Abstract
Objective
We aimed to establish an easy-to-use screening questionnaire with risk factors and suspected symptoms of COPD for primary health care settings.
Methods
Based on a nationwide epidemiological study of pulmonary health among adults in mainland China (China Pulmonary Health, CPH study) between 2012 and 2015, participants ≥40 years who completed the questionnaire and spirometry tests were recruited and randomly divided into development set and validation set by the ratio of 2:1. Parameters including sex, age, BMI, residence, education, smoking status, smoking pack-years, biomass exposure, parental history of respiratory diseases and daily respiratory symptoms were initially selected for the development of scoring system. Receiver operating characteristic (ROC) curve, area under curve (AUC), positive and negative predictive values were calculated in development set and validation set.
Results
After random split by 2:1 ratio, 22443 individuals were assigned to development set and 11221 to validation set. Ten variables were significantly associated with COPD independently in development set after a stepwise selection by multivariable logistic model and used to develop scoring system. The scoring system yielded good discrimination, as measured by AUC of 0.7737, and in the validation set, the AUC was 0.7711. When applying a cutoff point of ≥16, the sensitivity in development set was 0.69 (0.67 − 0.71); specificity 0.72 (0.71 − 0.73), PPV 0.25 (0.24 − 0.26) and NPV 0.94 (0.94 − 0.95).
Conclusion
We developed and validated a comprehensive screening questionnaire, COPD-CPHS, with good discrimination. The score system still needs to be validated by large cohort in the future.
Supplemental data for this article is available online at https://doi.org/10.1080/15412555.2022.2042504 .
Keywords:
Introduction
Chronic obstructive lung disease (COPD) is one of the most leading causes of death globally, especially in developing areas. The burden of COPD increases over decades for the constant exposure to COPD risk factors and the graying population structure. In China, the prevalence of spirometry-defined COPD was reported as high as 8.6% in population aged 20 years or older, while only 12.0% of them reported a previous pulmonary function test [Citation1]. In another recent study conducted in the primary care setting of Poland, 182 of 1960 patients (9.3%) were found to meet diagnostic criteria for COPD, but only 19% of those meeting the diagnostic criteria had been diagnosed and treated [Citation2]. Though spirometry is recommended by Global Initiative for Chronic Obstructive Lung Disease (GOLD) for the diagnosis of COPD, the spirometer is inaccessible or unaffordable in most primary care units of low-to-middle income countries. Therefore, detection of COPD is to be improved in primary care and a simple and affordable screening tool for detection of early symptomatic COPD prior to the onset of disabling symptoms is needed.
Considering the chronic, progressive but occult nature of the COPD, the development of a brief easy-to-use questionnaire for COPD assessment could improve patient care. Previous studies have explored some screening score-based questionnaires on exposures and symptoms, for example, the Lung Function Questionnaire (LFQ) [Citation3] and COPD Screening Questionnaire (COPD-SQ) [Citation4]. These scores were either population-specific or with relatively small sample size, and may not be applicable for general population in China. Here, by using the data from the China Pulmonary Health (CPH) study, the largest and representative COPD cohort in China, we aimed to establish a screening questionnaire (COPD-CPH score, COPD-CPHS) with risk factors and suspected symptoms of COPD for primary health care settings in Chinese population.
Methods
Design and population
This study was conducted based on a nationwide epidemiological study of pulmonary health among adults in mainland China (China Pulmonary Health, CPH study) between 2012 and 2015, details of which have been reported elsewhere [Citation1]. Participants ≥40 years who completed the questionnaire and spirometry tests were recruited from the CPH study and randomly divided into development set and validation set by the ratio of 2:1.
MasterScreen Pneumo PC spirometer (CareFusion, Yorba Linda, CA, USA) were used by train technicians for spirometry tests. The participants were required to do up to eight forced expiratory maneuvers until FVC and FEV1 were reproducible within 150 mL. For each participant, the bronchodilator (salbutamol 400 μg metered dose inhaler) was administered by inhalation through a 500 mL spacer and spirometry repeated 20 min later. All the spirometry data were reviewed centrally by an expert panel on the basis of the criteria of the American Thoracic Society and the European Respiratory Society [Citation5].
Subjects with post-bronchodilator FEV1/FVC < 0.7 were defined as having COPD, according to 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD) report [Citation6]. We also used lower limit of normal (LLN) of FEV1/FVC ratio [Citation7] as the alternative diagnosis criteria of COPD.
The study protocol was approved by the ethics review committee of the Beijing Capital Medical University and other participating institutes. We obtained written informed consent from all study participants.
Statistical analysis
Based on our previous findings and recent studies, parameters including sex, age, BMI, residence, education, smoking status, smoking pack-years, biomass exposure, parental history of respiratory diseases and daily respiratory symptoms were initially selected for the development of COPD scoring system. The raw database with contact data of these parameters then was randomly allocated by 2:1 into development set and validation set respectively. The characteristics between two sets were described as number (proportion) or mean ± standard deviation where appropriate, and compared by chi-square test or students’ t test separately. A multi-variable logistic regression was conducted by a stepwise selection to estimated risk scores of risk factors for COPD. We used the estimations of the regression coefficients of the logistic regression model to calculate an integer point for each level of the parameter in the screening questionnaire after determining a reference value. The score of each individual was then obtained by summing integer points [Citation8]. The discrimination of the scoring system was assessed by portraying receiver operating characteristic (ROC) curve and area under curve (AUC), accuracy, positive and negative predictive values were also calculated in development set and validation set.
A 2-sided α of <.05 was considered statistically significant. Statistical analyses were conducted using SAS software, version 9.4 (SAS Institute), unless otherwise indicated.
Results
Characteristics of study population
A total of 33664 individuals with contact information of key variables and aged over 40 years were involved in this study. After random split by 2:1 ratio, 22443 individuals were assigned to development set and 11221 to validation set. In total population, the COPD incidence was 12.0% and there was no significant difference between development and validation sets. There were 20436 (60.7%) females and mean age was 55.4 ± 9.6 years. No significant different was observed between two sets among each risk factor and the results are shown in (, ).
Figure 1. Flowchart of involving subjects. Abbreciation: COPD, chronic obstructive pulmonary disease.
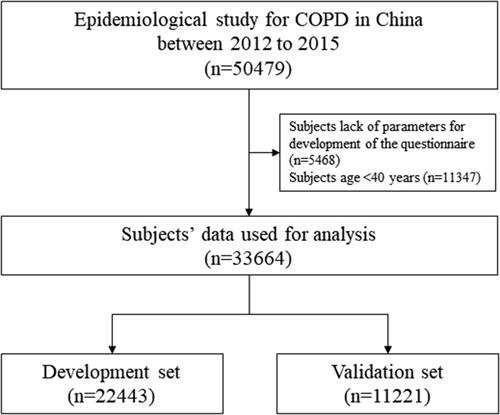
Table 1. Comparison of characteristics between development and validation dataset.
Development of risk scoring system
Ten variables were significantly associated with COPD independently in development set after a stepwise selection by multivariable logistic model and are shown in including sex, age, BMI, smoking history, education, cough during childhood, family history and daily respiratory symptoms. There variables were used to develop the predictive score according to their regression coefficients. After the minimum score was determined, score of each variable was assigned (). The score weight distribution of COPD-CPHS was shown in Supplementary Figure S1. Compared with COPD-SQ, daily respiratory symptoms and demographic predictors were taken more weights in the score items of COPD-CPHS (Supplementary Figure S1).
Table 2. Results of logistic regression and scores in development set.
By adding up each score point assigned above, every patient obtained a total score, by which, the distribution of predictor contributions by score groups in the development dataset was described. shows that as the score grew, the weights of BMI, education and disease history decreased and daily symptoms increased. The screening questionnaire yielded good discrimination, as measured by AUC of 0.7737, and in the validation set, the AUC was 0.7711 (). When applying a cutoff point of ≥16, the sensitivity in development set was 0.69 (0.67 − 0.71); specificity 0.72 (0.71 − 0.73), PPV 0.25 (0.24 − 0.26) and NPV 0.94 (0.94 − 0.95) (). The results of development and validation of the screening questionnaire using LLN as alternative diagnosis criteria of COPD were shown in Supplementary Table S2 and Figure S3.
Figure 2. Distribution of clusters of questions in the screening questionnair among different range of score in development dataset. BMI, body-mass index.
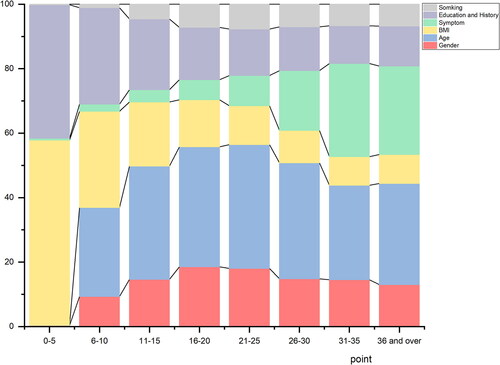
Figure 3. ROC for screening questionnaire in development set (A. AUC = 0.7737) and validation set (B. AUC = 0.7711). Abbreviation: AUC, area under curve.
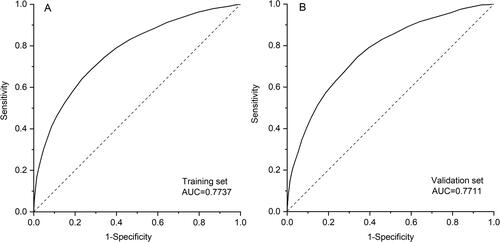
Table 3. Accuracy, Positive and negative predictive values for COPD across different score thresholds.
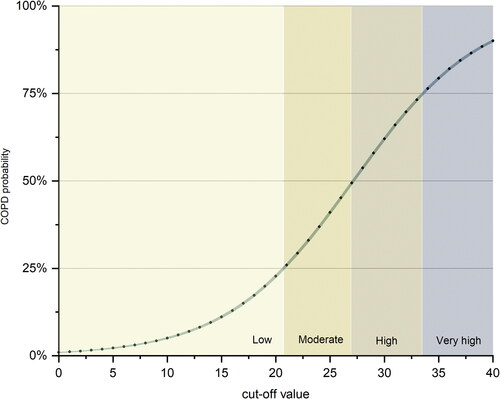
Predicted individual COPD probability regarding the screening questionnaire score was estimated and shown in . According to the probability of COPD occurrence, the population was divided into four probability levels, including low, moderate, high and very high probability (). As the total scores increased, patients with GOLD II-III gradually gained more proportions (Supplementary Figure S2).
Discussion
This study is based on the largest survey of COPD in a nationally representative sample of the general Chinese adult population. Based on our study population with spirometry data, we developed and validated a simple COPD score system, predicting individual COPD probability regarding the screening questionnaire score. With 10 items from 3 categories, the score system offered a reasonable and simplified way to identify individuals at risk of COPD with a recommendation of further lung function test. The discrimination of the score system, COPD-CPHS, was good and easy-to-use for clinical or online quick screening of COPD.
According to our previous study, among participants with spirometry-defined COPD, only 2.6% were aware of their condition, and previous spirometry had been done in only 9.7% of those patients, indicates an extremely low self-awareness of the disease in China [Citation1]. Since 2019, pulmonary function test has been recommended among routine health examinations in people aged 40 years or older in China. However, it is still difficult to be applied in the large scale of rural areas of the nation, which has higher prevalence of COPD than urban areas. Thus, a suitable and reliable screening questionnaire to identify people at high probability of COPD who need further spirometry is required, especially for primary health service in China. That would consequently improve patient–physician communication and patients’ awareness of COPD.
Several studies have demonstrated that symptom-based questionnaires such as IPAG, COPD-PS, COPD-AQ, LFQ and COPD-SQ could be useful in identifying individuals who are likely to have COPD in specific risk groups [Citation3, Citation4, Citation9–14], achieving sensitivities ranging from 54% to 86% and specificities from 40% to 85.2%. Consistent with our previous analysis and other large epidemiological study conducted in China, cigarette smoking attribute high weight in risk factors of COPD [Citation1, Citation15]. Indoor exposure of biomass for cooking or heating has been identified associated with COPD prevalence in China, especially in rural areas [Citation15] and was thus included in the COPD-SQ [Citation4]. However, in our study, biomass exposure was not included in COPD-CPHS, instead, more weights were laid on daily respiratory symptoms and demographic predictors. The difference on question distribution or different weight on same questions may due to the design of the questionnaire, sample population and the substantial decrease of biomass exposure since COPD-SQ was published.
BMI was found as one of the major predictors in our study, consistent with previous studies [Citation3, Citation4]. Notably, our study firstly found that BMI, education and history undertook most weight of the lower score, but their proportions shrank as the proportions of other risk factors, including daily symptoms and age, dilated. Lung capacity is known to diminish with age and daily symptoms (dyspnea, wheeze and phlegm) may be predictive for chronic airway limitation [Citation9]. A classification scheme relying on FEV1/FVC < 0.7 across all age groups is likely to result in large false-positive rates among elderly respondents (aged 65 and older). Therefore, a synthesized scheme combined with daily symptoms is recommended.
Smoking may also contribute to the high risk of COPD in elderly individuals, as smoking amount and tobacco damages may accumulate by age. Hence, early identification of airflow obstruction is likely to prove useful, as there are now data from population-based studies showing that individuals who have their airflow obstruction identified are more likely to quit smoking.
There were some limitations. Firstly, the PPV of the score was pretty low and the sensitivity was moderate, which may due to the low prevalence of COPD in the total population. A specific questionnaire focused on the high risk may improve the precision of the screening. Secondly, we did not include parameters such as air pollution in a questionnaire due to the difficulty a self-report and quantification. Thirdly, the questionnaire was not developed specifically for nonsmokers and ever-smokers because detailed information about smoking was insufficient in the current database. It is indeed to have questionnaires for more specific population (in particular, smokers, with more precise information of smoking), further modification is required for applications in particular population. Fourthly, only internal validation was performed and external validation was needed. However, by using the largest sample size so far, this study provided a simple way to predict individuals with high risk of COPD. Besides, by including variables from different categories, the predictive score was much more comprehensive.
Conclusion
We developed and validated a comprehensive screening questionnaire, COPD-CPHS, with good discrimination. The score system still needs to be validated by large cohort in the future.
Statement of ethics
The study protocol was approved by the ethics review committee of the Beijing Capital Medical University and other participating institutes. We obtained written informed consent from all study participants.
Author contributions
DW, GF, SW and CW provided the idea and designed the study and had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. DW, GF, SW, TY and CW drafted the paper. DW, GF, SW, TY and CW did the analysis and all authors critically revised the manuscript for important intellectual content and gave final approval for the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Enquiries for data generated or analyzed during this study can be directed to the corresponding author.
Additional information
Funding
References
- Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China pulmonary health [CPH] study): a national cross-sectional study. The Lancet. 2018;391(10131):1706–1717. DOI:10.1016/S0140-6736(18)30841-9
- Bednarek M, Maciejewski J, Fau-Wozniak M, Wozniak M, Fau-Kuca P, et al. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax. 2008;63(5):402–407. DOI:10.1136/thx.2007.085456
- Yawn BP, Mapel DW, Mannino DM, et al. Development of the lung function questionnaire (LFQ) to identify airflow obstruction. Int J COPD. 2010;5:10.
- Zhou YM, Chen SY, Tian J, et al. Development and validation of a chronic obstructive pulmonary disease screening questionnaire in China. Int J Tuberc Lung Dis. 2013;17(12):1645–1651. DOI:10.5588/ijtld.12.0995
- Miller MR, Hankinson J, Fau - Brusasco V, Brusasco V, Fau - Burgos F, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338.
- Vogelmeier CA-O, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. DOI:10.1164/rccm.201701-0218PP
- Jian W, Gao Y, Hao C, et al. Reference values for spirometry in Chinese aged 4-80 years. J Thorac Dis. 2017;9(11):4538–4549. : DOI:10.21037/jtd.2017.10.110
- Sullivan LM, Massaro JM, D’AgostinoSr.RB. Presentation of multivariate data for clinical use: the Framingham study risk score functions. Stat Med. 2004;23(10):1631–1660. DOI:10.1002/sim.1742
- Calverley P, Nordyke R, Halbert RJ, et al. Development of a population-based screening questionnaire for COPD. COPD: J Chronic Obstruct Pulmonary Dis. 2005;2(2):225–232. DOI:10.1081/COPD-57594
- Bailey WC, Sciurba FC, Hanania NA, et al. Development and validation of the chronic obstructive pulmonary disease assessment questionnaire (COPD-AQ). Primary Care Respir J. 2009;18(3):198–207. DOI:10.4104/pcrj.2009.00032
- Price DB, Tinkelman DG, Halbert RJ, et al. Symptom-Based questionnaire for identifying COPD in smokers. Respiration. 2006;73(3):285–295. DOI:10.1159/000090142
- Levy ML, Fletcher M, Price DB, et al. Yawn BP: International primary care respiratory group (IPCRG) guidelines: Diagnosis of respiratory diseases in primary care. Prim Care Respir J. 2006;15(1):20–34. DOI:10.1016/j.pcrj.2005.10.004
- Hanania NA, Mannino DM, Yawn BP, et al. Predicting risk of airflow obstruction in primary care: Validation of the lung function questionnaire (LFQ). Respir Med. 2010;104(8):1160–1170. DOI:10.1016/j.rmed.2010.02.009
- Kotz D, Nelemans P, van Schayck CP, et al. External validation of a COPD diagnostic questionnaire. Eur Respir J. 2008;31(2):298–303. DOI:10.1183/09031936.00074307
- Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China. Am J Respir Crit Care Med. 2007;176(8):753–760. DOI:10.1164/rccm.200612-1749OC