Abstract
Although fibrinogen is a FDA qualified prognostic biomarker in COPD, it still lacks sufficient resolution to be clinically useful. Next to replication of findings in different cohorts also the combination with other validated biomarkers should be investigated. Therefore, the aim of this study was to confirm in a large well-defined population of COPD patients whether fibrinogen can predict mortality and whether a combination with the biomarker MR-proADM can increase prognostic accuracy. From the COMIC cohort study we included COPD patients with a blood sample obtained in stable state (n = 640) and/or at hospitalization for an acute exacerbation of COPD (n = 262). Risk of death during 3 years of follow up for the separate and combined biomarker models was analyzed with Cox regression. Furthermore, logistic regression models for death after one year were constructed. When both fibrinogen and MR-proADM were included in the survival model, a doubling in fibrinogen and MR-proADM levels gave a 2.2 (95% CI 1.3–3.7) and 2.1 (95% CI 1.5–3.0) fold increased risk of dying, respectively. The prediction model for death after 1 year improved significantly when MR-proADM was added to the model with fibrinogen (AUC increased from 0.78 to 0.83; p = 0.02). However, the combined model was not significantly more adequate than the model with solely MR-proADM (AUC 0.83 vs 0.82; p = 0.34). The study suggests that MR-proADM is more promising than fibrinogen in prediciting mortality. Adding fibrinogen to a model containing MR-proADM does not significantly increase the predictive capacity of the model.
Keywords:
Introduction
COPD will be the third-leading cause for mortality worldwide by 2020 [Citation1]. Accurate prediction of mortality is important because it helps identify patients in whom the implementation of specific therapeutic measures can improve outcome [Citation1]. Several attempts have been made to identify risk factors for mortality.
Existing models to predict mortality use multiple parameters including lung function, exercise tests, reported dyspnea or patient characteristics like body-mass-index [Citation2] or age. Well known multidimensional tools are the BODE (Body-Mass Index, Obstruction, Dyspnea, Exercise) and ADO (Age, Dyspnea, Obstruction) index. These indices not only have limited predictive value, but may also be cumbersome to regularly determine in a clinical setting. Furthermore, while the models may incorporate parameters reflecting the disease state, none incorporates a direct reflection of the possible ongoing inflammatory process in COPD.
We, among others, have shown that the peptide mid-range proadrenomedullin (MR-proADM) is a valuable predictor for mortality in COPD [Citation3–6]. Adrenomedullin (ADM) has a wide range of actions including immune-modulating, metabolic and vascular actions. It can behave both as a hormone and as a cytokine [Citation7,Citation8]. After an inflammatory stimulus there is also an increased expression of this molecule in the lungs [Citation9]. Adding MR-proADM to various multidimensional indices such as the ADO and BODE improved their predictive power significantly and relevantly [Citation3, Citation5, Citation10, Citation11].
Another well studied biomarker in COPD is fibrinogen. Fibrinogen is considered as a prognostic biomarker in COPD by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) [Citation12]. Besides a blood clothing factor, fibrinogen is also an acute phase reactant. While the liver is the primary source of plasma fibrinogen, it has been shown that lung epithelium may also produce fibrinogen in response to an inflammatory stimulus [Citation13, Citation14]. Normal fibrinogen levels in blood range between 1.5 and 3.5 g/litre [Citation15]. Higher fibrinogen levels in the stable state have been shown to be related with an increased frequency of exacerbations [Citation16, Citation17] and increased mortality [Citation18–21] in COPD, although there are conflicting results [Citation22, Citation23]. The study of Mannino et al. that pooled 5 studies showed that increased fibrinogen is associated with increased risk of death [Citation19].
Replication of data in different cohorts is imperative as underlined by a recent study of Keene [Citation24]. In this study blood biomarkers were significantly associated with the occurrence of exacerbations but were not robust between the SPIROMICS and COPDGene cohort.
Combining two or more biomarkers for prediction of clinical outcome in COPD, including mortality, improved when two or more biomarkers were combined, but MR-proADM was never included [Citation1, Citation21, Citation25, Citation26]. Therefore, we hypothesized that, in a large well-defined population of COPD patients, the unique combination of fibrinogen and MR-proADM could increase the prognostic accuracy.
Materials and methods
Setting and study population
In this study we describe the results of a retrospective analysis of prospectively collected data from the COMIC study, a single center cohort study on the immune status of COPD patients as a determinant for survival. From December 2005 till April 2010, 795 patients were included with a follow-up period of three years. The COMIC study was approved by the medical ethical committee Twente, at Enschede (P05–49). All patients provided written informed consent. The COMIC study was initiated in a phase when registration was not necessary for non-interventional cohort studies.
Patients had to meet the following criteria; 1) a clinical diagnosis of COPD according to the GOLD recommendations [Citation27]; 2) current or former smoker; 3) age ≥40 years; 4) no medical condition compromising survival within the follow-up period, or serious psychiatric morbidity, 5) absence of any other active lung disease (e.g. sarcoïdosis), 6) no maintenance therapy with antibiotics, 7) ability to speak Dutch. Patients were enrolled when hospitalized for an acute exacerbation of COPD (AECOPD) or when visiting the outpatient clinic in stable state. To be included in the AECOPD group, patients had to be hospitalized for an AECOPD and be able to produce an adequate sputum sample at the day of hospitalization [Citation28]. An AECOPD was defined as an acute negative change from the baseline, reported by the patient, in dyspnea and/or sputum volume and/or color of sputum (yellowish or greenish sputum) and/or cough, which may warrant additional physician-initiated treatment of prednisolone with or without antibiotics. To be included in the stable state group patients had to meet the following criteria; no use of antibiotic and/or prednisolone 4 weeks prior to enrollment and no exacerbation less than 4 weeks before study entry. Also, data on common co-morbidities like hypertension; myocardial infarction and heart failure were obtained as they probably influence fibrinogen and MR-proADM level. Patients were asked to complete a questionnaire about their dyspnea in stable state (mMRC) [Citation29]. All patients were treated according to the COPD GOLD standard. Approximately eighty percent of the patients used inhaled corticosteroids during follow-up [Citation30]. The study was initiated to investigate the relationship between the immune response after influenza vaccination in COPD patients and mortality.
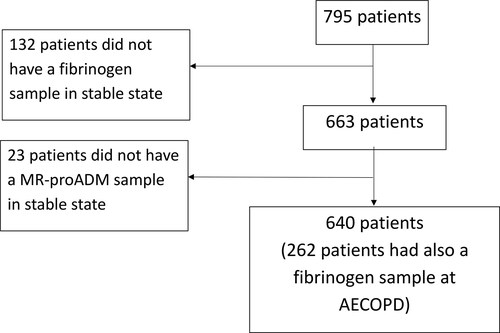
The study design is depicted in . Six hundred sixty-three of the 795 patients included in the COMIC study had a blood sample in stable state in which fibrinogen was measured. In 640 of these 663 patients, stable state MR-proADM was also measured. From 262 out of 640 patients we also obtained a blood sample at hospitalization for AECOPD in which fibrinogen was determined.
Outcomes
The primary outcome parameter was survival, based on all-cause mortality. Date of death was verified from the municipal administration.
Measurements of fibrinogen
Fibrinogen levels were measured on the STA-R (Stago) by the Clauss fibrinogen assay. This test method involves measuring the rate of fibrinogen to fibrin conversion in diluted sample under the influence of excess thrombin. Since under these conditions the fibrinogen content is rate limiting, the clotting time can be used as a measure of the concentration of the fibrinogen and in fact the clotting time is inversely proportional to the level of fibrinogen in the plasma. The reference value of fibrinogen is 2.0 − 4.0 g/l.
Measurements of MR-proADM
MR-proADM levels were measured in EDTA-plasma (ethylene diamine tetraacetic acid anticoagulant) with an automated sandwich immunoassay using a time-resolved amplified cryptate emission technology [Citation31] (KRYPTOR®; Thermo Scientific Biomarkers, [formerly B.R.A.H.M.S. AG] Hennigsdorf, Germany). The KRYPTOR MR-proADM assay has a measuring range of 0.05–100 nmol/l and a functional sensitivity of 0.25 nmol/l. The reference interval of MR-proADM in a healthy subset is 0.23 − 0.64 nmol/l (median 0.41 nmol/l) [Citation19, Citation32].
Statistical analysis
Continuous variables are expressed as mean (± standard deviation (SD)) in case of parametric data and as median (Interquartile range (IQR)) for non-parametric data. Categorical variables are expressed as counts with percentages. Differences in fibrinogen levels between survivors and non-survivors and between stable state and hospitalization for an AECOPD, were analyzed by Mann-Whitney U test. Since the levels of the biomarkers MR-pro-ADM and fibrinogen have a skewed distribution this data was transformed using a logarithm with base two for the other analyses. A Pearson correlation was used to determine the correlation between the transformed fibrinogen and MR-proADM levels. Time from stable state to death, was analyzed by Kaplan-Meier survival curves with log rank tests. Hazard ratio’s (HR) were determined by Cox regression analysis. All analyses were corrected for age, sex, BMI, and GOLD stage in a multivariate Cox regression analysis (the only confounders from the parameters named in ) and the C statistic was calculated [Citation33]. One-year survival after stable state was analyzed by logistic regression analyses and corrected for the same confounders. Furthermore, we used the biomarkers fibrinogen and proADM in constructing the prediction models. To test for differences between the AUC we calculated the standard error using a non-parametric test as described by Delong et al. [Citation34]. The confidence intervals of the AUC were calculated as exact binomial confidence intervals.
Table 1. Baseline characteristics.
All tests were two-sided and a p-value of 0.05 was considered statistically significant. The data were analyzed using SPSS, version 22 (SPSS Inc. Chicago IL). The standard error of the area under the curve and differences between the curves were analyzed using MedCalc 17.2 (Medcalc software, Ostend, Belgium).
Results
Baseline characteristics
The baseline characteristics of the patients in stable state (640) and in a subpopulation during an AECOPD (262) are presented in . The median follow-up after inclusion at stable state was 34 (IQR 27–35) months. Mortality was 21% during this period.
Fibrinogen levels in survivors and non-survivors
Fibrinogen levels were significantly higher in non-survivors (4.6 ± 3.9–5.4 g/L (median ± IQR)) compared to survivors (4.1 ± 3.6–4.7 g/L) when measured in stable state (p < 0.001). There was no significant difference in fibrinogen levels between survivors and non-survivors at hospitalization for AECOPD (p = 0.606) (). Since this significance in it was lacking, we chose not to further investigate the relation between fibrinogen and mortality in EACOPD. Subsequent analysis are on the biomarkers as measured in stable state.
Table 2. Fibrinogen.
Regression analysis
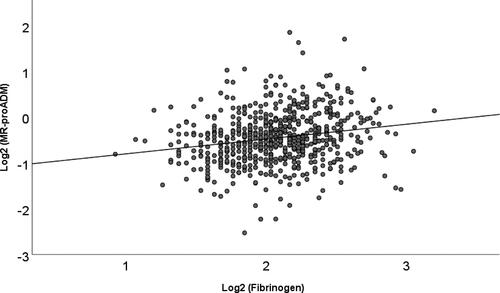
There was a very weak correlation of 0.2 between fibrinogen and MR-proADM (p < 0.001; r2=0.04) (see also ).
Prediction of mortality with fibrinogen and MR-proADM in stable state
shows univariate (data in table) and multivariate Cox regression. In the multivariate Cox regression with fibrinogen (as a continuous variable) a doubling of fibrinogen levels shows a 2.39 (95% CI 1.41–4.05) fold increased risk of dying, with a C-statistic of 0.73.
Table 3. Survival analysis.
Multivariate Cox regression with MR-proADM (as a continuous variable) shows a 2.22 (95% CI 1.59–3.10) fold increased risk of dying with a doubling of MR-proADM, with a C-statistic of 0.75.
When both fibrinogen and MR-proADM are included in the fully adjusted Cox regression model, a doubling in absolute fibrinogen levels is associated with a 2.17 (95% CI 1.27–3,7) fold increased risk of dying and a doubling of absolute MR-proADM levels with a 2.12 (95% CI 1.51–2.96) fold increased risk. The C-statistic of the combined model is 0.76 ().
After one year of follow-up, 46 of 640 patients (7.2%) died. When corrected for age, sex, BMI and GOLD, a doubling in absolute fibrinogen levels is associated with a 4.80 (95% CI 1.80–12.81) fold increased risk of dying within the first year of follow-up.
When fibrinogen is combined with MR-proADM, a doubling of fibrinogen shows a significant 4.39 (95% CI 1.60–12.05) fold increased chance of dying within one year, while doubling of MR-proADM shows a significant 3.59 (95%CI 1.90–6.78) fold increased risk of dying within one year (). presents the ROC curves of the models as shown in . When MR-proADM is added to the model with fibrinogen, the AUC increased significantly from 0.78 to 0.83 (p = 0.02). However, the combined model containing fibrinogen and MR-proADM is not significantly better than the model with solely MR-proADM (AUC 0.83 vs 0.82; p = 0.34).
Figure 3. ROC curves for prediction of death after 1 year.
Abbreviations; ROC: Receiver Operating Characteristic
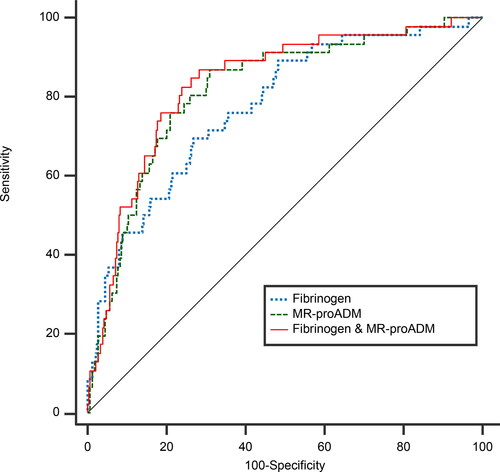
Table 4. Logistic regression analysis for death after one year.
Discussion
This study shows that fibrinogen and MR-proADM, measured in a well-characterised population in stable state, are predictors for mortality in COPD. The study suggests that MR-proADM is more promising than fibrinogen in predicting mortality. Adding fibrinogen, another well-known biomarker, to a model containing MR-proADM does not significantly increase the predictive capacity of the model. On the the contrary, adding MR-proADM to fibrinogen increases the accuracy of the short term mortality prediction model (one year follow-up) both relevantly and significantly.
The finding that fibrinogen is a predictor for mortality in stable state is in line with previous studies [Citation18–21]. E.g. a large meta-analysis [Citation18] focused on coronary heart disease and stroke showed people with high levels of fibrinogen had higher risks of dying from COPD. Mannino et al. [Citation19] combined 5 studies containing a total of 6376 individuals with COPD and reports significantly increased mortality (HR 1.94 (95%CI 1.62–2.31)) in individuals with fibrinogen above a level of 3–5 g/L.
Other studies used other cut off values, for example the reference value used by the FDA. In our study fibrinogen showed better results when used as a continuous variable (data not shown).
In the current cohort the median fibrinogen level is well over the upper limit of the normal reference values. This is in accordance with many cross-sectional studies in which blood fibrinogen in patients with COPD are higher than in healthy controls [Citation21, Citation35–37]. We also confirmed high levels of fibrinogen during exacerbations of COPD [Citation35, Citation38–40].
In a previous study we showed that MR-proADM is an accurate predictor for mortality and we validated this in a different population [Citation3, Citation32]. In the current study this predictive capability is reflected in a high C-statistic and AUC. Zeman et al. showed that combining several biomarkers may increase the predictive capability further [Citation25]. And although the model for one-year mortality risk with solely fibrinogen improved when MR-proADM was added, adding fibrinogen to a model already containing MR-proADM did not significantly improve the predictive power.
For mortality prediction in a clinical setting it seems therefore most promising to focus on MR-proADM in combination with other scores (ADO, BODE), by which the predictive power can be significantly and relevantly increased [Citation10,Citation11]. Furthermore, the combination of MR-proADM with other biomarkers for example S-RAGE [Citation41] could be investigated. This could increase the predictive power especially when measured longitudinal and in relation to clinical condition. However, fibrinogen may still have a role in prediction in COPD because it has been shown to be a relevant biomarker in many aspect of the disease for example exacerbations [Citation15], but also because this biomarker is already available for use in regular clinical setting. The COPD Foundation Biomarker Qualification Consortium (CBQC) proposes fibrinogen for drug development [Citation12].
Interestingly, the correlation between MR-proADM and fibrinogen was strikingly low (r = 0.2). From the lack of correlation, it may be hypothesized that both biomarkers reflect different biological processes. As suggested in the introduction it may be that such processes are directly related to pulmonary condition, but it may also reflect COPD as a systemic disease [Citation42, Citation43].
While fibrinogen and MR-proADM are hardly correlated, there was little benefit when the combined predictive capacity is expressed as AUC or c-statistic. This is explained by the fact that both the AUC and c-statistic are non-linear quantities. Apparently, the prediction-model containing MR-proADM is so superior that adding fibrinogen to the model does not lead to a significant increase in AUC.
The strength of the current study is that the results are based on data obtained from a large cohort study with a well-defined population of COPD patients from a general hospital with three years of follow-up. We recognize that in the current study only two biomarkers are studied. A direct comparison with other biomarkers for mortality prediction in COPD, is lacking. MR-proADM was only measured once in stable state. The next step would be to do longitudinal measurement to study MR-proADM over time, to detect whether changes in MR-proADM are also associated with changes in disease severity, health status and prognosis.
Increased fibrinogen is associated with increased mortality. Adding MR-proADM to fibrinogen increased the prognostic accuracy of the model for short term mortality prediction both relevantly and significantly. But the combination was not superior in predicting short term mortality compared to the model with solely MR-proADM.
We consider MR-proADM superior in predicting mortality in COPD compared to the FDA-approved biomarker fibrinogen. Not only is MR-proADM a strong predictor; it also has been well validated. We therefore advocate that MR-proADM should be included in future biomarker studies in COPD.
Acknowledgements
The authors thank the Research Department of Thermofischer, Hennigsdorf, Berlin for supplying the MR-proADM and the use of the Kryptor®. We thank B. Steenhuis for the measurement of MR-proADM and Dr. Ir. A.H.L. Mulder for the measurement of fibrinogen.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Celli BR, Locantore N, Yates J, for the ECLIPSE Investigators, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(10):1065–1072. DOI:https://doi.org/10.1164/rccm.201110-1792OC
- Lapolla A, Bonomo M, Dalfra MG, et al. Prepregnancy BMI influences maternal and fetal outcomes in women with isolated gestational hyperglycaemia: a multicentre study. Diabetes Metab. 2010;36(4):265–270. DOI:https://doi.org/10.1016/j.diabet.2010.01.008
- Zuur-Telgen MC, Brusse-Keizer MG, Vandervalk PD, et al. Stable state MR-proadrenomedullin level is a strong predictor for mortality in COPD patients. Chest. 2014;145(3):534–541. DOI:https://doi.org/10.1378/chest.13-1063
- Vizza CD, Letizia C, Sciomer S, et al. Increased plasma levels of adrenomedullin, a vasoactive peptide, in patients with end-stage pulmonary disease. Regul Pept. 2005;124(1-3):187–193. DOI:https://doi.org/10.1016/j.regpep.2004.07.021
- Stolz D, Christ-Crain M, Morgenthaler NG, et al. Plasma pro-adrenomedullin but not plasma pro-endothelin predicts survival in exacerbations of COPD. Chest. 2008;134(2):263–272. DOI:https://doi.org/10.1378/chest.08-0047
- Grolimund E, Kutz A, Marlowe RJ, ProHOSP Study Group, et al. Long-term prognosis in COPD Exacerbation: Role of biomarkers, clinical variables and exacerbation type. COPD. 2015;12(3):295–305. DOI:https://doi.org/10.3109/15412555.2014.949002
- Linscheid P, Seboek D, Zulewski H, et al. Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology. 2005;146(6):2699–2708. DOI:https://doi.org/10.1210/en.2004-1424
- Yoshibayashi M, Kamiya T, Kitamura K, et al. Plasma levels of adrenomedullin in primary and secondary pulmonary hypertension in patients <20 years of age. Am J Cardiol. 1997;79:1556–1558.
- Cheung BM, Hwang IS, Li CY, et al. Increased adrenomedullin expression in lungs in endotoxaemia. J Endocrinol. 2004;181(2):339–345. DOI:https://doi.org/10.1677/joe.0.1810339
- Brusse-Keizer M, Zuur-Telgen M, van der Palen J, et al. Adrenomedullin optimises mortality prediction in COPD patients. Respir Med. 2015;109(6):734–742. DOI:https://doi.org/10.1016/j.rmed.2015.02.013
- Stolz D, Kostikas K, Blasi F, et al. Adrenomedullin refines mortality prediction by the BODE index in COPD: the "BODE-A" index. Eur Respir J. 2014;43(2):397–408. DOI:https://doi.org/10.1183/09031936.00058713
- Miller BE, Tal-Singer R, Rennard SI, et al. Plasma fibrinogen qualification as a drug development tool in chronic obstructive pulmonary disease. Perspective of the chronic obstructive pulmonary disease biomarker qualification consortium. Am J Respir Crit Care Med. 2016;193(6):607–613. DOI:https://doi.org/10.1164/rccm.201509-1722PP
- Haidaris PJ. Induction of fibrinogen biosynthesis and secretion from cultured pulmonary epithelial cells. Blood. 1997;89(3):873–882.
- Simpson-Haidaris PJ, Courtney MA, Wright TW, et al. Induction of fibrinogen expression in the lung epithelium during Pneumocystis carinii pneumonia. Infect Immun. 1998;66(9):4431–4439. DOI:https://doi.org/10.1128/IAI.66.9.4431-4439.1998
- Duvoix A, Dickens J, Haq I, et al. Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax. 2013;68(7):670–676. DOI:https://doi.org/10.1136/thoraxjnl-2012-201871
- Groenewegen KH, Postma DS, Hop WC, COSMIC Study Group, et al. Increased systemic inflammation is a risk factor for COPD exacerbations. Chest. 2008;133(2):350–357. DOI:https://doi.org/10.1378/chest.07-1342
- Hurst JR, Vestbo J, Anzueto A, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. DOI:https://doi.org/10.1056/NEJMoa0909883
- Danesh J, Lewington S, Thompson SG, Fibrinogen Studies Collaboration, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294(14):1799–1809. DOI:https://doi.org/10.1001/jama.294.14.1799
- Mannino DM, Tal-Singer R, Lomas DA, et al. Plasma fibrinogen as a Biomarker for Mortality and Hospitalized Exacerbations in People with COPD. Chronic Obstr Pulm Dis. 2015;2(1):23–34.
- Mannino DM, Valvi D, Mullerova H, et al. Fibrinogen, COPD and mortality in a nationally representative U.S. cohort. COPD. 2012;9(4):359–366. DOI:https://doi.org/10.3109/15412555.2012.668249
- Sand JM, Leeming DJ, Byrjalsen I, et al. High levels of biomarkers of collagen remodeling are associated with increased mortality in COPD - results from the ECLIPSE study. Respir Res. 2016;17(1):125. DOI:https://doi.org/10.1186/s12931-016-0440-6
- Vestbo J, Edwards LD, Scanlon PD, ECLIPSE Investigators, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184–1192. DOI:https://doi.org/10.1056/NEJMoa1105482
- Miller J, Edwards LD, Agusti A, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med. 2013;107(9):1376–1384. DOI:https://doi.org/10.1016/j.rmed.2013.05.001
- Keene JD, Jacobson S, Kechris K, COPDGene and SPIROMICS Investigators ‡, et al. Biomarkers predictive of Exacerbations in the SPIROMICS and COPDGene Cohorts. Am J Respir Crit Care Med. 2017;195(4):473–481. DOI:https://doi.org/10.1164/rccm.201607-1330OC
- Zemans RL, Jacobson S, Keene J, et al. Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir Res. 2017;18(1):117. DOI:https://doi.org/10.1186/s12931-017-0597-7
- Mendy A, Forno E, Niyonsenga T, et al. Blood biomarkers as predictors of long-term mortality in COPD. Clin Respir J. 2018;12(5):1891–1899. DOI:https://doi.org/10.1111/crj.12752
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease [GOLD] 2011; 2011. Available from: http://www.goldcopd.org/.
- Telgen MC, Brusse-Keizer MG, van der Valk P, et al. Impact on clinical decision making of quality control standards applied to sputum analysis in COPD. Respir Med. 2011;105(3):371–376. DOI:https://doi.org/10.1016/j.rmed.2010.10.009
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. DOI:https://doi.org/10.1378/chest.93.3.580
- Kort S, Ter Huurne K, van der Palen J, et al. Quality of life and adherence to inhaled corticosteroids and tiotropium in COPD are related. COPD. 2016;11:1679–1688. DOI:https://doi.org/10.2147/COPD.S107303
- Morgenthaler NG, Struck J, Alonso C, et al. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51(10):1823–1829. DOI:https://doi.org/10.1373/clinchem.2005.051110
- Zuur-Telgen M, VanderValk P, van der Palen J, et al. Stable state proadrenomedullin level in COPD Patients: A Validation Study. COPD. 2017;14(2):219–227. DOI:https://doi.org/10.1080/15412555.2016.1250254
- Nam B-H, D’Agostino R, et al. The area under the ROC curve. In: Huber-Carol C, Balakrishnan N, Nikulin MS, editors. Goodness-of-fit tests and model validity. Boston: Birkhäuser; 2002. p. 273–277.
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845.
- Polatli M, Cakir A, Cildag O, et al. Microalbuminuria, von Willebrand factor and fibrinogen levels as markers of the severity in COPD exacerbation. J Thromb Thrombolysis. 2008;26(2):97–102. DOI:https://doi.org/10.1007/s11239-007-0073-1
- Alessandri C, Basili S, Violi F, et al. Hypercoagulability state in patients with chronic obstructive pulmonary disease. Chronic Obstructive Bronchitis and Haemostasis Group. Thromb Haemost. 1994;72(3):343–346.
- Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and markers of inflammation: data from the Third National Health and Nutrition Examination. Am J Med. 2003;114(9):758–762. DOI:https://doi.org/10.1016/S0002-9343(03)00185-2
- Wedzicha JA, Seemungal TA, MacCallum PK, et al. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost. 2000;84(8):210–215. DOI:https://doi.org/10.1055/s-0037-1613998
- Saldias PF, Diaz PO, Dreyse DJ, et al. [Etiology and biomarkers of systemic inflammation in mild to moderate COPD exacerbations]. Rev Med Chil. 2012;140(1):10–18.
- Valipour A, Schreder M, Wolzt M, et al. Circulating vascular endothelial growth factor and systemic inflammatory markers in patients with stable and exacerbated chronic obstructive pulmonary disease. Clin Sci (Lond). 2008;115(7):225–232. DOI:https://doi.org/10.1042/CS20070382
- Stockley RA, Halpin DMG, Celli BR, et al. Chronic obstructive pulmonary disease biomarkers and their interpretation. Am J Respir Crit Care Med. 2019 May;199(10):1195–1204. DOI:https://doi.org/10.1164/rccm.201810-1860SO
- Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):71–86. DOI:https://doi.org/10.1016/j.ccm.2013.10.004
- Koutsokera A, Stolz D, Loukides S, et al. Systemic biomarkers in exacerbations of COPD: the evolving clinical challenge. Chest. 2012;141(2):396–405. DOI:https://doi.org/10.1378/chest.11-0495