and obesity-hypoventilation syndrome (OHS), congestive heart failure (CHF) and small airways diseases. We considered chronic diseases reported in the charts as previously known to be ‘diagnosed’, and conditions that were not known at the time of index admission but were suspected by the physicians based on patients’ characteristics, clinical presentation and risk factors to be ‘suspected’. Patients could have more than one underlying chronic disease.
We defined as “index admission” the first ED admission for AHRF as per our criteria. Subsequent admissions for AHRF were considered as an outcome of interest and each patient contributed one case. After the ED admission, patients could be directly discharged (“ED visit”) or could be admitted to the hospital (“inpatient admission”). We collected the following data at the time of the index admission: length of stay in emergency department and in-hospital, hospital location, main reason for admission, admission and discharge location, patient code status (DNR, DNI, full code), arterial and venous blood gases at ED admission and during the hospital stay, type of respiratory support, respirology consult, referral to respirology, in-hospital mortality.
We followed surviving patients for one year after discharge from the index admission. Additionally, we recorded (1) unplanned readmissions to St. Michael’s Hospital (due to any reason) and (2) death at one year. We excluded elective admissions. Mortality was collected from the hospital death registration and from the Ontario necrology database [Citation23].
Data analysis
Continuous variables are expressed as mean ± standard deviation or median and 25th–75th quartile according to their distribution; categorical variables are expressed as a percentage. We compared patients who were and were not readmitted a using the t-test or Mann-Whitney test and the Chi-square or the Fisher test, where appropriate. Comparisons between more than two groups were done using the variance analysis (ANOVA) or the Kruskal-Wallis test for non-paired data. All timed variables were considered from the date of discharge from index admission.
We defined as event a readmission or death (the first one of the two) that occurred within the follow-up period. We analyzed event-free survival using the Kaplan-Meier statistic with the log-rank test and the Wilcoxon test, by dividing our cohort into different groups according to the type of respiratory support. In order to evaluate predictors of readmission and mortality at one year, we calculated the hazard ratios with 95% interval of confidence (IC) of each variable and variables with a p value < 0.05 were inserted in a multivariate Cox model. P values < 0.05 were considered statistically significant. The statistical analysis was conducted using “R,” version 3.5.2.
Results
Patients
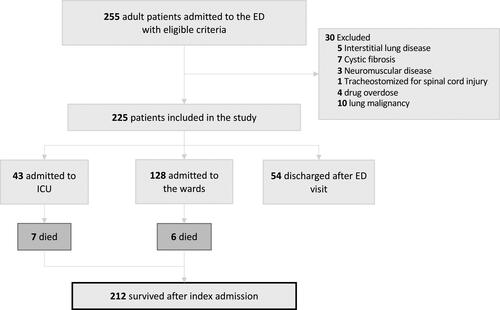
Over a 12-month time period, we identified 255 patients who were admitted with AHRF. We excluded 30 patients including five with interstitial lung disease, seven with cystic fibrosis, three with neuromuscular disease, ten with lung malignancy, four with drug overdose and one tracheostomized for a spinal cord injury. Of the 225 patients included, 54 were discharged home after presentation to the ED, 128 were admitted to a general ward, and 43 were admitted to the ICU. Of the patients admitted to the hospital or to ICU, 13 (6%) died during the hospital stay. Consequently 212 patients were available for analysis and one-year follow-up (). Among survivors, 62/212 (29%) received ventilatory support and 36/212 (17%) were admitted to ICU.
Baseline and in-hospital characteristics of the cohort are shown in detail in . When compared with patients that were not readmitted, patients readmitted within one year from the index admission had a significantly lower BMI (25.2 vs. 29.1, p = 0.035), higher Charlson comorbidity index (2 vs. 1, p = 0.013), higher rate of cardiovascular comorbidities (71.6% vs. 54.7%, p = 0.025) and previous echocardiography available (64.9% vs. 43.8%, p = 0.007) and higher use of long-acting bronchodilators (56.1% vs. 39.1%, p = 0.034). Only 82 (38.6%) patients had a spirometry available and 11 (5.2%) patients had a polysomnography performed before being admitted. Readmitted patients also had higher rate of hospital admissions in the previous year (50.7% vs. 17.2%, p < 0.001) with higher number of patients that needed ICU admission and NIV in the year prior to the index admission. We did not identify differences between the two groups with regard to their in-hospital characteristics.
Table 1. Patients baseline and in-hospital characteristics.
Chronic underlying diseases
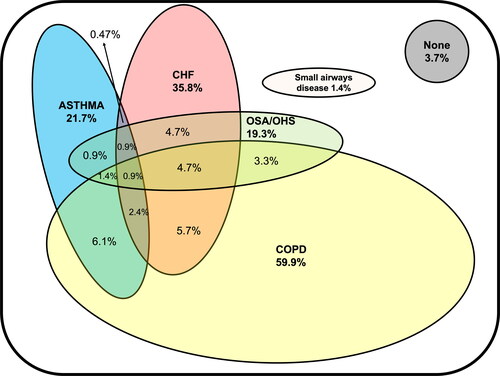
We depict the types of chronic diseases that led to AHRF in . COPD was the most frequent chronic disease among our population (59.9%) followed by CHF (35.8%), asthma (21.7%), OSA/OHS (19.3%) and small airways diseases (1.4%); more than one third of patients had more than one coexisting chronic illness. Only 3.7% didn’t have any known chronic disease that could lead to an exacerbation of hypercapnic respiratory failure.
Figure 2. Main diseases that can lead to AHRF in our population (n = 212). Each oval figure represents a disease; overlapping of figures indicates the presence of more than one disease. Areas in the figures represent the prevalence of the diseases. COPD = chronic obstructive pulmonary disease, CHF = congestive heart failure, OSA = obstructive sleep apnea, OHS = obesity-hypoventilation syndrome. Numbers in bold represent the total number in a given category.
Primary analyses
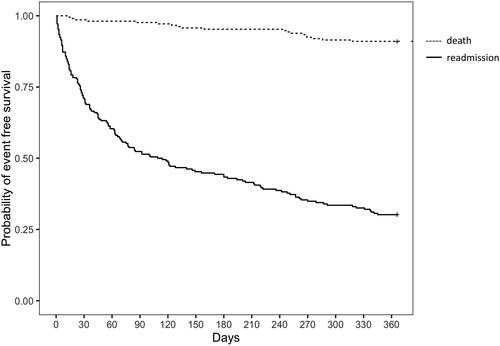
We analyzed outcomes in all patients who survived their index admission. During the one-year follow-up period, 150 patients (70.8%) were readmitted at least once (as ED visit only or ED visit and admission as inpatient). The one-year readmission rate was 73.2% (93/127) for COPD patients, 69.7% (53/76) for CHF patients, 70.7% (29/41) for OSA/OHS patients, 67.4% (31/46) for asthma patients and 54.5% (6/11) for others. The mean time to the first readmission was 88 days (SD 97.1) and the median time was 45 days (IQR 14–121.5). The median number of readmissions in the year following discharge was 2 (IQR 1–4.8). One year mortality was nearly 9%. Data pertaining to one year mortality and readmissions (considered as ED visits and inpatient admissions), are shown in . The rate of previous admissions was 41.7% (53/127) for COPD patients, 47.4% (36/76) for CHF patients, 56.1% (23/41) for OSA/OHS patients, 45.6% (21/46) for asthma patients and 30% (9/30) for others.
Table 2. Cumulative number of patients readmitted and deceased due to all causes at different times after discharge from index event (n = 212).
Univariate and multivariate analyses
In the univariate analysis, Charlson comorbidity index, cardiovascular comorbidities, severe renal insufficiency, home use of long-acting bronchodilators, having an unplanned hospital admission in the previous year and being admitted to the ICU during index admission were associated with readmission and death at one year.
For the multivariate analysis, we created two models as shown in , using variables either well known to influence outcome (e.g. age), or relevant to patients’ baseline status and severity of hospital admission or statistically significant in the univariate analysis. We created two models using either a score (Charlson comorbidity index) or the presence of comorbidities as indicators of the burden of chronic diseases. Only a previous history of admissions was significantly associated with a recurrent readmission or death within one year. pH at index admission was not significant in the univariate analysis nor significantly changed the model when inserted in the multivariate one.
Table 3. Predictors of adverse outcomes (readmissions and death) at one year.
When considering mortality alone, although both age and unplanned hospital admissions in the previous year were associated with higher risk of one-year mortality in the univariate analysis, only age was associated with one-year mortality in the multivariate analysis.
Influence of initial severity on outcomes
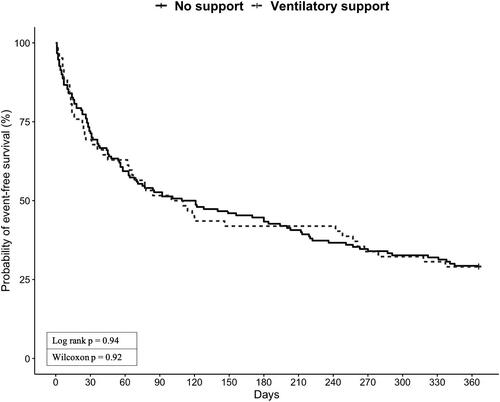
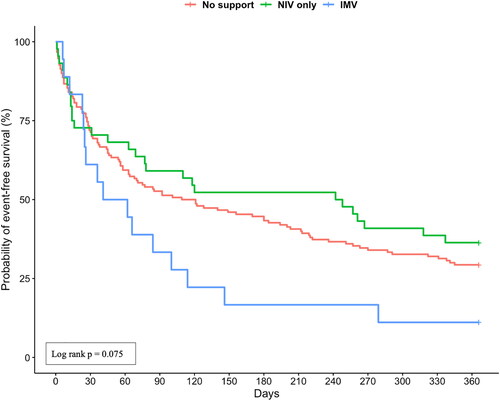
The Kaplan-Meier plots for readmission and survival at one year are shown in . Patients who received ventilatory support had a similar event-free survival probability as those who did not receive ventilator support (). The probability of event-free survival of invasively ventilated patients was 11% (2/18) compared with 36% (16/44) for patients who received NIV and 29% (44/150) for patients that didn’t receive any respiratory support (). In a post hoc analysis, we did not find significant difference between the 3 curves (no support vs. NIV: p = 0.375; no support vs. IMV: p = 0.078; NIV vs. IMV: p = 0.078).
Discussion
We identified that patients who present to the hospital with AHRF include predominantly patients with COPD, obesity-related respiratory disorders, heart failure, and asthma. The prevalence of comorbidities in this cohort is extremely high, with more than 80% of patients having at least one comorbidity, as reported in previous studies limited to COPD patients [Citation19, Citation24, Citation25]. Patients who survive an episode of AHRF have 70% rate of readmission to the same hospital and 9% mortality within one year. Notwithstanding, the severity of AHRF was not associated with worst outcomes. Among the predictors of these adverse outcomes, only the history of an unplanned admission in the previous year was an independent predictor of adverse outcomes at one year in our population and not the disease associated with the AHRF.
Our study is among the first studies to analyze outcomes of patients who presented to the ED with undifferentiated AHRF. As such, we used hypercapnia at presentation as a way to identify patients who are at risk of worse outcomes (readmission, mortality) at the time of initial presentation. We further characterized that approximately 60% of patients presenting with AHRF will have COPD and 40% will have other cardiac or respiratory diagnoses. These findings are important as they highlight the diversity of illnesses that may present to the ED with AHRF. Moreover, they highlight the potential need for (i) better diagnostic pathways to elucidate the causes of AHRF and (ii) for preventing readmission in a high-risk patient cohort. Unlike our study, previous studies focused on convenience cohorts of patients with predominantly or solely COPD, those discharged from the ICU, or those treated with NIV [Citation10, Citation11, Citation13, Citation24]. We believe that these studies left behind a high number of patients that didn’t get tested, thus not having a precise diagnosis, or that were wrongly diagnosed. We showed, in fact, that many patients didn’t have, for instance, a spirometry or polysomnography available before index admission, and that many patients have more than one chronic disease, thus making it difficult to classify them.
The high readmission rate in some groups of patients with AHRF has been noted previously in the literature. Several studies have shown similar results in patients with COPD who were treated with NIV and survived an episode of AHRF [Citation10, Citation14]. Hypercapnia, in turn, is a well-described predictor of readmissions in COPD patients [Citation26]. Nevertheless, we demonstrated a high prevalence of readmissions in patients with undifferentiated AHRF. Furthermore, we highlighted that although COPD is the most prevalent chronic disease in our population, obesity-related diseases, as well as, asthma and CHF are also prevalent in patients presenting with AHRF. We also showed that interestingly these patients have very similar one-year readmission rates. At least 35% of patients in our cohort had overlapping chronic diseases that likely contributed to their hypercapnia. Given the known underestimation and underdiagnosis of comorbidities in the COPD population, we believe that this number could be an underestimate simply because they had not been assessed.
In univariate and the multivariate analyses, we did not find that the type of chronic cardio-respiratory disease was a predictor of readmission and death at one year. This finding suggests that hypercapnic patients can be considered as a single group in terms of instituting a care pathway to prevent readmission. A prior history of hospital admissions was the only predictor of readmission and death at one year in our Cox regression model (28). Notwithstanding our study differs from the “ECLIPSE” study, one of the largest observational studies, that was limited to patients with COPD [Citation27]. Lastly, the level of care required at admission, as reflected by the need for ventilator support was not a predictor of worse outcomes, suggesting the importance of illness chronicity in patients presenting with AHRF. Although the use of NIV has been shown to be effective in improving clinically important outcomes in patients with COPD, the findings of this observational study do not support confirm that readmission rates remain very high in patients with AHRF independently of the initial use of NIV.
In their prospective study, Chu and colleagues reported one-year readmission rate for COPD patients treated with NIV of up to 80%, similar to ours [Citation10]. Their reported mortality rate at one year, though, was much higher, with a death rate of almost 50% (10). The cohort in their study was on average older (mean age 73.2 vs. 67.6 years) and more cachectic (mean BMI 20 vs. 25.8 in our study), and our cohort is more heterogeneous and with less severe exacerbations. Having spent more than 21 days in the hospital in the previous year was an independent risk factor for readmission in their study, as well as a higher Katz score [Citation10]; independent risk factors for recurrent episodes of AHRF requiring ventilation were home oxygen therapy, higher APACHE II score and lower BMI, whereas a higher MRC dyspnea score predicted death. We did not find similar associations in our study. Another study by Fazekas and colleagues studied long term outcomes of COPD patients treated with NIV for AHRF, and found a readmission rate due to respiratory reasons of only of 40% at one year, with a mortality around 20% at one year. Independent risk factors for readmission were lower BMI, older age, lower PaO2/FiO2 ratio at admission and hypercapnia at discharge. When we looked at the relationship between severity at index admission, with regard to receipt of respiratory support, and readmission and death at one year we did not find differences in the event-free survival curves of patients treated with ventilatory support and patients treated without support. An analysis comparable to ours was conducted in the European COPD Audit by Hartl and colleagues. These investigators found that COPD patients treated with ventilatory support for an exacerbation had higher risk of readmission and death at 90 days after discharge [Citation12]. This difference could be explained by the fact that Hartl and colleagues compared exacerbations treated with NIV with exacerbations without hypercapnia, whereas our patients were all hypercapnic at admission, suggesting they had a higher chronic disease severity. Similar to our study, Lindenauer and colleagues [Citation9] found that the 30 day readmission rate didn’t differ between COPD patients treated with NIV and those treated with IMV.
Our results have several clinical implications. Firstly, we showed how hypercapnia could be an easy and simple tool to identify patients at risk of readmission at an early stage, in order to be able to target them with interventions to reduce readmissions. Secondly, since hypercapnic patients are at very high risk of readmissions, these patients should be targeted as a group in the process of assessing and improving outcomes. All could benefit from individualized programs of readmission-reducing interventions, regardless whether they received ventilatory support during the index admission. Readmission-reducing programs, however, have proven to be challenging in these patients [Citation28]. Our data suggest the possibility that early identification targeting a larger group than only those with COPD during their index admission could help in finding new approaches. Our data show that the prevalence of comorbidities is very high and that patients with overlapping diseases are common. Physicians should be aware of the high probability of the presence of more than one contributing disease in these patients and every patient should be assessed for comorbidities, such as sleep disorders, heart failure and COPD, as soon as possible. We believe that the early identification of comorbidities and their management could reduce readmissions.
This study as several limitations, particularly due to its retrospective nature. Firstly, we defined our cohort with specific criteria for hypercapnia, with venous blood gas being much more frequently available at admission than the arterial one. Additionally, we only captured patients that were admitted to our hospital and consequently the incidence is likely an underestimate. The presence of missing data didn’t permit us to include all variables in the Cox regression model, such as BMI, thus we may have missed some significant predictors of adverse outcomes. Moreover, it is possible that we couldn’t find differences in the event-free survival rates between groups based on the type of ventilator support utilized due to the size of our cohort.
Conclusion
Our findings show that patients with undifferentiated AHRF are at high risk of hospital readmission, whatever their precise underlying diagnosis, thus suggesting to consider them as a group with similar outcomes. When looking for risk factors for complicated one-year outcome hypercapnic patients having at least one admission in the previous year should be considered at high risk of readmission. On the other hand, the use of noninvasive ventilation does not influence outcomes in these patients. These easily collectable information could help physicians to identify and target patients at higher risk in a pragmatic manner.
Author’s contribution
GC and LB conceived the study. GC, VD, TP, OS, DAH, KEAB and LB participated in its design and coordination. GC, RC and FV conducted the statistical analysis. GC and LB drafted the manuscript. All authors helped to revise the draft of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors are grateful to the discussion in the meetings of the Centre of Excellence in Mechanical Ventilation, especially with Kari White, RRT, Hilary Every, RRT, and Pamela Greco, RRT, for helpful suggestions.
Declaration of interest
LB’s laboratory received research grants from Medtronic Covidien, Draeger and Fisher Paykel, as well as non-financial support from Sentec, Philips, Air Liquide and General Electric outside the submitted work. RC received grants from the European Respiratory Society and from the French Intensive Care Society outside the submitted work. TP received personal fees from Drager, Philips and Fisher & Paykel outside the submitted work. No potential competing interests were reported by other authors.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Roussos C, Koutsoukou A. Respiratory failure. Eur Respir J. 2003;22(Suppl 47):3s–14s. DOI:https://doi.org/10.1183/09031936.03.00038503
- Epstein SK, Singh N. Respiratory acidosis. Respir Care. 2001;46(4):366–383.
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. DOI:https://doi.org/10.1164/rccm.201701-0218PP
- Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333(13):817–822. DOI:https://doi.org/10.1056/NEJM199509283331301
- Kramer N, Meyer TJ, Meharg J, et al. Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995;151(6):1799–1806. DOI:https://doi.org/10.1164/ajrccm.151.6.7767523
- Celikel T, Sungur M, Ceyhan B, et al. Comparison of noninvasive positive pressure ventilation with standard medical therapy in hypercapnic acute respiratory failure. Chest. 1998;114(6):1636–1642. DOI:https://doi.org/10.1378/chest.114.6.1636
- Martin TJ, Hovis JD, Costantino JP, et al. A randomized, prospective evaluation of noninvasive ventilation for acute respiratory failure. Am J Respir Crit Care Med. 2000;161(3 Pt 1):807–813. DOI:https://doi.org/10.1164/ajrccm.161.3.9808143
- Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet Lond Engl. 2000;355(9219):1931–1935. DOI:https://doi.org/10.1016/S0140-6736(00)02323-0
- Lindenauer PK, Stefan MS, Shieh M-S, et al. Outcomes associated with invasive and noninvasive ventilation among patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174(12):1982–1993. DOI:https://doi.org/10.1001/jamainternmed.2014.5430
- Chu CM, Chan VL, Lin AWN, et al. Readmission rates and life threatening events in COPD survivors treated with non-invasive ventilation for acute hypercapnic respiratory failure. Thorax. 2004;59(12):1020–1025. DOI:https://doi.org/10.1136/thx.2004.024307
- Connors AF, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (study to understand prognoses and preferences for outcomes and risks of treatments). Am J Respir Crit Care Med. 1996;154(4 Pt 1):959–967. DOI:https://doi.org/10.1164/ajrccm.154.4.8887592
- Hartl S, Lopez-Campos JL, Pozo-Rodriguez F, et al. Risk of death and readmission of hospital-admitted COPD exacerbations: European COPD audit. Eur Respir J. 2016;47(1):113–121. DOI:https://doi.org/10.1183/13993003.01391-2014
- Ankjaergaard KL, Rasmussen DB, Schwaner SH, et al. COPD: mortality and readmissions in relation to number of admissions with noninvasive ventilation. COPD J Chronic Obstr Pulm Dis. 2017;14(1):30–36. DOI:https://doi.org/10.1080/15412555.2016.1181160
- Fazekas AS, Aboulghaith M, Kriz RC, et al. Long-term outcomes after acute hypercapnic COPD exacerbation: first-ever episode of non-invasive ventilation. Wien Klin Wochenschr. 2018;130(19–20):561–568. DOI:https://doi.org/10.1007/s00508-018-1364-6
- Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the-Art review. Respir Care. 2010;55(10):1347–1362.
- Miller MR, Levy ML. Chronic obstructive pulmonary disease: missed diagnosis versus misdiagnosis. BMJ. 2015;351:h3021. DOI:https://doi.org/10.1136/bmj.h3021
- Hangaard S, Helle T, Nielsen C, et al. Causes of misdiagnosis of chronic obstructive pulmonary disease: a systematic scoping review. Respir Med. 2017;129:63–84. DOI:https://doi.org/10.1016/j.rmed.2017.05.015
- Adler D, Pépin J-L, Dupuis-Lozeron E, et al. Comorbidities and subgroups of patients surviving severe acute hypercapnic respiratory failure in the intensive care unit. Am J Respir Crit Care Med. 2017;196(2):200–207. DOI:https://doi.org/10.1164/rccm.201608-1666OC
- Thille AW, Córdoba-Izquierdo A, Maitre B, et al. High prevalence of sleep apnea syndrome in patients admitted to ICU for acute hypercapnic respiratory failure: a preliminary study. Intensive Care Med. 2018;44(2):267–269. DOI:https://doi.org/10.1007/s00134-017-4998-3
- Spece LJ, Epler EM, Donovan LM, et al. Role of comorbidities in treatment and outcomes after chronic obstructive pulmonary disease exacerbations. Ann Am Thorac Soc. 2018;15(9):1033–1038. DOI:https://doi.org/10.1513/AnnalsATS.201804-255OC
- Kendzerska T, Leung RS, Aaron SD, et al. Cardiovascular outcomes and all-cause mortality in patients with obstructive sleep apnea and chronic obstructive pulmonary disease (overlap syndrome). Ann Am Thorac Soc. 2018;16:71–81.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. DOI:https://doi.org/10.1016/0021-9681(87)90171-8
- Ontario | Canada Obituaries [Internet]. [cited 2019 Jul 18]. Available from: https://necrocanada.com/obituaries-2018/necrology/province/ontario/#.XTCETi0ZOCQ.
- Adler D., Pépin JL, Dupuis-Lozeron E, Espa-Cervena K, et al. Comorbidities and subgroups of patients surviving severe acute hypercapnic respiratory failure in the intensive care unit. Am J Respir Crit Care Med. 2017;196(2):200–207.
- Divo M, Cote C, de Torres JP; BODE Collaborative Group, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. DOI:https://doi.org/10.1164/rccm.201201-0034OC
- Garcia-Aymerich J, Monsó E, Marrades RM, EFRAM Investigators, et al. Risk factors for hospitalization for a chronic obstructive pulmonary disease exacerbation. EFRAM study. Am J Respir Crit Care Med. 2001;164(6):1002–1007. DOI:https://doi.org/10.1164/ajrccm.164.6.2006012
- Vestbo J, Anderson W, Coxson HO, ECLIPSE investigators, et al. Evaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE). Eur Respir J. 2008;31(4):869–873. DOI:https://doi.org/10.1183/09031936.00111707
- Puebla Neira DA, Hsu ES, Kuo Y-F, et al. Readmissions reduction program: mortality and readmissions for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(4):437–446. DOI:https://doi.org/10.1164/rccm.202002-0310OC