Abstract
Alpha-1 Antitrypsin deficiency (AATD) is a genetic condition that can lead to Chronic Obstructive Pulmonary Disease. The burden of psychological disease, its impact and contributing factors in patients with AATD are largely unknown. This study determined the prevalence of depression and anxiety in AATD and its clinical impact. All subjects with PiZZ/PiZnull (n = 635) and PiSZ (n = 111) genotypes within the AATD registry who had sufficient data to calculate pulmonary physiological and health status (HS) decline were grouped as those with or without a diagnosis of depression and/or anxiety. Univariate and multivariate analyses were performed on physiological, demographic and HS parameters. Depression and/or anxiety was present in 16.4% overall in both PiSZ and PiZZ/PiZnull cohorts and was associated with lower baseline pulmonary function and worse HS. In the multivariable analysis of the PiZZ/PiZnull cohort, a greater average decline in FEV1% predicted was observed in those with depression and/or anxiety than those without (–1.53 SD ± 2.26 per year, −0.99 ± 1.79, respectively; p = 0.03) but there was no difference in HS decline (p = 0.33). No differences were seen in the PiSZ cohort. Dyspnoea (mMRC score) was generally worse in those with depression and/or anxiety than those without. Comorbidity burden did not differ between those with or without depression and/or anxiety. Disease severity and progression may be contributing to the prevalence of psychological factors in PiZZ/PiZnull patients. Patients who are declining rapidly should be actively monitored for psychological co-morbidity and treated by cognitive or pharmacological means.
Supplemental data for this article is available online at https://doi.org/10.1080/15412555.2021.1991904 .
Introduction
Alpha-1 Antitrypsin deficiency (AATD) is a genetic condition that increases susceptibility to Chronic Obstructive Pulmonary Disease (COPD), and also Liver disease [Citation1], Bronchiectasis [Citation2], Vasculitis [Citation3], Panniculitis [Citation4] and Inflammatory Bowel Disease [Citation5]. It is clinically characterised by symptoms of chest tightness, wheezing, increased cough, sputum production and shortness of breath [Citation6]. Whilst pulmonary emphysema is the most common manifestation in AATD, there are differences in the development of emphysema from individuals with usual non-deficient COPD. This difference reflects the distribution and the enhanced susceptibility to damage which presents at a younger age in AATD patients [Citation7]. Early onset of pulmonary disease at a younger age and more generally active stage of life could therefore be associated with worse psychological as well as clinical outcomes [Citation8].
Psychological problems have been recognised and widely studied in patients with COPD, with a systematic review showing a general prevalence of 27% for depression and a relationship between depression or anxiety and poor health status [Citation9]. However the psychological impact and contributing factors in patients with AATD, many of whom have COPD at younger age than non-deficient patients, are largely unknown. In non-deficient COPD depression and anxiety are associated with an impact on everyday activities, more severe symptoms (e.g. shortness of breath and fatigue) [Citation10], increased hospitalisation, mortality and morbidity rates, as well as non-compliance with treatment [Citation11]. Furthermore, undiagnosed depression and anxiety in COPD patients may also impair social interactions, physical functioning and lead to a downward psychological spiral and increased use of healthcare systems [Citation10,Citation12].
Increased feelings of panic, fear and hopelessness in addition to dyspnoea and fatigue, may amplify a cycle that modulates anxiety and depression [Citation13]. The overlap of COPD and depression and anxiety symptoms highlights the challenges for diagnosing and treating coexistent psychological disorders [Citation14].
In COPD, reduced lung function (specifically FEV1 – forced expiratory volume in 1 s) is related to health status and mortality rates [Citation15–17] therefore slowing physiological progression is a key objective of current and future therapies [Citation18]. COPD patients have more rapid decline of lung function beyond normal ageing [Citation18,Citation19] especially in AATD [Citation20].
We hypothesised that anxiety and depression would be at least as common in AATD, as in non-deficient COPD, and relate to severity and decline in lung function and health status, as well as overall co-morbidity burden. The current paper explores these possibilities in a highly characterised patient population followed prospectively in a single centre.
Methods
Patient characteristics
All AATD subjects with the PiZZ, PiZnull and PiSZ genotype enrolled into the UK ADAPT AATD registry from 1996 to 2016 were included in the study. The study was approved by South Birmingham Research and Ethics Committee (LREC number: 3359) and written informed consent was obtained from all subjects. Once identified from the database, subjects were grouped into those with or without a clinical diagnosis of depression or anxiety, as documented in the medical records. Depression and/or anxiety was previously diagnosed by a Clinician or the patient’s GP either by diagnostic interviews or by using psychological assessment tools.
Data collected at baseline and follow up visits has been described previously [Citation20,Citation21]. In brief this included medical and family history, COPD related symptoms including cough, sputum volume and colour, mMRC score (modified Medical Research Council), exacerbation history, smoking history and current status, concomitant medication and occupational status. Subjects completed COPD Assessment Test (CAT), EQ5D and SGRQ (St George’s Respiratory Questionnaire) to assess annual health status.
For the current study lung function (FEV1, FVC – forced vital capacity, TLCO - transfer factor of the lungs for carbon monoxide, KCO- transfer coefficient for carbon monoxide) and HS (SGRQ) data collected at baseline and annual assessments were collated. Decline in lung function was expressed as the change of the % predicted for age, sex, height and ethnicity per year, using linear regression established for all annual data points (≥four data points over ≥ 3 years) for each patient as reported previously [Citation18]. Rapid decline in lung function (FEV1 and Kco change) was expressed as the change of 1.0% predicted per year (loss of FEV1 or Kco greater than >1.0% predicted per year) [Citation22]. Abnormal lung function is expressed as predicted values <80% (FEV1 and Kco).
The present study assessed data from 840 (PiZZ/PiZnull) and 111 (PiSZ) eligible subjects from an existing database as part of the ADAPT AATD registry. In the PiZZ/PiZnull cohort, a total of 205 subjects were excluded including subjects lost to follow up (n = 73) with insufficient data (<3 years) to determine decline of lung function and health status. There were no subjects excluded from the PiSZ cohort. Augmentation is not routinely available in the UK, outside of a clinical trial. None of the patients included in this cohort had received augmentation therapy as treatment or part of a clinical trial.
Charlson Comorbidity Index (CCI) was used to assess the burden of comorbidities and predict 10 year survival [Citation23]. A higher total CCI score reflects greater disease burden and higher risk of death [Citation24]. The mMRC dyspnoea scale was used to assess restricted baseline activity due to breathlessness (dyspnoea) [Citation25].
Statistical analysis
All statistical analyses were carried out in IBM SPSS Statistics for Windows, version 24.0, Armonk, NY: IBM Corp. Univariate analyses were performed on a range of physiological, demographic and HS parameters, including (but not limited to) age, FEV1% predicted, SGRQ, as well as lung function and HS decline, to determine associations with psychological co-morbidity. Normality was assessed by visual inspection of Q-Q plots, and normal data is reported as mean (+/- standard deviation) and nonparametric data as median and interquartile range (IQR). Comparisons of categorical groups were assessed with Fisher’s exact test and Kendall’s tau b test and continuous variables were assessed using Mann Whitney U tests and t-tests, respectively for PiZZ/PiZnull and PiSZ cohorts. All candidate variables were then included in a multivariable multiple regression to assess independent associations with depression and/or anxiety, with FEV1% predicted decline and SGRQ decline as the dependant variables. Variables including sex, smoking status (ever smoked/never smoked), baseline lung function such as FEV1% predicted and KCO% predicted (multicollinearity was assessed), baseline total SGRQ scores and baseline mMRC scores were adjusted to account for factors that relate to decline. A statistical significance value of p < 0.05 was used for all analyses.
Results
Subject characteristics
Subjects with PiZZ/PiZnull (n = 635) and PiSZ (n = 111) AATD deficiency were included in the final analysis. A clinical diagnosis of depression and/or anxiety was present in 16.4% overall (PiZZ/PiZnull and PiSZ and cohorts combined), with 16.4% in the PiZZ/PiZnull subjects and 16.2% in the PiSZ subjects. In the PiZZ/PiZnull cohort, depression and/or anxiety was associated with lower baseline FEV1 (43% predicted (34–74) v 56 (40–84); p = 0.01, respectively) and worse baseline SGRQ total (57 (34–70) v 43 (28–58); p < 0.001). In the PiSZ cohort, baseline lung function (FEV1) was also lower in those with depression and/or anxiety compared to those without (73 (48–102) v 101 (74–116); p = 0.04) as well as worse SGRQ total (54 (23–67) v 21 (8–53); p = 0.01). Baseline subject demographics, and decline in lung function and HS for both PiZZ/PiZnull and PiSZ cohorts are shown in and respectively.
Table 1. Data is shown for the PiZZ/PiZnull AATD cohort at baseline and subsequent annual decline in spirometry and HS for those with and without a clinical diagnosis of depression and anxiety.
Table 2. Data is shown for the PiSZ AATD cohort at baseline and subsequent annual decline in spirometry and HS for those with and without a clinical diagnosis of depression and anxiety.
Smoking status in PiZZ/PiZnull and PiSZ cohorts
In the PiZZ/PiZnull cohort, there was a higher proportion of ex-smokers amongst those with depression and/or anxiety compared to those without (83% v 70%; p < 0.01). This association was reversed in the PiSZ cohort where there were more ex-smokers in those without depression and/or anxiety (82% v 55%; p = 0.03). There were no current smokers in either the PiZZ/PiZnull or PiSZ cohort.
Antidepressant use in PiZZ/PiZnull and PiSZ cohorts
In the PiZZ/PiZnull cohort, 61% of subjects with anxiety or depression (n = 63) were on active pharmacological management for their symptoms (see supplementary data Table S1). In the PiSZ cohort 72% (n = 13) were on active treatment. The most common antidepressants/anxiolytics used in both cohorts were Citalopram, Fluoxetine, Paroxetine, Sertraline and Diazepam.
Breathlessness (modified MRC score) in the PiZZ/PiZnull and PiSZ cohort
In the PiZZ/PiZnull cohort, there was a statistically significant difference in the distribution of mMRC scores (τb = 0.140, p < 0.001) between subjects with and without depression and/or anxiety. Subjects with depression and/or anxiety tended to have higher scores, most frequently being 3 in those with psychological co-morbidity, but 1 in those without (see supplementary data Table S2). In the PiSZ cohort, although there was numerically a larger proportion of patients with an mMRC score of 0 with no depression and/or anxiety (and more with depression and/or anxiety in mMRC score of 1), this was not statistically significant (τb = −0.176, p = 0.07). and display the distribution of mMRC scores in both PiZZ/PiZnull and PiSZ cohorts.
Figure 1. The distribution of mMRC scores in the PiZZ/PiZnull cohort in those with and without depression and/or anxiety.
Notes: The bar chart demonstrates the proportion of mMRC scores in both groups with a higher percentage of mMRC score of 1 in those with no depression or anxiety and a higher percentage of mMRC score of 3 in those with depression and/or anxiety.
Abbreviations: mMRC score, modified Medical Research Council score.
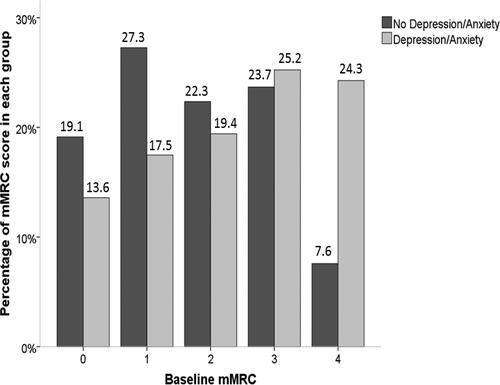
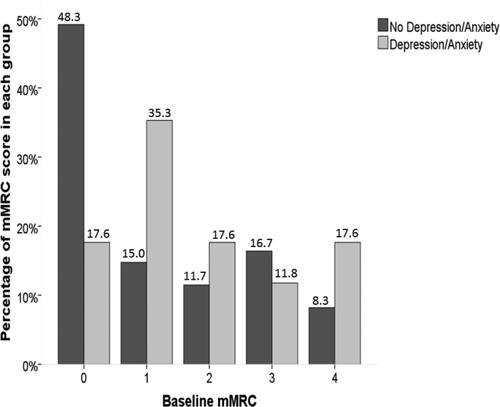
Figure 2. The distribution of mMRC scores in the PiSZ cohort in those with and without depression and/or anxiety.
Notes: The bar chart shows the proportion of mMRC scores in both groups with a higher percentage of mMRC score of 0 in those with no depression or anxiety and a higher percentage of mMRC score of 1 in those with depression and/or anxiety.
Abbreviations: mMRC score, modified Medical Research Council score.
Impact of comorbidities
The most common co-morbidities present in subjects in both cohorts (see supplementary data Table S3) were confirmed liver disease (n = 37, 9%), chronic kidney disease (n = 7, 3.6%), malignancy (n = 39, 13%) and diabetes (n = 30, 8%). The frequency distribution of CCI scores ( and ) indicated that there were no differences in comorbidity burden between those with or without depression and/or anxiety (see supplementary data Table S4) in either the PiZZ/PiZnull (τb = −0.029, p = 0.49) or the PiSZ cohorts (τb = −0.086, p = 0.27).
Lung function and health status decline in PiZZ/PiZnull and PiSZ cohorts
Univariate analyses
The decline in FEV1% predicted per year was greater in those with established depression and/or anxiety (–1.53 ± 2.26 v −0.99 ± 1.79; p = 0.03) but there were no differences in annual increase in SGRQ total score (0.14 ± 3.69 and 0.46 ± 2.85; p = 0.33) in the PiZZ/PiZnull cohort. There was no difference in the annual decline of FEV1% predicted for PiSZ subjects in those with or without depression and/or anxiety (–1.49 ± 2.13 v −0.65 ± 2.19, respectively; p = 0.32), nor worsening in total SGRQ score (–0.20 ± 2.33 v 0.43 ± 1.80; p = 0.54). and display FEV1 and Kco data (expressed as % predicted and the rate of decline) in patients with and without depression and/or anxiety for both the PiZZ/PiZnull and PiSZ cohorts. In every comparison, there were fewer patients with depression and/or anxiety than without. However, the proportion of patients with depression and/or anxiety compared to those without was only significantly different for Kco % predicted, where there was less depression and/or anxiety in patients with a normal Kco (12.2%) compared to those with an abnormal Kco (22.3%) (p = 0.02).
Figure 3. Lung function and rate of decline in AATD subjects (PiZZ/PiZnull cohort) in those with or without depression and/or anxiety.
Notes: demonstrates the number of subjects (in those with or without depression and/or anxiety) displaying normal or abnormal FEV1. demonstrates the number of subjects (in those with or without depression and/or anxiety) displaying rapid or non-rapid decline in FEV1. demonstrates the number of subjects (in those with or without depression and/or anxiety) displaying normal or abnormal Kco. demonstrates the number of subjects (in those with or without depression/anxiety) displaying rapid or non-rapid decline in Kco.
Abbreviations: FEV1, Forced Expiratory Volume in 1 Second; Kco, Transfer Coefficient for carbon monoxide; PRED, Predicted.
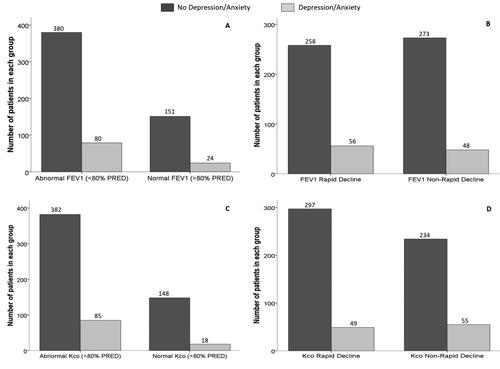
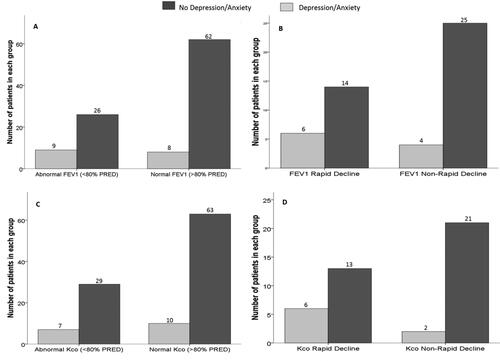
Figure 4. Lung function and rate of decline in AATD subjects (PiSZ cohort) in those with or without depression and/or anxiety.
Notes: demonstrates the number of subjects (in those with or without depression and/or anxiety) displaying normal or abnormal FEV1. demonstrates the number of subjects (in those with or without depression and/or anxiety) displaying rapid or non-rapid decline in FEV1. demonstrates the number of subjects (in those with or without depression and/or anxiety) displaying normal or abnormal Kco. demonstrates the number of subjects (in those with or without depression and/or anxiety) displaying rapid or non-rapid decline in Kco.
Abbreviations: FEV1, Forced Expiratory Volume in 1 Second; Kco, Transfer Coefficient for carbon monoxide; PRED, Predicted.
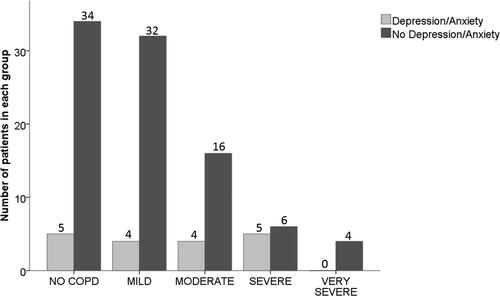
Similar data for patients with and without spirometric COPD (as defined by GOLD [Citation21,Citation26]) is displayed in and , where patients have been further classified by GOLD severity.
Figure 5. Different severity stages of COPD and no COPD in those with or without depression and/or anxiety PiZZ/PiZnull cohort.
Notes: The bar chart demonstrates the number of those with or without COPD and those with different severity stages of COPD as defined by GOLD in those with or without depression and/or anxiety.
Abbreviations: COPD, Chronic Obstructive Pulmonary Disease; GOLD, Global initiative for Chronic Obstructive Lung Disease.
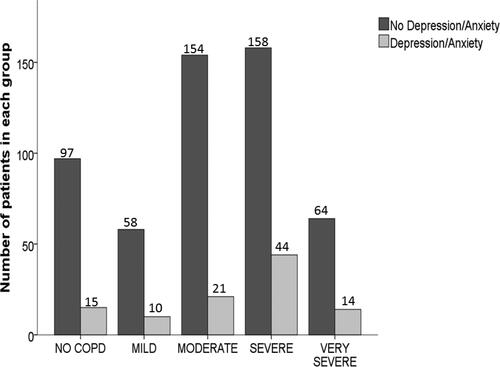
Figure 6. Different severity stages of COPD in those with or without depression and/or anxiety PiZZ/PiZnull cohort.
Notes: The bar chart demonstrates the number of those with or without COPD and those with different severity stages of COPD as defined by GOLD in those with or without depression and/or anxiety.
Abbreviations: COPD, Chronic Obstructive Pulmonary Disease; GOLD, Global initiative for Chronic Obstructive Lung Disease.
Analysis of depression and anxiety separately in PiZZ/PiZnull cohort indicated that subjects with depression alone also had a more rapid decline in median FEV1% predicted than those without (–1.32, IQR −2.78 to −0.45 v − 0.89 IQR −1.95 to 0.02, p < 0.01), but not HS decline. For subjects with or without anxiety alone, there were no differences in FEV1% or SGRQ decline.
Multivariable analyses
In the PiZZ/PiZnull cohort, after adjusting for smoking status, baseline lung function and baseline mMRC scores, multivariate analyses demonstrated that those with depression and/or anxiety still had greater decline rates (FEV1% predicted coef −0.499; 95% CI −0.895 to –0.104, R2 = .057; p = 0.01) although the proportion attributable to psychological comorbidity was low (5.7%).The rate of HS decline was not independently significant following adjustment (coef −0.240; 95% CI −0.880 to 0.399; R2 = .027 p = 0.46).
In the PiSZ cohort, there were no differences in FEV1 or HS decline related to the presence of psychological comorbidity alone (FEV1; coef −0.053; 95% CI –1.94 to 1.84; p = 0.96, SGRQ; coef 0.499; 95% CI −1.83 to 2.83; p = 0.66). Multivariable models are described more fully in supplementary data Tables S5–S8.
Discussion
The present study of AATD subjects is consistent with observations in non-deficient COPD that those with depression and/or anxiety exhibit a higher symptom burden and are likely to have more severe disease. However, uniquely the longitudinal data indicated this was also independently associated with the subsequent rate of deterioration of lung function (decline of FEV1) in the PiZZ/PiZnull cohort. This association suggests that the perception of past (or future likelihood) has an impact irrespective of baseline disease severity, and is reflected by their depression and/or anxiety.
Greater decline in FEV1% predicted was a feature of the PiZZ/PiZnull with depression and/or anxiety even after adjustment for baseline severity and other co-variates. Given that we have shown decline tends to be linear and stable over time in individuals with AATD [Citation17] this might indicate declining lung function contributes to development of psychological impact, particularly depression. In those with moderate AAT deficiency (PiSZ) no such relationship was seen; which may reflect lower decline rates or reduced power due to the smaller cohort size.
In the general population, studies assessing lung function and depression have proposed that it relates to reduced lung function and associated dyspnoea and social isolation [Citation27]. Whilst differences in disease severity and subsequent decline between PiZZ/PiZnull and PiSZ AATD patients are well recognised [Citation18,Citation28] the relationship between decline and psychological disorders is novel and likely reflects recognition that the baseline state reflects previous perceived or documented progression that persists prospectively.
Our results also indicate the psychological impact of comorbidity in AATD which has not been reported in detail previously. In a systematic review of prevalence of depression in non-deficient COPD, 27% of patients and 10% of control patients [Citation9] had evidence of a psychological impact of comorbidity which is less in our AATD subjects (16% for depression and/or anxiety) albeit higher than in health. The reasons for this are unclear, but younger age, fewer comorbidities and potentially less social consequences of illness (e.g. isolation) may influence this outcome. Nevertheless, clinicians caring for AATD patients should be aware of the early presence of depression and anxiety which can have impact on poor quality of life, abandonment of treatment and poor compliance. A diagnosis and treatment of psychological disorders may also help to reduce hospitalisations, exacerbation rates, mortality and morbidity and improve quality of life as seen in studies of non-deficient COPD [Citation10–12]. However further work is required to evaluate the above outcomes, as well as non-adherence to specific treatment, in AATD patients with depression and/or anxiety.
In both cohorts, impaired HS was worse in those with depression and/or anxiety at baseline although no relationship to subsequent decline was observed. Whilst studies have reported relationships between symptoms of depression and anxiety and HS [Citation29,Citation30], meta-analysis in non-deficient COPD indicated that other factors such as dyspnoea and exercise tolerance are the important determinants of HS [Citation31]. Consequently our adjustment for disease severity (which is strongly linked to symptomology) likely accounts for the lack of relationship with HS decline, suggesting it is more dependent on respiratory symptoms than psychological factors. This would be understandable given that the HS score used is specific to respiratory disease. In non-deficient COPD patients it has been suggested that the impact of COPD is likely to depend on non-respiratory factors such as patient’s social circumstances, expectations to coping skills and psychological wellbeing in addition to the clinical burden [Citation32]. In our study, generic HS data, such as that derived from EQ5D, is limited as this data was added to our protocol at a later point in time, and the data that EQ5D provides is nor very granular relative to other generic HS questionnaires, such as SF36 [Citation33]. Further studies of the impact of psychological morbidity on HS in AATD may clarify this by using detailed generic questionnaires rather than disease specific ones [Citation34].
Previous smoking was more common in those with depression and/or anxiety in the PiZZ/PiZnull cohort. Research has indicated that depression and smoking may exhibit a bidirectional association in which patients with depression are at an increased risk of smoking [Citation35] and then find smoking cessation more challenging [Citation35,Citation36]. However, the interaction between psychological disorders and COPD is unlikely to be related to confounding factors such as nicotine dependence and cigarette smoking alone [Citation37]. Furthermore, in our cohort many subjects were never smokers and almost all smokers had ceased, irrespective of presence of psychological comorbidity and no relationship was found between smoking burden and psychological morbidity.
The major strength of the current study is the number of subjects and longitudinal data available for analysis. However, it has several potential limitations. In particular the number of subjects in the PiSZ cohort was relatively small, especially in those with depression and/or anxiety group limiting comparisons between both groups. Thus the findings should be reviewed and validated in a larger cohort as available in international registries. In addition, there may be an acquisition bias as subjects may be less likely to come for repeated follow-up either if they remain subsequently well or their psychological status is markedly impaired. Either could affect the relationship to subsequent decline in lung function and HS, as greater than four data points over 3 or more years are necessary to determine decline. It is also possible that beyond these initial 4 data points, subjects who had no depression or anxiety at baseline may have subsequently developed such changes but not been identified. It is suggested that relevant questions related to psychological health should become a part of routine care in follow up.
Also the effects of depression and anxiety were studied in subjects categorised into two categorical groups (with or without) a diagnosis which would not reflect the severity of illness (mild, moderate or severe). The use of additional psychological assessment tools such as Hospital Anxiety and Depression Scale [Citation38], Hamilton Rating Scale [Citation39] and Geriatric Mental Health Scale [Citation40], may help to establish the true impact of depression and anxiety in AATD patients. In view of the prevalence of these psychological issues in AATD this is important for future studies as it is recognised that psychological issues in COPD patients may remain undiagnosed and untreated [Citation41] leading to a manageable impact on the disease [Citation13]. This is likely in AATD subjects, albeit partly managed in 67% were at least on appropriate therapy. Effective treatment options for psychological morbidity in COPD include Cognitive Behavioural Therapy (as the most beneficial), pulmonary rehabilitation and education, relaxation therapy including breathing exercises and mindfulness [Citation42]. A combination of antidepressants with such therapies would be a holistic intervention which is likely to be beneficial in the management of relevant patients with AATD.
Conclusion
These data suggest that disease severity and progression, as measured by the annual decline of FEV1% predicted, may be contributing to the prevalence of psychological disorders in PiZZ/PiZnull patients. This implies that patients who are declining rapidly should be actively monitored for psychological co-morbidity and treated by relevant cognitive or pharmacological means.
Author contributions
FM and AMT were instrumental in the conception and design of the study and FM, AMT, RE, AP and RAS contributed towards data acquisition, analysis and interpretation. All authors contributed to drafting and critically revising the paper and approved the final submitted version.
Acknowledgements
The authors would like to thank Peter Nightingale (University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK) for his statistical advice in the analysis of the data. During the part of the conduct of this research Ross Edgar was supported by a Clinical Doctoral Research Fellowship (CDRF‐2014‐05‐044), supported by the National Institute for Health Research (NIHR) and Health Education England (HEE). This report is an independent research arising from this support. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, Health Education England, or the Department of Health. The ADAPT programme was an investigator lead project funded by Talecris the West Midlands Chest fund and Griffols.
Disclosure statement
AMT has had grants or honoraria from CSL Behring and Vertex within the last 2 years, and grant funding from Alpha 1 Foundation, ATS Foundation and Chest Foundation for work in AATD. RAS has received grants or honoraria from AstraZeneca, MedImmune, Almirall, Nycomed, Takeda, Boehringer Ingelheim, CSL Behring, Kamada, Baxter, Chiesi, Polyphor and GlaxoSmithKline.
Data availability statement
Data from this study will not be publicly available due to the terms of the patient consent to participation.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Eriksson S, Carlson J, Velez R. Risk of cirrhosis and primary liver cancer in alpha 1-antitrypsin deficiency. N Engl J Med. 1986;314(12):736–739. DOI:https://doi.org/10.1056/NEJM198603203141202
- Shin MS, Ho KJ. Bronchiectasis in patients with alpha 1-antitrypsin deficiency. A rare occurrence? Chest. 1993;104(5):1384–1386. DOI:https://doi.org/10.1378/chest.104.5.1384
- Mahr AD, Edberg JC, Stone JH, et al. Alpha₁-antitrypsin deficiency-related alleles Z and S and the risk of Wegener’s granulomatosis. Arthritis Rheum. 2010;62(12):3760–3767. DOI:https://doi.org/10.1002/art.27742
- Smith KC, Pittelkow MR, Su WP. Panniculitis associated with severe alpha 1-antitrypsin deficiency. Treatment and review of the literature. Arch Dermatol. 1987;123(12):1655–1661.
- Yang P, Tremaine WJ, Meyer RL, et al. Alpha1-antitrypsin deficiency and inflammatory bowel diseases. Mayo Clin Proc. 2000;75(5):450–455.
- Institute NHLaB. Alpha-1 Antitrypsin deficiency. Published 2019. Available from: https://www.nhlbi.nih.gov/health-topics/alpha-1-antitrypsin-deficiency.
- Zacherle EN, Runken MC, Blanchette CM. Health care cost and utilization associated with alpha-1 antitrypsin deficiency among a cohort of medicare beneficiaries with COPD. Value Health. 2015;18(7):A664.
- Beiko T, Strange C. Anxiety and depression in patients with alpha-1 antitrypsin deficiency: current insights and impact on quality of life. Ther Clin Risk Manag. 2019;15:959–964. DOI:https://doi.org/10.2147/TCRM.S175369
- Matte DL, Pizzichini MM, Hoepers AT, et al. Prevalence of depression in COPD: a systematic review and meta-analysis of controlled studies. Respir Med. 2016;117:154–161. DOI:https://doi.org/10.1016/j.rmed.2016.06.006
- Doyle T, Palmer S, Johnson J, et al. Association of anxiety and depression with pulmonary-specific symptoms in chronic obstructive pulmonary disease. Int J Psychiatry Med. 2013;45(2):189–202. DOI:https://doi.org/10.2190/PM.45.2.g
- Yohannes AM, Willgoss TG, Baldwin RC, et al. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry. 2010;25(12):1209–1221. DOI:https://doi.org/10.1002/gps.2463
- Dalal AA, Shah M, Lunacsek O, et al. Clinical and economic burden of depression/anxiety in chronic obstructive pulmonary disease patients within a managed care population. Copd. 2011;8(4):293–299. DOI:https://doi.org/10.3109/15412555.2011.586659
- Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133):345–349. DOI:https://doi.org/10.1183/09059180.00007813
- Willgoss TG, Yohannes AM. Anxiety disorders in patients with COPD: a systematic review. Respir Care. 2013;58(5):858–866.
- Ketelaars CA, Schlosser MA, Mostert R, et al. Determinants of health-related quality of life in patients with chronic obstructive pulmonary disease. Thorax. 1996;51(1):39–43. DOI:https://doi.org/10.1136/thx.51.1.39
- Ebi-Kryston KL. Respiratory symptoms and pulmonary function as predictors of 10-year mortality from respiratory disease, cardiovascular disease, and all causes in the Whitehall study. J Clin Epidemiol. 1988;41(3):251–260. DOI:https://doi.org/10.1016/0895-4356(88)90129-1
- Tockman MS, Comstock GW. Respiratory risk factors and mortality: longitudinal studies in Washington county, Maryland. Am Rev Respir Dis. 1989;140(3 Pt 2):S56–S63. DOI:https://doi.org/10.1164/ajrccm/140.3_Pt_2.S56
- Stockley RA, Edgar RG, Pillai A, et al. Individualized lung function trends in alpha-1-antitrypsin deficiency: a need for patience in order to provide patient centered management? COPD. 2016;11:1745–1756. DOI:https://doi.org/10.2147/COPD.S111508
- Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med. 2006;174(8):886–893. DOI:https://doi.org/10.1164/rccm.200509-1374OC
- Green CE, Vayalapra S, Hampson JA, et al. PiSZ alpha-1 antitrypsin deficiency (AATD): pulmonary phenotype and prognosis relative to PiZZ AATD and PiMM COPD. Thorax. 2015;70(10):939–945. DOI:https://doi.org/10.1136/thoraxjnl-2015-206906
- Pillai AP, Turner AM, Stockley RA. Relationship of the 2011 global initiative for chronic obstructive lung disease strategy to clinically relevant outcomes in individuals with alpha1-antitrypsin deficiency. Annals Ats. 2014;11(6):859–864. DOI:https://doi.org/10.1513/AnnalsATS.201311-380OC
- Stockley RA, Miravitlles M, Vogelmeier C, et al. Augmentation therapy for alpha-1 antitrypsin deficiency: towards a personalised approach. Orphanet J Rare Dis. 2013;8:149. DOI:https://doi.org/10.1186/1750-1172-8-149
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. DOI:https://doi.org/10.1016/0021-9681(87)90171-8
- Sundararajan V, Henderson T, Perry C, et al. New ICD-10 version of the charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57(12):1288–1294. DOI:https://doi.org/10.1016/j.jclinepi.2004.03.012
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. DOI:https://doi.org/10.1378/chest.93.3.580
- RA S. Proteases and antiproteases in chronic obstructive pulmonary disease: Pathogenesis to treatment. Chisester (UK): Wiley; 2001.
- Park Y, Jung JY, Kim YS, et al. Relationship between depression and lung function in the general population in Korea: a retrospective cross-sectional study. COPD. 2018;13:2207–2213. DOI:https://doi.org/10.2147/COPD.S169025
- Lara B, Miravitlles M. Spanish registry of patients with alpha-1 antitrypsin deficiency; comparison of the characteristics of PISZ and PIZZ individuals. COPD. 2015;12 Suppl 1:27–31. DOI:https://doi.org/10.3109/15412555.2015.1021912
- Hajiro T, Nishimura K, Tsukino M, et al. Stages of disease severity and factors that affect the health status of patients with chronic obstructive pulmonary disease. Respir Med. 2000;94(9):841–846. DOI:https://doi.org/10.1053/rmed.2000.0804
- Gudmundsson G, Gislason T, Janson C, et al. Depression, anxiety and health status after hospitalisation for COPD: a multicentre study in the Nordic countries. Respir Med. 2006;100(1):87–93. DOI:https://doi.org/10.1016/j.rmed.2005.04.003
- Tsiligianni I, Kocks J, Tzanakis N, et al. Factors that influence disease-specific quality of life or health status in patients with COPD: a review and meta-analysis of pearson correlations. Prim Care Respir J. 2011;20(3):257–268. DOI:https://doi.org/10.4104/pcrj.2011.00029
- Hynninen MJ, Pallesen S, Nordhus IH. Factors affecting health status in COPD patients with co-morbid anxiety or depression. Int J Chron Obstruct Pulmon Dis. 2007;2(3):323–328.
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. DOI:https://doi.org/10.1097/00005650-199206000-00002
- Hobbins S, Chapple IL, Sapey E, et al. Is periodontitis a comorbidity of COPD or can associations be explained by shared risk factors/behaviors? Int J Chron Obstruct Pulmon Dis. 2017;12:1339–1349. DOI:https://doi.org/10.2147/COPD.S127802
- Glassman AH, Helzer JE, Covey LS, et al. Smoking, smoking cessation, and major depression. JAMA. 1990;264(12):1546–1549.
- Glassman AH, Covey LS, Stetner F, et al. Smoking cessation and the course of major depression: a follow-up study. Lancet. 2001;357(9272):1929–1932. DOI:https://doi.org/10.1016/S0140-6736(00)05064-9
- Goodwin RD, Lavoie KL, Lemeshow AR, et al. Depression, anxiety, and COPD: the unexamined role of nicotine dependence. Nicotine Tob Res. 2012;14(2):176–183. DOI:https://doi.org/10.1093/ntr/ntr165
- Dowson C, Laing R, Barraclough R, et al. The use of the hospital anxiety and depression scale (HADS) in patients with chronic obstructive pulmonary disease: a pilot study. N Z Med J. 2001. 114(1141):447–449.
- Stage KB, Middelboe T, Pisinger C. Measurement of depression in patients with chronic obstructive pulmonary disease (COPD). Nord J Psychiatry. 2003;57(4):297–301. DOI:https://doi.org/10.1080/08039480310002147
- Yohannes AM, Baldwin RC, Connolly MJ. Depression and anxiety in elderly outpatients with chronic obstructive pulmonary disease: prevalence, and validation of the BASDEC screening questionnaire. Int J Geriat Psychiatry. 2000;15(12):1090–1096. DOI:https://doi.org/10.1002/1099-1166(200012)15:12<1090::AID-GPS249>3.0.CO;2-L
- Mignogna J, Cully J. Depression and anxiety in patients with COPD: a focus on psychological treatments in ambulatory care settings. Curr. Respir. Med. Rev. 2012;8:137–144.
- Tselebis A, Pachi A, Ilias I, et al. Strategies to improve anxiety and depression in patients with COPD: a mental health perspective. Neuropsychiatr Dis Treat. 2016;12:297–328. DOI:https://doi.org/10.2147/NDT.S79354
