Abstract
Conventional pulmonary rehabilitation programs are used as therapies for the treatment of chronic obstructive pulmonary disease (COPD). However, this modality presents barriers that make rehabilitation difficult. For this reason, home-based pulmonary rehabilitation (HBPR) has been used to overcome these barriers. The objective was to systematically compare a structured program with HBPR or a control group for participants with COPD. The primary outcome was an improvement in symptoms in the level of dyspnea and secondary outcomes were parameters in lung function, exercise capacity, health-related quality of life (HRQoL) and the impact of the disease on the individual. The Medline (via PubMed), Virtual Health Library and Cochrane Library databases were searched until May 10, 2021. Randomized controlled trials were included without restrictions on the year of publication or language. The risk of bias was evaluated using the Cochrane risk-of-bias tool for randomized trials (RoB). Our results showed that there was a significant decrease in the level of dyspnea, (MD: 5.46; 95% CI: 1.97 to 8.96), increased distance covered (MD: 61.75; 95% CI: 42, 94 to 80.56, significant improvement in HRQoL (MD: −11.30; 95% CI: −19.81 to −2.79) and reduction in the impact of the disease (DM: −4.71; 95% CI: −7.95 to −1.47). All results found were comparing the intervention group versus the control group. To conclude we found a reduction in the levels of dyspnea, an increase in the distance covered on the six-minute walk test, improving HRQoL and decreasing the impact of the disease in COPD patients in home-based pulmonary rehabilitation.
Supplemental data for this article is available online at https://doi.org/10.1080/15412555.2021.2020234 .
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable and treatable disease characterized by persistent respiratory symptoms and airflow limitation caused by airway and/or alveolar abnormalities [Citation1]. The prevalence of COPD has increased worldwide, and the disease is now considered the third leading cause of death [Citation2] and represents 3.64% of the total years lived with disability [Citation3].
Dyspnea is the most common clinical manifestation of COPD [Citation4]. In addition, symptoms promote progressive impairment of the ability to perform activities of daily living and physical activities, impairing sleep quality, and are associated with a decline in health-related quality of life (HRQoL) [Citation5]. One should use the best tools available to diagnose and evaluate COPD and combine pharmacological and no pharmacological measures, such as pulmonary rehabilitation, for effective management [Citation6].
Previous studies have shown that at any stage of COPD, physical training performed in the context of a pulmonary rehabilitation program is effective in improving exercise tolerance, muscle strength, physical capacity, HRQoL, reducing levels of dyspnea and fatigue, and presents the potential to restore mental and social functioning [Citation7–10]. In the context of pulmonary rehabilitation, several items are recommended, such as warm-up exercises, strengthening of the upper limbs and lower limbs, aerobic exercises, and stretching. In addition, energy conservation techniques and information about the disease and health self-management are necessary [Citation11–13].
One of the major obstacles to pulmonary rehabilitation is the difficulties that patients have in accessing conventional pulmonary rehabilitation (CPR) programs [Citation12]. Such difficulties occur around trips to rehabilitation centers, lack of transport, diseases and comorbidities, scarcity of programs, mainly in rural areas and insufficient number of health professionals [Citation14–16].
To eliminate these obstacles, home-based pulmonary rehabilitation (HBPR) has been used, since it is an acceptable, easy and accessible method that is addressed at home. The activities are the same as those performed in ambulatorial rehabilitation and can result in clinical benefits equivalent to those obtained through CPR for COPD patients [Citation17].
From this perspective, in 2014, a systematic review showed that HBPR programs are effective in relieving respiratory symptoms in COPD patients, improving HRQoL and exercise capacity [Citation18]. However, some methodological issues related to the quality of the included studies with small sample sizes, study randomization process and descriptions of the allocation concealment unclear highlight the importance of a new systematic review in this field. Determining responses to HBPR will help us to assist with an individualized prescription recommendation for exercise in COPD patients.
Thus, the main objective of this systematic review and meta-analysis was to synthesize the efficacy of HBPR compared to CPR or without rehabilitation therapy in reducing the levels of dyspnea. The secondary outcomes were improvements in lung function, exercise performance, endurance, muscle strength, health-related quality of life and disease impact.
Methodology
Protocol and registry
The review methodology was registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42020151669) and followed the recommendations of the Cochrane Handbook [Citation19] and PRISMA statement [Citation20].
Eligibility criteria
We considered randomized clinical trials (RCTs) that compared a structured HBPR with CPR or without rehabilitation therapy. Inclusion criteria: studies on individuals with a diagnosis of COPD (mild, moderate, or severe) confirmed by spirometry [Citation21, Citation22] (American Thoracic Society [Citation23], British Thoracic Society [Citation24], or Global Initiative for Chronic Obstructive Lung Disease [Citation6]), over the age of 20 years old; Studies that exposure included any type of activity, including but not limited to aerobic and resistance training/activities Exclusion criteria: Studies including a population diagnosed with COPD associated with another respiratory disease, such as asthma, were excluded if the data could not be obtained separately; study maintenance pulmonary rehabilitation; papers which clearly demonstrate that patients attended less than 70% of the total rehabilitation sessions. In addition, duplicate studies, conference abstracts, letters to the editor, case reports, studies with a design incompatible with the purpose of this review and those that did not clarify the interventions used were excluded.
Search strategy and selection of articles
Searches were performed from inception until October 28, 2019 (updated May 10, 2021), without date limits or language restrictions, in the following databases: Medline via PubMed (www.pubmed.gov), Lilacs via Virtual Health Library (www.bvsalud.org) and CENTRAL via Cochrane Library (www.cochranelibrary.com). The ClinicalTrials.gov was assessed to identify potential ongoing studies. Moreover, the initial search was complemented by a manual search of reference lists from retrieved articles. To ensure literature saturation, comprehensive searches were constructed of both index terming’s (MeSH terms), “free text” terms, and synonyms. The search strategies and filters applied are described in Appendix 1, Supplementary Material. To avoid publication bias, the gray literature (Google Scholar) was also consulted.
The electronic searches were performed by two independent authors (DMX and AAF). Articles found in the databases were exported to Rayyan, a web and mobile app for systematic reviews (http://rayyan.qcri.org), for management and the removal of duplicates and selection of manuscripts. First, the manuscripts were selected by titles and abstracts based on the eligibility criteria. Then, the selected studies were read in full to confirm their eligibility. Disagreements on the inclusion process were resolved by a third reviewer (VPL).
Data extraction
Data were extracted from the included studies by two independent reviewers (DMX and AAF). Any disagreements that arose between the reviewers were resolved through discussion with a third reviewer (VPL). Authors of papers were contacted to request missing or additional data when necessary. The following information was extracted: Condition of participants (COPD classification and smoking participants); Symptoms (subjective ratings of exertional symptoms; i.e. dyspnea, secretion, cough, wheezing); Cardiorespiratory and metabolic responses (heart rate (HR), breathing frequency, peripheral capillary oxygen saturation (SpO2%), VO2, carbon dioxide production (VCO2) and concentration of blood lactate. Forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), inspiratory and expiratory maximal pressure (MIP and MEP), inspiratory capacity, residual volume and total lung capacity (TLC)); Physical capacity (distance walked on the six-minute walk test (6MWT)); Composite mortality indices (BODE index (iBODE) variation or other indices with similar properties); Health status (health-related quality of life); and Impact of COPD (COPD Assessment Test (CAT)).
Risk of bias in individual studies
Assessment of the methodological quality of the studies was performed using the Cochrane risk-of-bias tool for randomized trials. Based on this tool, the following criteria were evaluated: random sequence generation, allocation concealment, blinding of outcome assessor, incomplete outcome data, and selective outcome reporting. The domain blinding of patients and personnel was not considered for analysis once it is not possible to blind patients and staff. The quality assessment was guided by Rev-Man 5.3 software (Cochrane Collaboration, Oxford, UK).
Summarized measures and synthesis of results
When appropriate, we performed a meta-analysis using R software, version 3.6.2 (meta package). The meta-analysis was considered when at least two studies reported sufficient data for the same outcome in the exposure and control groups.
The data referring to mean difference and standard deviation (SD) from baseline to post-intervention follow-up were collected. Effect sizes were expressed as weighted (or standardized) final post-intervention mean differences (for continuous data) and their 95% confidence intervals. Heterogeneity was assessed statistically using the I2 tests: random effects meta-analysis was used if I2 ≥ 30% and fixed effects models if I2 < 30%. Subgroup analyses were conducted where there were sufficient data to investigate the impact between the types of rehabilitation programs (HBPR, CPR and no rehabilitation) regarding the follow-up period. Funnel plots to assess publication bias were not generated once there were 10 or fewer studies included in the meta-analyses. In addition, descriptive analyses are reported in tables.
Reliability of cumulative evidence
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach for grading the certainty of evidence and a Summary of Findings (SoF) were created using GRADEPro GDT (McMaster University, ON, Canada) for each outcome that allowed meta-analyses. The ranking of the quality of the evidence was based on five criteria: risk of bias, inconsistency, indirect evidence, imprecision, and publication bias. The SoF tables were generated at the end of the analysis according to the certainty of the evidence among the studies and classified as high, moderate, low, or very low certainty of the evidence.
Results
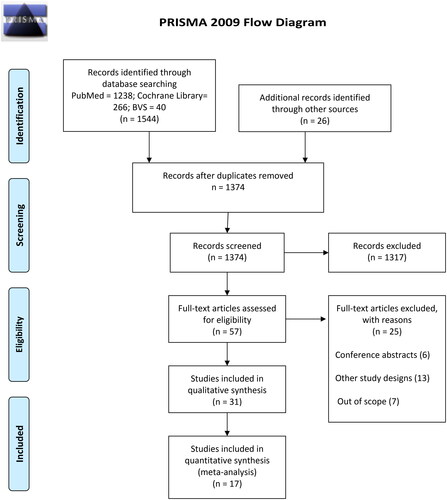
In total, 1570 articles were found from electronic databases, manual searches and gray literature. After screening and selection process based on eligibility criteria, 31 studies [Citation12, Citation25–54] were included in qualitative analysis involving 2531 COPD patients. However, only 17 articles (including 898 COPD patients) provided enough information to be included in the meta-analyses [Citation26, Citation28–33, Citation35, Citation37–39, Citation41, Citation42, Citation44, Citation46, Citation52, Citation53]. presents the flowchart of the study selection process.
Description of the studies
The studies included were published between 1996 and 2021. The pulmonary rehabilitation program duration ranged from five weeks to a maximum of 18 months. The sample size of the included studies ranged from 20 to 305 patients, the mean age ranged from 56.69 to 76.70, and the mean FEV1 ranged from 23.21% predicted to 68.00% predicted (moderate, severe, and very severe). Among the studies, 27 reported data on the participants’ sex [Citation12, Citation25–27, Citation29–34, Citation36, Citation37, Citation40–43, Citation45–54] 1159 males and 765 females with COPD (proportion of 1.65:1). In addition, 16 studies [Citation26, Citation30, Citation32, Citation36, Citation42–49, Citation52, Citation54] reported the number of participants with COPD (n = 612) who still had smoking habits.
HBPR was considered a therapy based on any type of strength and resistance exercise for upper limbs, lower limbs, trunk and also cardiopulmonary exercises and specific exercises for respiratory muscles. As the activities were carried out at home, the HBPR could be supervised through calls and/or visits by the study team, as a way to verify compliance with what was proposed to the participants. In addition, health education and self-management of the disease can also be part of the HBPR, as it contributes to the participants’ autonomy process. Participants who were allocated to the CPR group perform the same strength and/or resistance exercises proposed for the HBPR group, however, the place of performance was in a clinic/specialized outpatient clinic. The control group received health education sessions and standard medical follow-up.
The main characteristics of the included studies, characteristics of the participants, interventions and clinical characteristics are presented in , respectively.
Table 1. Main methodological characteristics of the included studies.
Table 2. Characteristic of the interventions and the population enrolled in included studies.
Table 3. Clinical characteristics of the population enrolled in included studies.
Table 4. Characteristics of the intervention.
Data extraction and meta-analysis
Dyspnea level
Seventeen studies (54.83%) evaluated dyspnea as an outcome [Citation28–30, Citation32–36, Citation38, Citation39, Citation41, Citation44, Citation46, Citation47, Citation50–53]. The scales used to assess dyspnea were Borg scale [Citation30, Citation33], Chronic Respiratory Questionnaire (CRQ/CRDQ) [Citation29, Citation35, Citation36, Citation38, Citation44, Citation47, Citation51], Medical Research Council (MCRC) or the modified version (MMRC) [Citation28, Citation32, Citation34, Citation41, Citation46, Citation52, Citation53], and Baseline Dyspnea Index (BDI) [Citation39].
BORG scale scores
Two RCTs, (6.45%) used the Borg scale to compare dyspnea level between HBPR and control group after two months [Citation30] and five weeks [Citation33] of rehabilitation from the baseline. In the experimental groups, the score on the Borg scale decreased, indicating a decrease in dyspnea levels, while there was an increase in dyspnea level in the control group [Citation30]. There were no differences between the beginning and the end of the study [Citation33].
CRQ/CRDQ scores
Seven studies (22.58%) used the CRQ/CRDQ scores to assess the level of dyspnea [Citation29, Citation35, Citation36, Citation38, Citation44, Citation47]. Six studies (20%) [Citation29, Citation35, Citation36, Citation38, Citation44, Citation51] compared HBPR and control group; however, only two of these studies were evaluated in the same follow-up period [Citation38, Citation44]. Only one study (3.22%) [Citation47], whose follow-up period were 18 months, compared HBPR with CPR, showing favorable results for the group treated with HBPR.
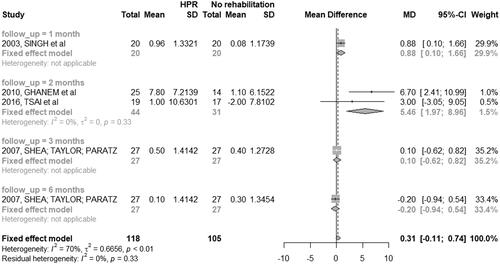
There was a significant decrease in dyspnea level among the patients classified as severe and very severe in the HBPR group when compared to the control group after two months of training (MD: 5.46; 95% CI: 1.97 to 8.96). One study assessed dyspnea levels after one month [Citation29], while another study assessed after three and six months [Citation35] from the baseline ().
MCRC or a modified version of mMRC scores
Dyspnea was evaluated in five studies (16.12%) using the MCRC or MMRC scales comparing HBPR to patients of the control group [Citation28, Citation32, Citation41, Citation46, Citation53]. One study also included a group of CPRs [Citation46], while two studies (6.45%) compared the HBPR to CPR [Citation34, Citation52].
There was no significant difference in dyspnea levels reported in the third month of follow-up between participants from HBPR and the control group (MD: −0.41; 95% CI: −0.82 to 0.01) ().
BDI scores
Only one study [Citation39] (3.22%) used the BDI scale for the assessment of dyspnea at the beginning of the study and after three months. The dyspnea level was lower in patients in the HBPR group than in the control group at the end of the follow-up period.
Lung function
Forced expiratory volume in 1 s
Three studies (9.67%) [Citation27, Citation33, Citation53] presented the result of FEV1 differently from other studies, so they did not enter the meta-analyses. The results obtained in these studies demonstrate that there was no significant difference between the values of this variable at the beginning and at the end of the programs between both groups evaluated (HBPR and control group). In addition, one study [Citation36] did not present the standard deviation values, and for this reason, it did not enter the meta-analysis. This was done in an intervention period of 3 and 6 months, and in both periods, the HBPR obtained better results than the control group. Only one study that reported FEV1 results compared HBPR and CPR [Citation47], and in 18 months, there was a small improvement in HBPR results, while in CPR, there was no change.
Three (9.67%) RCTs [Citation29, Citation30, Citation39] compared FEV1 values between the HBPR and control groups but with different follow-up periods. The study with the intervention of one month [Citation29] had an increase of 29.10% (SD: 10.40) for the HBPR group and 26.90% (SD: 9.20) for the control group. The two-month [Citation30] intervention study had an increase of 41.91% (SD: 17.90) for the HBPR group and 53.89% (SD: 24.53) for the control group. The three-month [Citation39] intervention study had an increase of 40.70% (SD: 13.20) for the HBPR group and 39.30% (SD: 12.10) for the control group.
Forced vital capacity
Two studies (6.45%) evaluated FVC, with a follow-up of 12 months [Citation37] and 2 months [Citation38]. In the first study, there was an increase of 57% (SD: 17) for the BPH group and a reduction of 66% (SD: 24) for the control group. In the second study, there was an increase of 40.40% (SD: 16.16) for the BPH group and of 26.57% (SD: 7.13) for the control group. The study included patients classified as moderate, severe and very severe COPD.
Residual volume
Only one (3.22%) study evaluated residual volume [Citation37] pre and post-intervention, with an increase of 145% (SD: 34) for HBPR group and 148% (SD: 24) for the control group. The study included patients classified as moderate, severe and very severe COPD.
Total lung capacity
Only one (3.22%) study evaluated Total lung capacity [Citation37] pre and post-intervention, with an increasing of 94% (SD: 15) for HBPR group and 108% (SD: 15) for control group. The study included patients classified as moderate, severe and very severe COPD.
Respiratory muscle strength
Inspiratory and expiratory maximal pressure
Two studies (6.45%) evaluated respiratory muscle strength, with an intervention period of 12 months [Citation37] and two months [Citation40]. There was an improvement in MIP and MEP in the HBPR group in comparison to the control group after the rehabilitation program. The population included in these studies obtained a classification of mild, moderate, severe and very severe COPD.
Oxygenation and BODE index
SpO2%
SpO2% showed no differences at three, six, 12 and 18 months from the baseline in comparison to HBPR with control group [Citation26, Citation39]. Compiled analysis of the data was only possible for the three-month follow-up time ().
iBODE
Only one study evaluated iBode [Citation47] before and after the intervention, with a reduction in the score of 4.10 (SD: 1.8) for the HBPR group and 4.40 (SD: 2.10) for the control group. The study included patients classified as moderate and severe COPD.
Physical capacity
6MWT
The distance walked in a 6MWT was evaluated in 18 studies (58.06%) [Citation29–31, Citation33, Citation35, Citation39, Citation41, Citation42, Citation44, Citation46–48, Citation50, Citation51, Citation53]. Of these, four (12.90%) [Citation36, Citation48, Citation50, Citation51] presented only the value of mean distance obtained, and the results were individually better in the HBPR group than in the CPR group. In addition, eight studies (25.80%) compared HBPR to control group [Citation29–31, Citation33, Citation35, Citation37–39, Citation41, Citation42, Citation44, Citation53]. A study (3.22%) with a 14-month intervention reported the distance covered in the three groups (HBPR, CPR and control group) [Citation46].
The distance covered after two months of intervention [Citation30, Citation38, Citation44, Citation46] was significantly greater for the HBPR group than for the control group (MD: 61.75 m; 95% CI: 42.94 m to 80.56 m). In a three-month intervention period [Citation31, Citation35, Citation39, Citation41], the difference between the groups (participants with mild, moderate, severe, and very severe classification of COPD) remained significant, but the distance covered was shorter when compared to a two-month intervention period (MD: 40.24 m; 95% CI: 5.52 m to 74.97 m). After twelve months, the difference between groups was not significant (MD: 26.97 m; 95% CI: −12.02,52 m a 65.96 m) ().
Figure 5. Mean difference (and 95% confidence interval) regarding the results of 6MWT between groups of HBPR (Home pulmonary rehabilitation) versus CG (Control group).
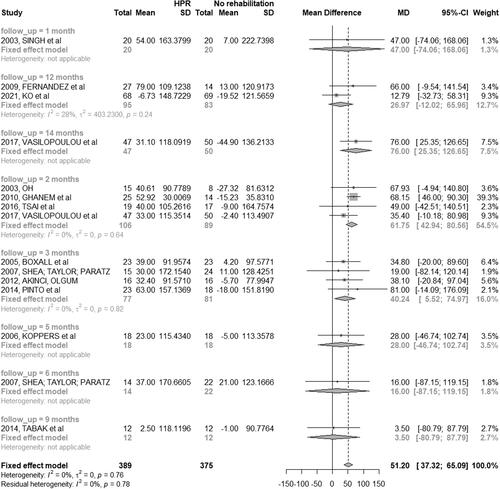
It was not possible to perform a meta-analysis between the HBPR and CPR groups because the studies [Citation46, Citation47] presented different follow-up periods.
HRQoL
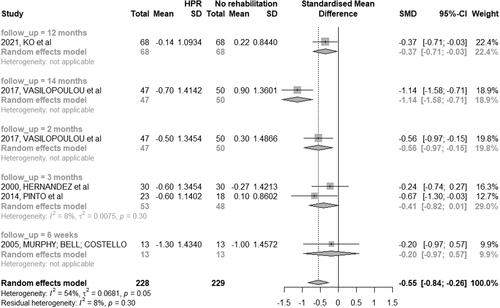
Twenty-two studies (70.96%) assessed HRQoL in COPD patients [Citation28–41, Citation44–46, Citation48, Citation51–54]. There was no homogeneity in the time of follow-up between studies among patients in the HBPR and CRP groups; thus, a meta-analysis was performed only between HBPR and control group ().
Figure 6. Mean difference (and 95% confidence interval) regarding the results of HRQoL by SGRQ between groups of HBPR (Home pulmonary rehabilitation) versus CG (Control group).
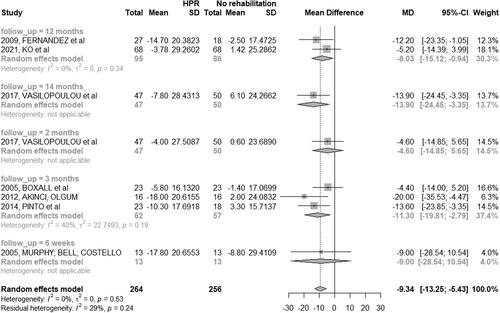
St. George’s respiratory questionnaire
Eight studies (25.80%) used the St. George’s Respiratory Questionnaire (SGRQ). Three studies (9.67%) including population classified as mild, moderate, severe, or very severe, were carried out in three months [Citation31, Citation39, Citation41], with a significant improvement in HRQoL in the HBPR group compared to the control group (MD: −11.30; 95% CI: −19.81 to −2.79). In one study, therapists assessed the intervention group after six weeks [Citation32] with no difference between groups (HBPR and CPR).
Two studies (6.45%) [Citation37, Citation53] carried out the study in 12 months (MD: −08.03; 95% CI: −15.12 to −0.94) and showed a significant improvement in HRQoL in the HBPR group compared to the control group. One study [Citation46] had the longest follow-up period, with the assessment of HRQoL after two and 14 months ().
Short form 36
A single study (3.22%) [Citation38] used SF-36 to assess HRQoL in individuals classified as severe and very severe; the total score and the domains were higher in the HBPR group than in the control group after two months from baseline.
Airways questionnaire 20
One study (3.22%) [Citation40] used the AQ-20 with a population having COPD classification as mild, moderate, and severe. There was an increase in the score of 15 (0–18 minimum and maximum value) for the HBPR group and 11 (3–20 minimum and maximum value) for the control group. The study included patients classified as moderate, severe, and very severe COPD.
Chronic respiratory questionnaire (CRQ/CRDQ)
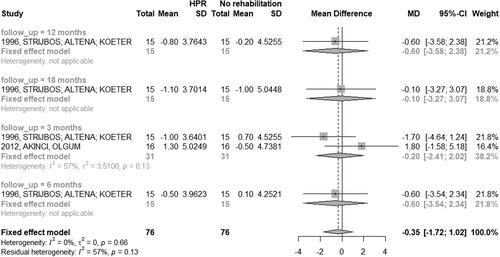
Nine studies (29.03%) evaluated HRQoL between the HBPR and control group using CRQ or CRDQ scales [Citation28–30, Citation33–36, Citation44, Citation45]. The meta-analysis was not performed due to the variation in the follow-up period. Three studies verified the comparison between HBPR and CPR. In the study [Citation33], the score in the five-week intervention period was higher in the HBPR, and in the others [Citation34, Citation45], the score was higher in CPR, in an interval period of seven weeks and six months, and nine weeks and six months, respectively.
CAT
Four studies (12.90%) performed assessments related to the impact of COPD using the CAT score among participants in the HBPR and control groups [Citation44, Citation46]. The results of the meta-analysis () showed that the patients included in the groups treated with HBPR reported a decrease in impact of disease. The studies were addressed in a population with moderate, severe and very severe COPD - after two months of intervention (MD: −4.71; 95% CI: −7.95 to −1.47) when compared to the participants included in the control group. Only one study [Citation46] evaluated the impact of the disease after 14 months from baseline, showing a reduction of the impact in this population.
Figure 7. Mean difference (and 95% confidence interval) regarding the results of impact of the disease by CAT between groups of HBPR (Home pulmonary rehabilitation) versus CG (Control group).
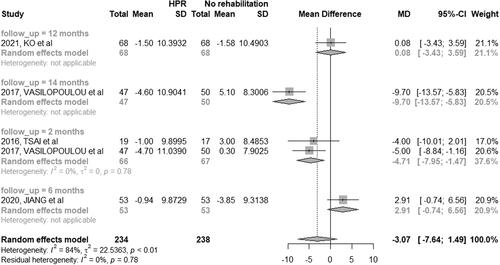
The RCTs evaluating HBPR and CPR groups [Citation46, Citation48, Citation54] had different follow-up periods, and meta-analyses were not performed.
Other findings
Wijkstra PJ et al., [Citation27], performed an intervention with a 12-month follow-up comparing HBPR and CG. This RCT was the only one that performed an evaluation and reevaluation of the participants in the following variables: heart rate, breathing frequency, VO2, VCO2, and blood lactate concentration. Only one study evaluated the heart rate at the beginning and at the end of the study. In this case the heart rate in the intervention group had a small increase at the end of the study when compared to the initial values; however, this increase was not significant: baseline: 120 (SD:13) beats·min-1 and post intervention: 122 (SD:17) beats·min-1. Although the post-intervention values of the conventional rehabilitation group were reduced, this result was also not significant: baseline: 126 (SD: 15) beats·min-1 and post-intervention: 121 (SD: 16) beats·min-1. Both groups performed the same activities only the settings were different (home-based or clinic/ambulatory). For breathing frequency there was a reduction of 29 (SD: 4) breaths·min-1 in the HBPR group and reduction of 28 (SD: 3) breaths·min-1 in the control group. There was a reduction in blood lactate of 3.10 (SD: 1.30) mEq·L-1 in the HBPR group and a reduction of 3.4 (SD: 1.7) mEq·L-1 in the control group. In addition, for VO2 there was an increase of 1.1 (SD: 0.40) L·min-1 in the HBPR group and reduction of 1.0 (SD: 0.30) L·min-1 in the control group. For VCO2, was an increase of 1 (SD: 0.40) in the HBPR group and reduction of 0.90 (SD: 0.30) in the control group.
Quality assessment
The quality assessment of the included studies is presented in Appendice 2 and 3, Supplementary Material. Twenty-two articles (70.96%) showed a high risk of bias in ‘blinding of outcome assessment’. The domain in which the studies had the lowest risk of methodological bias was ‘generation of random sequence’, for which all had low risk of bias.
Reliability of cumulative evidence
The evaluation of the quality of the evidence through GRADE showed very low certainty of evidence for the dyspnea levels measured by CRQ/CRDQ; low certainty for the dyspnea levels measured by MRC/mMRC, as well for the outcomes FEV1 and SpO2% and moderate certainty of evidence for 6MWT and HRQoL. The main reasons for the decrease in the certainty of the evidence of the evaluated outcomes were the possibility of risk of bias, inconsistency, and imprecision (Appendix 4, Supplementary Material).
Discussion
COPD is a health condition characterized by persistent limitation of expiratory airflow [Citation55]. Individuals diagnosed with COPD may have peripheral muscle deconditioning, dyspnea, decreased exercise tolerance and reduced HRQoL [Citation56, Citation57]. In this systematic review, 31 RCTs were conducted to evaluate a HBPR program as an alternative treatment choice for COPD patients. Our results showed that HBPR programs reduce dyspnea levels, improve physical capacity (increasing the distance covered in the 6MWT), improving HRQoL and reduce the impact of the disease compared to participants who received only education and standard medical care.
The number of weekly sessions in the studies ranged from 2 to 7 days a week, with a mean of 4.29 (SD:1.76). All included studies counted only patients who were able to attend at least 70% of the treatment sessions, as availability to undergo treatment was one of the main inclusion criteria. Although 6 (19.35%) articles [Citation25, Citation31, Citation33, Citation35, Citation37, Citation49] not reporting this information, it was clear that, in general, the sessions were held, as in most cases there was the supervision of a therapist performing stimuli and motivation.
Furthermore, in some studies [Citation29, Citation32, Citation33, Citation36, Citation38, Citation39, Citation42, Citation43, Citation45, Citation47–49, Citation52–54] the protocol continuous exercises were used throughout the study, that is, the load, number of repetitions and sets were defined after the first assessment and followed the same values until the end of the study. Differently, the other studies [Citation12, Citation25–28, Citation30, Citation31, Citation34, Citation35, Citation40, Citation41, Citation44, Citation46, Citation49, Citation50] included chose to establish protocols with gradual increases in workloads according to the results obtained in the first assessment. In these studies, the load is gradually increased according to the condition of the participants.
The HBPR programs use several methods to achieve the goals, such as breathing exercises, resistance exercises of the upper and lower limbs and aerobic exercises. In addition, it is recommended to carry out education, self-management and follow-up activities through visits or telephone calls to monitor and motivate patients [Citation58, Citation59]. When these methods are combined, they can result in better lung functions, symptoms, muscle respiratory strength, exercise capacity, dyspnea and HRQoL in patients with COPD [Citation51, Citation60, Citation61].
The use of upper limbs is essential in activities of daily living, such as eating, combing hair and brushing teeth, and therefore, it is related to functionality and quality of life [Citation62]. Previous studies report that the muscular oxidative capacity of the upper limbs, in people with COPD, has lower levels when compared to individuals with normal spirometry [Citation63]. Thus, in order to mitigate the effects of reduced oxidative capacity, one of the approaches used within home programs is the use of resistance and aerobic exercises for the arms. These exercise modalities contribute to reduced perception of dyspnea and arm fatigue during exercises, increased peripheral muscle strength, improved performance in daily activities [Citation64, Citation65], directly affecting quality improvement of life [Citation60].
We can note that in recent years, measuring HRQOL in multicenter studies of patients with COPD has been a constant and common practice, having a very important meaning in clinical practice [Citation66]. Most of the included studies in this review measured HRQoL with different instruments: AQ-20, SGRS, CRQ/CRDQ and SF-36. Among these, the most commonly used was the SGRS, which made it possible to carry out a meta-analysis. In the included studies, intervention periods of a maximum of two months proved not to be sufficient to achieve statistically significant changes. However, when the intervention programs are longer than two months, we notice statistically positive changes in HRQoL for patients with COPD.
To assess physical capacity in rehabilitation programs, several tests can be used, including the 6MWT, which is considered a cheap and easy test to apply, being used as a complement in the assessment of patients with pulmonary and cardiovascular diseases [Citation67]. In this review, the patients included in the HBPR group demonstrated improvement in functional performance, since the distance covered after two and three months was greater in the HBPR group when compared to the control group. In two months of rehabilitation, the patients obtained an average of 61.75 m of in walking distance and in 3 months this average was 40.24 m. According to the literature, an increase in the distance walked of 30 m is considered a minimally important difference (MID) intervention in the analysis of adults with COPD [Citation68]. In our study, the performance for the 6MWT after the HBPR exceeded the minimal important difference (MID). After five months of intervention, the HBPR did not show better results when compared to the control group. Two studies [Citation43, Citation69], had shown that the HBPR was not effective in maintaining gains in the areas of functional testing over long periods.
The increase obtained in the distance covered in the 6MWT of the participants present in the HBPR group when compared to the control group can be explained by the type of activities that each of these groups was assigned to do during the study. Participants in the control group were instructed to maintain their daily activities, taking their medication properly; in addiction, the participants in the group HBPR entered the group that followed a structured format, with exercises that varied from standardized or with increased weekly workload, depending on the protocol established by the RCT; moreover, the home group received phone calls conducted by therapists trained and specialized in motivational interviewing techniques. Setting exercise goals was an essential component of all telephone consultations, and participants were also encouraged to set other health goals. In addition, participants were encouraged to exercise using equipment that was readily available in the home environment (e.g. resistance exercises with water bottles). Thus, it is clear that the best result obtained in the 6MWT may be closely related to the daily exercises and stimuli present in the HBPR group. Furthermore, when related to the HBPR group, one of the major difficulties found in the studies is the participants’ inability to attend treatment sessions, which can negatively influence the results of a rehabilitation program [Citation70]. We noticed an increase in the distance walked in CG participants, although it was not greater than the distance walked by participants in the HBPR group. This fact can be explained by the method used to treat these patients, which was based on drug treatment. The correct use of medication contributes to the reduction of symptoms and exacerbations of COPD, which directly interferes with the physical capacity of the participants [Citation6].
All studies included in this systematic review investigated the impact of COPD using the CAT scale. This is a reliable, valid, and good answer tool for assessing the health status of patients with COPD [Citation71]. The HBPR program was effective in reducing the CAT scores after two months of follow-up. Our meta-analysis identified an average of −4.71 points reduced in this questionnaire after the HBPR. This result was superior to MCID, as it represents a reduction of 2 to 3 points [Citation72, Citation73].
Dyspnea levels after two months of HBPR were also improved. The MRC/mMRC - are traditionally used because it is easy to apply and understand [Citation74] and the CRQ/CRDQ - is the most responsive instrument among the existing ones [Citation75]. Reducing of dyspnea symptoms leads to less restrictions on activities of daily living, triggered by shortness of breath, consequently, health-related quality of life improves [Citation76].
In general, the included studies presented a high age range related to participants: the minimum age was 38 years [Citation33], and the maximum was 85 years [Citation31]. Current evidence suggests that early COPD is associated with ineffective clinical results and leads to early detection, diagnosis, and maintenance of COPD, combined with smoking cessation and exercise, to ensure symptom control, disease progress and COPD results [Citation77]. Thus, although COPD is considered a disease that affects the elderly population, it is also present in younger individuals.
Even though another review on this topic was published in 2014, the present review highlighted some points and updated information about HBPR. In this systematic review it was included a total of 31 RCTs and the number of studies selected to carry out the meta-analysis was also higher. A total of 2235 COPD patients were included in the qualitative description and 898 in the meta-analysis. In addition, this new study obtained updated results from the measurement of dyspnea levels using the CRQ/CRDQ instrument and another scale that had not been used previously, the mMRC/MRC. Another issue addressed in this review that had not been addressed previously was the results on SPO2 levels. It was also possible to carry out new meta-analyses evaluating the physical capacity of the participants using the 6WMT. Due to the number of RCTs, analyses of the results obtained at two and three months of intervention were performed. Furthermore, this review presents the results of the meta-analysis on the impact of the disease on the patient using the COPD Assessment Test (CAT). Finally, GRADE system was used to assess the overall quality of the body of evidence associated with the main results.
Study limitations
There are some concerns to consider as limitations of the present review. Although the included studies had similar primary objectives, the methodology, follow-up periods, and assessment of outcomes were far different among them. Thus, few studies were included in our meta-analyses. In addition, no meta-analysis could be performed to compare HBPR and CPR groups. Thus, it is not possible to include patients with HBPR who have the same performance and improve the outcomes that patients receive in an outpatient setting. In this context, the scientific literature will benefit from new studies that compare these two approaches for COPD patients with protocols for evaluating primary and secondary outcomes considering the gold standard instruments and longer follow-up periods. In addition, although the PROSPERO protocol did not include secondary outcomes, the research team responsible for preparing the systematic review decided to include these other variables. Several of these variables were cited in the majority of studies included here.
Conclusion
There is a limited number of studies comparing patients with COPD performing HBPR vs standard treatment. However, in our systematic review we found a reduction in the levels of dyspnea, an increase in the distance covered on the six-minute walk test, improving HRQoL and decreasing the impact of the disease in COPD patients in home-based pulmonary rehabilitation. Nonetheless, these results are based on evidence of very low to moderate quality.
Ethics approval
The systematic review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) platform on 20 September 2019 (PROSPERO; CRD42020151669).
Acknowledgements
We offer our deepest thanks to the FAPEMIG for the scholarships and to the to the Programa de Pós-graduação em Reabilitação e Desempenho Funcional (PPGReab), Physiotherapy Department of the Universidade Federal dos Vales do Jequitinhonha e Mucuri, that provided technical support for the development and implementation of this study.
Disclosure statement
We declare that there are no conflicts of interest.
Additional information
Funding
References
- GOLD. Global strategy for the diagnosis, management, and prevention of Chronic Obstructive Pulmonary Disease – 2020 Report [Internet]. Global Initiative for Chronic Obstructive Lung Disease, Inc. 2020. Available from: https://copd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf.
- Burney PGJ, Patel J, Newson R, et al. Global and regional trends in COPD mortality, 1990-2010. Eur Respir J. 2015;45(5):1239–1247. DOI:10.1183/09031936.00142414
- Institute for Health Metrics and Evaluation (IHME). GBD compare data visualization. Seattle, WA: IHME, University of Washington, 2018. http://vizhub.healthdata.org/gbd-compare
- Cukier A, De Godoy I. Variabilidade dos sintomas diários de pacientes com DPOC estável no brasil: um estudo observacional de vida real. SciELO. 2019;46(3):1–9.
- Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Respir Res. 2017;18(1):1–11. DOI:10.1186/s12931-017-0548-3
- Vogelmeier CF, Román-Rodríguez M, Singh D, et al. Goals of COPD treatment: Focus on symptoms and exacerbations. Respir Med. 2020;166:105938. (March). DOI:10.1016/j.rmed.2020.105938
- Gloeckl R, Marinov B, Pitta F. Practical recommendations for exercise training in patients with COPD. Eur Respir Rev. 2013;22(128):178–186. DOI:10.1183/09059180.00000513
- Paneroni M, Simonelli C, Vitacca M, et al. Aerobic exercise training in very severe chronic obstructive pulmonary disease: A systematic review and meta-analysis. Am J Phys Med Rehabil. 2017;96(8):541–548. DOI:10.1097/PHM.0000000000000667
- Jones R, Muyinda H, Nyakoojo G, et al. Does pulmonary rehabilitation alter patients’ experiences of living with chronic respiratory disease? A qualitative study. Int J Chron Obstruct Pulmon Dis. 2018;13:2375–2385. DOI:10.2147/COPD.S165623
- Marillier M, Bernard A-C, Vergès S, et al. Locomotor muscles in COPD: the rationale for rehabilitative exercise training. Front Physiol. 2019;10:1590. DOI:10.3389/fphys.2019.01590
- Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;4:CD003793.
- Mendes De Oliveira JC, Studart FS, Filho L, et al. Outpatient vs. home-based pulmonary rehabilitation in COPD: a randomized controlled trial. Multidiscip Respir Med. 2010;5(6):401–408. DOI:10.1186/2049-6958-5-6-401
- Holland AE. Pulmonary rehabilitation for chronic obstructive pulmonary disease: Has it peaked? Respirology. 2019;24(2):103–104. DOI:10.1111/resp.13447
- Arnold E, Bruton A, Ellis-Hill C. Adherence to pulmonary rehabilitation: a qualitative study. Respir Med. 2006;100(10):1716–1723. DOI:10.1016/j.rmed.2006.02.007
- Keating A, Lee A, Holland AE. What prevents people with chronic obstructive pulmonary disease from attending pulmonary rehabilitation? A systematic review. Chron Respir Dis. 2011;8(2):89–99. DOI:10.1177/1479972310393756
- Cox NS, Oliveira CC, Lahham A, et al. Pulmonary rehabilitation referral and participation are commonly influenced by environment, knowledge, and beliefs about consequences: a systematic review using the theoretical domains Framework. J Physiother. 2017;63(2):84–93. DOI:10.1016/j.jphys.2017.02.002
- Lahham A, McDonald CF, Mahal A, et al. Home-based pulmonary rehabilitation for people with COPD: a qualitative study reporting the patient perspective. Chron Respir Dis. 2018;15(2):123–130. DOI:10.1177/1479972317729050
- Liu X-L, Tan J-Y, Wang T, et al. Effectiveness of home-based pulmonary rehabilitation for patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Rehabil Nurs. 2014;39(1):36–59. DOI:10.1002/rnj.112
- Higgins J, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). 2019. www.training.cochrane.org/handbook. (Accessed 7 April 2020).
- Moher D, Liberati A, Tetzlaff J, for the PRISMA Group, et al. Preferred reporting items for systematic reviews and Meta-analyses: the PRISMA statement. BMJ. 2009;339(jul21 1):b2535–336. DOI:10.1136/bmj.b2535
- Siafakas NM, Vermeire P, Pride NB, et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task ForceEur Respir J. 1995;8(8):1398–1420. DOI:10.1183/09031936.95.08081398
- Celli BR, MacNee W, Agusti A, ATS/ERS Task Force, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. DOI:10.1183/09031936.04.00014304
- American thoracic society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152:S77–S121.
- Boote MG, Calverley PMA, Mcgavin CR, et al. BTS guidelines for the management of chronic obstructive pulmonary disease. Thorax. 1997;52(SUPPL5):S1–S28. DOI:10.1136/thx.52.suppl_5.1
- Bauldoff GS, Hoffman LA, Sciurba F, et al. Home-based, upper-arm exercise training for patients with chronic obstructive pulmonary disease. Heart Lung. 1996;25(4):288–294. DOI:10.1016/s0147-9563(96)80064-1
- Strijbos JH, Postma DS, van Altena R, et al. A comparison between an outpatient hospital-based pulmonary rehabilitation program and a home-care pulmonary rehabilitation program in patients with COPD. A follow-up of 18 months. Chest. 1996;109(2):366–372. DOI:10.1378/chest.109.2.366
- Wijkstra PJ, van der Mark TW, Kraan J, et al. Effects of home rehabilitation on physical performance in patients with chronic obstructive pulmonary disease (COPD). Eur Respir J. 1996;9(1):104–110. DOI:10.1183/09031936.96.09010104
- Hernández MT, Rubio TM, Ruiz FO, et al. Results of a home-based training program for patients with COPD. Chest. 2000;118(1):106–114. DOI:10.1378/chest.118.1.106
- Singh V, Khandelwal DC, Khandelwal R, et al. Pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci. 2003;45(1):13–17.
- Oh E-G. The effects of home-based pulmonary rehabilitation in patients with chronic lung disease. Int J Nurs Stud. 2003;40(8):873–879. https://pesquisa.bvsalud.org/portal/resource/pt/mdl-14568368. DOI:10.1016/S0020-7489(03)00071-3
- Boxall AM, Barclay L, Sayers A, et al. Managing chronic obstructive pulmonary disease in the community. A randomized controlled trial of home-based pulmonary rehabilitation for elderly housebound patients. J Cardiopulm Rehabil. 2005;25(6):378–385. DOI:10.1097/00008483-200511000-00012
- Murphy N, Bell C, Costello RW. Extending a home from hospital care programme for COPD exacerbations to include pulmonary rehabilitation. Respir Med. 2005;99(10):1297–1302. DOI:10.1016/j.rmed.2005.02.033
- Koppers RJ, Vos PJ, Boot CR, et al. Exercise performance improves in patients with COPD due to respiratory muscle endurance training. Chest. 2006;129(4):886–892. DOI:10.1378/chest.129.4.886
- Resqueti VR, Gorostiza A, Gáldiz JB, et al. Benefits of a home-based pulmonary rehabilitation program for patients with severe chronic obstructive pulmonary disease. Arch Bronconeumol. 2007;43(11):599–604. DOI:10.1016/S1579-2129(07)60136-0
- O’Shea SD, Taylor NF, Paratz JD. A predominantly home-based progressive resistance exercise program increases knee extensor strength in the short-term in people with chronic obstructive pulmonary disease: a randomised controlled trial. Aust J Physiother. 2007;53(4):229–237. DOI:10.1016/S0004-9514(07)70003-X
- Du Moulin M, Taube K, Wegscheider K, et al. Home-based exercise training as maintenance after outpatient pulmonary rehabilitation. Respiration. 2009;77(2):139–145. DOI:10.1159/000150315
- Fernández AM, Pascual J, Ferrando C, et al. Home-based pulmonary rehabilitation in very severe COPD: is it safe and useful? J Cardiopulm Rehabil Prev. 2009;29(5):325–331. DOI:10.1097/HCR.0b013e3181ac7b9d
- Ghanem M, ELaal EA, Mehany M, et al. Home-based pulmonary rehabilitation program: Effect on exercise tolerance and quality of life in chronic obstructive pulmonary disease patients. Ann Thorac Med. 2010;5(1):18–25. DOI:10.4103/1817-1737.58955
- Akinci AC, Olgun N. The effectiveness of nurse-led, home-based pulmonary rehabilitation in patients with COPD in Turkey. Rehabil Nurs. 2011;36(4):159–165. DOI:10.1002/j.2048-7940.2011.tb00084.x
- Dias FD, Malosá LM, Pereira ES, et al. HBPR with COPD patients. Int J Chron Obstruct Pulmon Dis. 2013;8:537–544. DOI:10.2147/COPD.S50213
- De Sousa Pinto JM, Martín-Nogueras AM, Calvo-Arenillas JI, et al. Clinical benefits of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2014;34(5):355–359. DOI:10.1097/HCR.0000000000000061
- Tabak M, Brusse-Keizer M, van der Valk P, et al. A telehealth program for self-management of COPD exacerbations and promotion of an active lifestyle: a pilot randomized controlled trial. Int J Chron Obstruct Pulmon Dis. 2014;9:935–944. DOI:10.2147/COPD.S60179
- Holland AE, Mahal A, Hill CJ, et al. Home-based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial. Thorax. 2017;72(1):57–65. DOI:10.1136/thoraxjnl-2016-208514
- Tsai LLY, McNamara RJ, Moddel C, et al. Home-based telerehabilitation via real-time videoconferencing improves endurance exercise capacity in patients with COPD: the randomized controlled TeleR study. Respirology. 2017;22(4):699–707. DOI:10.1111/resp.12966
- Horton EJ, Mitchell KE, Johnson-Warrington V, et al. Comparison of a structured home-based rehabilitation programme with conventional supervised pulmonary rehabilitation: a randomised non-inferiority trial. Thorax. 2018;73(1):29–36. DOI:10.1136/thoraxjnl-2016-208506
- Vasilopoulou M, Papaioannou AI, Kaltsakas G, et al. Home-based maintenance tele-rehabilitation reduces the risk for acute exacerbations of COPD, hospitalisations and emergency department visits. Eur Respir J. 2017;49(5):1602129. DOI:10.1183/13993003.02129-2016
- Coultas DB, Jackson BE, Russo R, et al. Home-based physical activity coaching, physical activity, and health care utilization in chronic obstructive pulmonary disease chronic obstructive pulmonary disease self-management activation research trial secondary outcomes. Ann Am Thorac Soc. 2018;15(4):470–478. DOI:10.1513/AnnalsATS.201704-308OC
- Maltais F, Bourbeau J, Shapiro S, et al. Chronic obstructive pulmonary disease axis of respiratory health network, fonds de recherche en santé du québec. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2008;149(12):869–878. DOI:10.7326/0003-4819-149-12-200812160-00006
- Lahham A, McDonald CF, Mahal A, et al. Participation in physical activity during center and Home-Based pulmonary rehabilitation for people with COPD: a secondary analysis of a randomized controlled trial. J Cardiopulm Rehabil Prev. 2019;39(2):E1–E4. DOI:10.1097/HCR.0000000000000373
- Burge AT, Holland AE, McDonald CF, et al. Home-based pulmonary rehabilitation for COPD using minimal resources: an economic analysis. Respirology. 2020;25(2):183–190. DOI:10.1111/resp.13667
- Lahham A, McDonald CF, Moore R, et al. The impact of home-based pulmonary rehabilitation on people with mild chronic obstructive pulmonary disease: a randomised controlled trial. Clin Respir J. 2020;14(4):335–344. DOI:10.1111/crj.13138
- Jiang Y, Liu F, Guo J, et al. Evaluating an intervention program using WeChat for patients with chronic obstructive pulmonary disease: Randomized controlled trial. J Med Internet Res. 2020;22(4):e17089. DOI:10.2196/17089
- Ko FW, Tam W, Siu EHS, et al. Effect of short-course exercise training on the frequency of exacerbations and physical activity in patients with COPD: a randomized controlled trial. Respirology. 2021;26(1):72–79. DOI:10.1111/resp.13872
- Hansen H, Bieler T, Beyer N, et al. Supervised pulmonary tele-rehabilitation versus pulmonary rehabilitation in severe COPD: a randomised multicentre trial. Thorax. 2020;75(5):413–421. DOI:10.1136/thoraxjnl-2019-214246
- Vogelmeier CF. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary desease, updated 2011. Am J Respir Crit Care Med. 2017;195(5):1–74.
- Maltais F, Decramer M, Casaburi R, et al. An official american thoracic Society/European respiratory society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–e62. DOI:10.1164/rccm.201402-0373ST
- Miravitlles M, Worth H, Soler Cataluña JJ, et al. Observational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: Results from the ASSESS study. Respir Res. 2014;15(1):1–13. DOI:10.1186/s12931-014-0122-1
- Viniol C, Vogelmeier CF. Exacerbations of COPD. Eur Respir Rev. 2018;27(147):170103. DOI:10.1183/16000617.0103-2017
- Aboumatar H, Naqibuddin M, Chung S, et al. Effect of a Hospital-Initiated program combining transitional care and long-term self-management support on outcomes of patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. J Am Med Assoc. 2019;322(14):1371–1380. DOI:10.1001/jama.2019.11982
- Silva CM, da S. e, Gomes Neto M, Saquetto MB, et al. Effects of upper limb resistance exercise on aerobic capacity, muscle strength, and quality of life in COPD patients: a randomized controlled trial. Clin Rehabil. 2018;32(12):1636–1644. DOI:10.1177/0269215518787338
- Lu Y, Li P, Li N, et al. Effects of Home-Based breathing exercises in subjects with COPD. Respir Care. 2020;65(3):377–387. DOI:10.4187/respcare.07121
- Couser JI, Jr, Martinez FJ, Celli BR. Respiratory response and ventilatory muscle recruitment during arm elevation in normal subjects. Chest. 1992;101(2):336–340. DOI:10.1378/chest.101.2.336
- Adami A, Corvino RB, Calmelat RA, et al. Muscle oxidative capacity is reduced in both upper and lower limbs in COPD. Med Sci Sports Exerc. 2020; 52(10):2061–2068. DOI:10.1249/MSS.0000000000002364
- Calik-Kutukcu E, Arikan H, Saglam M, et al. Arm strength training improves activities of daily living and occupational performance in patients with COPD. Clin Respir J. 2017; 11(6):820–832. DOI:10.1111/crj.12422
- Holland AE, Cox NS, Houchen-Wolloff L, et al. Defining modern pulmonary rehabilitation. An official american thoracic society workshop report. Ann Am Thorac Soc. 2021;18(5):e12–e29. DOI:10.1513/AnnalsATS.202102-146ST
- Ferreira CASA, Cukier A. Avaliando a DPOC pela perspectiva do paciente. J Bras Pneumol. 2006;32(2):7–8. DOI:10.1590/S1806-37132006000200001
- Morales-Blanhir, JE, Vidal CDP, de Jesús Rosas Romero M, et al. Six-minute walk test: a valuable tool for assessing pulmonary impairment. J Bras Pneumol 2013;37(1):73–84. DOI:10.5935/abc.20160042
- Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017;23(2):377–381. DOI:10.1111/jep.12629
- Grosbois JM, Gicquello A, Langlois C, et al. Long-term evaluation of home-based pulmonary rehabilitation in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:2037–2044. DOI:10.2147/COPD.S90534
- Cameron-Tucker HL, Wood-Baker R, Owen C, et al. Chronic disease self-management and exercise in COPD as pulmonary rehabilitation: a randomized controlled trial. Int J Chron Obstruct Pulmon Dis. 2014;9:513–523. DOI:10.2147/COPD.S58478
- Gupta N, Pinto LM, Morogan A, et al. The COPD assessment test: a systematic review. Eur Respir J. 2014;44(4):873–884. DOI:10.1183/09031936.00025214
- Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203. DOI:10.1016/S2213-2600(14)70001-3
- Smid DE, Franssen FM, Houben-Wilke S, et al. Responsiveness and MCID estimates for CAT, CCQ, and HADS in patients with COPD undergoing pulmonary rehabilitation: a prospective analysis. J Am Med Dir Assoc. 2017;18(1):53–58. DOI:10.1016/j.jamda.2016.08.002
- Bestall JC, Paul EA, Garrod R, et al. Usefulness of the medical research council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. DOI:10.1136/thx.54.7.581
- Puhan MA, Guyatt GH, Goldstein R, et al. Relative responsiveness of the chronic respiratory questionnaire, St. Georges respiratory questionnaire and four other health-related quality of life instruments for patients with chronic lung disease. Respir Med. 2007;101(2):308–316. DOI:10.1016/j.rmed.2006.04.023
- Karapolat H, Eyigor S, Atasever A, et al. Effect of dyspnea and clinical variables on the quality of life and functional capacity in patients with chronic obstructive pul monary disease and congestive heart failure. Chin Med J. 2008;121(7):592–596. DOI:10.1097/00029330-200804010-00004
- Soriano JB, Polverino F, Cosio BG. What is early COPD and why is it important? Eur Respir J. 2018;52(6):1801448. DOI:10.1183/13993003.01448-2018