Abstract
Chronic obstructive pulmonary disease (COPD) is a major burden of healthcare worldwide. We aimed to determine the effects of PDE-5 inhibitors on clinical outcomes and haemodynamic parameters in patients with COPD. A PROSPERO-registered systematic review and meta-analysis (identification number CRD42021227578) were performed to analyse the effects of PDE-5 inhibitors in patients with COPD. Data were sourced from MEDLINE, EMBASE, Cochrane Register of Controlled Trials and "ClinicalTrials.gov." Randomised controlled trials (RCTs) comparing PDE-5 inhibitors with control in patients with COPD were included. Quality assessment was carried out using the Cochrane Collaboration’s tool for assessing the risk of bias in randomised trials. The pooled mean difference of 6-minute walk distance (6MWD) and mean pulmonary arterial pressure based on inverse variance estimation were analysed with a fixed-effect model or random-effects model meta-analysis. Nine RCTs involving 414 patients were included in the review. There was no significant difference in 6MWD (mean difference = 22.06 metres, 95% confidence interval (CI), −5.80 to 49.91). However, there was a statistically significant difference between PDE-5 inhibitor and control groups in mean pulmonary artery pressure (mean difference = −3.83 mmHg, 95% CI, −5.93 to −1.74). Headaches were the most common adverse event, occurring significantly in the PDE-5 inhibitor intervention group (odds ratio 3.83, 95% CI, 1.49 to 9.86). This systematic review indicates that PDE-5 inhibitors do not improve exercise capacity despite some possible improvements in haemodynamic parameters in COPD patients.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable, and treatable disease of the lower respiratory system, characterised by persistent respiratory symptoms and airflow limitation [Citation1]. In 2015, COPD was accountable for the death of 3.2 million individuals [Citation2] and is recognised as the third leading cause of death worldwide [Citation3]. In the UK, COPD is affecting 9% of those aged 70 years old and at least 1.2 million people in the total population, establishing COPD as the second most common lung disease after asthma [Citation4]. Moreover, COPD accounts for an excess of 140,000 hospital admissions and beyond one million bed-days each year across the UK [Citation4].
The main aims of current COPD treatment are to reduce symptoms, exacerbations, and improve the quality of life. Current COPD treatments revolve around lifestyle choices such as smoking cessation, pulmonary rehabilitation, and teaching active cycles of breathing techniques to help better manage the condition [Citation5, Citation6]. The drug treatments currently available such as bronchodilators and anti-inflammatory agents are used for symptomatic relief of COPD, and prophylactic antibiotics can be used to reduce the risk of exacerbations in COPD patients [Citation7–9]. However, there is a fundamental need for more novel drug treatments.
Recent biological evidence suggested promising roles for phosphodiesterase-5 (PDE-5) inhibitors for pulmonary diseases since PDE-5 is present in the pulmonary vasculature on the lungs’ arterial wall smooth muscle [Citation10]. PDE-5 inhibitors can be used to prevent the breakdown of cGMP resulting in an increase of cGMP in pulmonary vascular smooth muscle cells, leading to smooth muscle relaxation and increased blood flow. Additionally, experimental data also demonstrated the effects of PDE-5 inhibitors on inflammation suppression and airway dilatation [Citation11, Citation12], possibly associated with their cross activities to various other PDE subtypes [Citation13]. PDE-5 inhibitors, such as sildenafil and tadalafil, are established treatments for pulmonary arterial hypertension and may be beneficial to pulmonary hypertension caused by COPD [Citation14–16]. However, there is a lack in the establishment of a direct effect of PDE-5 inhibitors on COPD per se. Given the above situation, we conducted a systematic review and meta-analysis to assess the effects of PDE-5 inhibitors on exercise capacity and pulmonary arterial pressure in patients with COPD.
Materials and methods
This systematic review has been registered with the International Prospective Register of Systematic Reviews (PROSPERO, https://www.crd.york.ac.uk/PROSPERO) under the identification number CRD42021227578, and was conducted according to the latest Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement [Citation17].
Eligibility criteria
The main inclusion criteria of this review comprised randomised controlled trials (RCTs) (parallel-group trials or cross-over trials) that assessed PDE-5 inhibitors (sildenafil and tadalafil) by comparing them with a control in patients with COPD, with or without pulmonary hypertension. We only included studies on sildenafil and tadalafil as these are the only PDE-5 inhibitors licenced for treating pulmonary hypertension. The control could be consisted of placebo or standard optimal COPD therapy. Additionally, trial eligibility considered the inclusion of adults (≥18 years of age) and quantitative, measurable outcomes such as exercise capacity. Publications that were case reports or conference abstracts and articles that involved studies in children or animals, or cells as opposed to humans, were excluded.
Search strategy and information sources
Studies applying PDE-5 inhibitor-specific therapy in COPD patients were searched. The search was conducted in Ovid-MEDLINE (from 1946 to 26th October 2020) and Ovid-EMBASE (from 1974 to 26th October 2020) by using the search filter in the Ovid database. A search of the Cochrane Register of Controlled Trials (CENTRAL) of Cochrane Library (2020) was also performed. The key search terms were: (COPD OR "airways disease" OR "airway disease" OR "chronic lung disease" OR "chronic obstructive pulmonary disease" OR "chronic bronchitis" OR "pulmonary emphysema") AND (PDE5 inhibitors OR PDE-5 inhibitors OR PDE5 inhibitor OR PDE-5 inhibitor OR "Phosphodiesterase 5 inhibitor" OR "Phosphodiesterase Type 5 inhibitors" OR "Phosphodiesterase Type V inhibitors" OR sildenafil OR tadalafil OR Viagra). The comprehensive search string used is included in the Appendix, detailing the search with included MeSH terms. Additionally, ongoing and completed registered clinical trials were searched for on “ClinicalTrials.gov.” The searches were performed without any language restriction or restriction on years of publication.
Selection process
Two authors (NI and DM) independently searched for publications in the databases and yielded search results from applying the search strategy. Any disagreements were resolved through discussion or the involvement of a senior author (LW). All search results were exported to EndNote X9, bibliographic management software (Clarivate Analytics) to de-duplicate the search results. After removing duplicate articles, the titles and abstracts of all articles were screened by two authors (NI and DM) independently to determine eligibility for inclusion in the review. The possibly eligible studies’ full texts were obtained and assessed according to the inclusion and exclusion criteria. Any disagreements concerning the eligibility of studies were committed through discussion or with a senior author’s (LW) inclusion and consensus.
Data collection process and data items
Data were extracted from the included studies applying PDE-5 inhibitor therapy in patients with COPD and were successively put into a pre-designed data extraction sheet. Two authors (NI and DM) independently collected data from each report. Any disagreements concerning the data collection were committed through discussion or with a senior author’s (LW) consensus. The extracted data summarised variables of each study including first author and year of publication, location of study, study design, trial duration, number of patients and sex, definition of COPD, intervention strategy, the dose of treatment and final mean or net changes in mean for the outcomes. Exercise capacity was the primary outcome, measured by 6-minute walk distance (6MWD). Secondary outcomes included mean pulmonary arterial pressure (mPAP), mean pulmonary arterial systolic pressure (mPASP), dyspnoea scales including the Borg scale and modified Medical Research Council (MMRC), oxygenation parameters including the partial pressure of arterial oxygen (PaO2) and the partial pressure of arterial carbon dioxide (PaCO2). All results that were compatible with each outcome domain in each study were sought.
Study risk of bias assessment
Two authors (NI and DM) independently assessed the studies for risk of bias using the Cochrane Collaboration’s tool for assessing the risk of bias in randomised trials, as recommended in the Cochrane Handbook of Systematic Reviews [Citation18]. Bias from the following sources were assessed: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete outcome data, selective reporting, and other bias. Each bias source of each study was assessed as low risk of bias, unclear risk of bias or high risk of bias. Unclear risk of bias intends that there is insufficient information to conclude the risk of bias. When assessing each study for bias, evidence was extracted from the article for the risk judgement and comments were made in the risk of bias table, as recommended by the Cochrane Collaboration. Authors compared their judgements and consulted a senior author (LW) to resolve any disagreements. The risk of bias assessment was summarised as the proportion of information from trials with low, unclear or high risk of bias within each domain. The risk of bias was considered when analysing PDE-5 inhibitors’ effect on patients’ outcomes in each study.
Data synthesis and analysis
Review Manager 5.4.1 (RevMan 5.4.1; The Cochrane Collaboration, Oxford, UK) was used to conduct meta-analyses. Most of the data from studies were reported as mean ± standard deviation (SD). Conversely, some data points were reported as median, standard error (SE) or 95% confidence interval (CI).
The pooled mean difference and 95% CI were estimated based on the inverse variance estimation method after PDE-5 inhibitor treatment in patients with COPD. p < 0.05 indicated a statistically significant effect. Heterogeneity of the included studies was assessed using the Cochran’s Q test and the I2 statistics. p < 0.1 or I2 ≥ 50% indicated statistically significant and a random-effects model for analysis was most appropriate. Otherwise a fixed-effect model was selected for analysis. For dichotomous safety outcomes, the odds ratios (ORs) and 95% CIs were used as summary statistics based on an inverse variance estimation method.
Ethics
This work was based on published studies; hence, it does not require institutional review board approval.
Results
Selection of studies
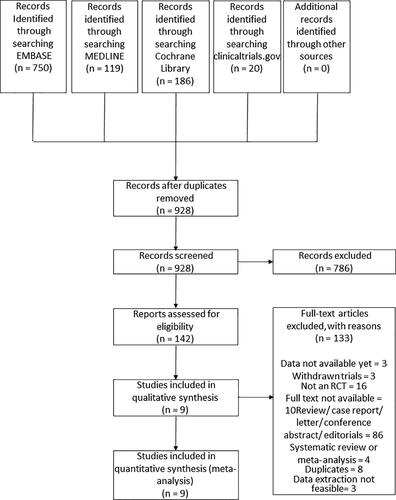
There were 1,075 publications identified from electronic databases after applying the search strategy. Removal of duplicates left 928 articles for screening the titles and abstracts. Hereafter, 142 studies were selected for full-text review. In the final review, nine publications were used. summarises the process of finding eligible papers for the study. details the characteristics of the nine articles [Citation19–27], which were all RCTs included in this review.
Table 1. Summary characteristics of included studies.
Characteristics of included studies
Five studies included patients diagnosed with COPD according to the Global Initiative for Chronic Obstructive Lung Disease criteria. The remaining trials included patients clinically diagnosed with COPD, diagnosed with class II stage disease according to American Society of Anaesthesiologists, diagnosed with COPD according to American Thoracic Society, European Respiratory Society criteria and patients with secondary pulmonary hypertension with the underlying aetiology of COPD according to the New York Heart Association functional class. One study, which evaluated PaCO2, was on the patients with COPD admitted to intensive care unit [Citation26]; while the rest of the studies included patients with stable COPD status.
Subjects in the trials were treated with a PDE-5 inhibitor where seven trials compared sildenafil to placebo, one trial compared sildenafil and pulmonary rehabilitation to placebo, and one trial compared tadalafil to placebo.
Risk of bias in the included studies
The risk of bias of the included RCTs was assessed accordingly.18 summarises the findings from the risk of bias assessment on each source of bias of individual studies. depicts the authors’ risk of bias judgement as to the proportion of information at low, unclear, or high risk of bias for each source across the included studies. Most information (>50%) was from trials with low risk of selection bias, attrition bias, reporting bias, and other bias, but with unclear risk of performance and detection bias. The proportions of information from trials with high risk of bias are low across all domains.
Figure 2. Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
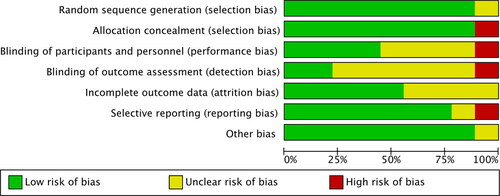
Table 2. Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Efficacy of PDE-5 inhibitor therapy
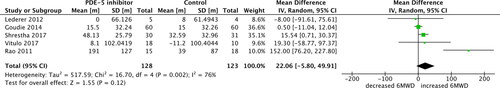
Six studies assessed 6MWD; however, one study reported median instead of mean, thus was excluded from the meta-analysis. Five studies compared the changes in 6MWD in PDE-5 inhibitor and control groups. There was no statistically significant difference for 6MWD [pooled mean difference 22.06 metres, 95% CI −5.80 to 49.91; p = 0.12; 5 trials, 251 participants; ]. There was high heterogeneity indicated for 6MWD in the meta-analysis (I2 = 76%, p = 0.002).
Figure 3. 6-minute walk distance (6MWD) outcome with phosphodiesterase-5 inhibitor treatment versus control in COPD patients. SD, standard difference; IV, inverse variance; CI, confidence interval; df, degree of freedom.
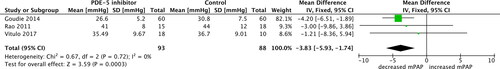
Three studies assessed the change in mPAP. All three studies measured the mPAP using echocardiography. The baseline mPAP (SD) in mmHg for the PDE-5 group and control group was 30.0 (5.0) versus 31.0 (7.0) in Goudie et al., 39.1 (12.5) versus 39.3 (7.6) in Vitulo et al., and 52.7 (11.9) versus 47.8 (13.4) in Rao et al., respectively [Citation20, Citation21, Citation23]. There was a statistically significant difference between the PDE-5 inhibitor and control groups in mPAP (pooled mean difference −3.83 mmHg, 95% CI −5.93 to −1.74; p = 0.0003; 3 trials, 181 participants; ). Thus, results indicate that PDE-5 inhibitors decrease mPAP. There was no heterogeneity indicated for mPAP in the meta-analysis (I2 = 0%, p = 0.72).
Figure 4. Mean pulmonary artery pressure (mPAP) outcome with phosphodiesterase-5 inhibitor treatment versus control in COPD patients. SD, standard difference; IV, inverse variance; CI, confidence interval; df, degree of freedom.
There was no statistically significant difference between the PDE-5 inhibitor group and the control group for mPASP [mean difference (MD) −4.34 mmHg, 95% CI −16.26 to 7.57, p = 0.47]; Borg scale (MD 0.66, 95% CI 0.00 to 1.32; p = 0.05); MMRC (MD 0.09, 95% CI −0.42 to 0.61, p = 0.72); PaCO2 (MD 0.80 mmHg, 95% CI −1.29 to 2.88; p = 0.45) and PaO2 (MD −2.79 mmHg, 95% CI −7.62 to 2.04; p = 0.26). The figures for these efficacy outcomes are included in the Appendix (Appendix Figures 1–5).
Safety of PDE-5 inhibitor therapy
Seven of nine RCTs reported adverse effects in the PDE-5 inhibitor and placebo groups. The adverse events reported are summarised in .
Table 3. Summary of adverse events reported in treatment and control groups.
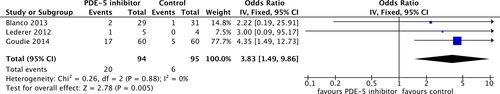
Headaches were reported in three studies entailing 189 patients and 26 total events. There was a difference between the PDE-5 inhibitor and control groups (OR 3.83, 95% CI 1.49 to 9.86; p = 0.005; ), indicating that PDE-5 inhibitors increase the risk of headaches in patients with COPD. There was no heterogeneity (I2 = 0%, p = 0.88).
Figure 5. Headache adverse effect outcome in phosphodiesterase-5 inhibitor treatment versus control in COPD patients. SD, standard difference; IV, inverse variance; CI, confidence interval; df, degree of freedom.
There was no statistically significant between PDE-5 inhibitor group and control group for COPD exacerbations (OR 0.76, 95% CI 0.42 to 1.38; p = 0.37; 3 studies entailing 213 patients and 71 total events) and hospitalisation after COPD exacerbation (OR 1.03, 95% CI 0.31 to 3.37; p = 0.96; 2 studies entailing 180 patients and 12 total events). The figures for these adverse effect outcomes are included in the Appendix (Appendix Figures 6 and 7).
Discussion
This review evaluated the effects of PDE-5 inhibitors in treating patients with COPD and found that PDE-5 inhibitors are effective in improving haemodynamic parameters by reducing pulmonary artery pressure. However, there was no clear benefit for PDE-5 inhibitors to be used for the improvement of exercise capacity or tolerance.
Pulmonary arterial pressure is measured in COPD patients as pulmonary hypertension is presented in many cases of severe COPD. Patients presenting with pulmonary hypertension associated with COPD have been considered for PDE-5 inhibitor therapy [Citation28]. This meta-analysis found a significant decrease in mPAP, indicating some improvements in haemodynamic parameters in COPD patients. However, this did not lead to an increased exercise capacity that was measured in 6MWD, especially in patients with borderline or mild pulmonary hypertension [Citation19, Citation21, Citation24]. This finding is consistent with previous evidence that the exercise tolerance in COPD patients is more likely limited by ventilatory reserve [Citation19–21, Citation24, Citation29]. 6MWD is widely reported to predict patients’ mortality and morbidity with lung disease and heart disease. It is a good measure of functional capacity and is useful for measuring the response to therapeutic interventions for pulmonary disease, hence, is an appropriate test to carry out when considering the effects of PDE-5 inhibitors on patients with COPD [Citation30]. Research showed that an increase in 6MWD of 30 metres or greater is clinically relevant in adult patients with chronic respiratory disease when evaluating a therapeutic agent [Citation31]. Six publications reported 6MWD [Citation19–24], yet Blanco 2013 [Citation24] was excluded from pooled analysis as it did not report the mean and SD. There was no difference found between the PDE-5 inhibitor group and the placebo from the five studies included in the meta-analysis. While there was a mean increase of 22.06 metres in 6MWD across the pooled studies, albeit being non-significant. Thereby, the current data cannot support the use of PDE-5 inhibitors to improve exercise tolerance in COPD patients.
It is noteworthy that in Vitulo 2016, which focussed on patients with severe pulmonary hypertension associated with COPD, PDE-5 inhibitor therapy for 16 weeks improved the BODE index and quality of life, although no improvement in exercise tolerance was observed [Citation20]. The effects were not seen in other trials that included patients with mild pulmonary hypertension [Citation21, Citation24]. However, due to the small size of Vitulo 2016, the benefits of PDE-5 inhibitors in patients with severe pulmonary hypertension in COPD remained unclear and warranted further investigations.
There are concerns about the worsening of gas exchange in COPD patients with the use of PDE-5 inhibitors [Citation32]. Such concerns stem from the inhibition of hypoxic pulmonary vasoconstriction [Citation32, Citation33] by PDE-5 inhibitors such as sildenafil. However, the meta-analysis of arterial oxygenation parameters did not support this concern as there was no difference found between the PDE-5 inhibitor group and placebo group for PaCO2 and PaO2.
Furthermore, there was a difference between the treatment and placebo groups for the occurrence of headaches in patients in data pooled from Blanco 2013 [Citation24], Lederer 2012 [Citation19] and Goudie 2014 [Citation21]. Headaches were reported as the most common side effect by Rao 2011 [Citation23] and were experienced by at least one in five patients experiencing adverse events by Vitulo 2017 [Citation20]. Additionally, a substantial number of patients experienced dyspepsia in the tadalafil group as reported by Goudie 2014 [Citation21] (nineteen in the tadalafil group compared to five in the placebo group). However, this adverse effect was not comparable in a pooled analysis as other studies did not report the number of participants who experienced dyspepsia. The reporting of this adverse effect may be primarily associated with tadalafil, rather than sildenafil. Hence, this suggests that perhaps different PDE-5 inhibitors have different adverse effect profiles, which need to be further investigated. Accordingly, more data is needed concerning adverse effects of PDE-5 inhibitors in patients with pulmonary disease to help establish safety in patients and assist professionals in considering this drug class in COPD patients.
Strength and limitations
This is the first review considers PDE-5 inhibitors in the context of effectiveness for patients with COPD, rather than targeting pulmonary hypertension in COPD. Thus, it differs from other existing systematic reviews. This meta-analysis considered only RCTs in determining the effects of PDE-5 inhibitors in patients with COPD since RCTs typically deliver a high level of evidence due to potential limits to several bias domains.
There are limitations to this study. Firstly, although the majority of trials reported mean and SD of outcomes, some trials reported changes from baseline values. Thus, change values had to be calculated to pool data for the analysis. Such calculations may have introduced statistical inaccuracies and heterogeneity between studies. Blanco 2013 [Citation24] reported a median and 95% CI instead of mean and SD, therefore, while the RCT was well designed, its results were difficult to pool. Further, there are limitations concerning randomisation methods since some studies did not achieve balanced baseline characteristics between PDE-5 inhibitor and control groups. Baseline results of some trials seemed to be imbalanced between treatment arms; thus, any effect of PDE-5 inhibitors may have been masked. Thirdly, most of the RCTs had small sample sizes that are not fully representative of the population of COPD patients. Lastly, heterogeneity was a significant factor in assessing the primary outcome and it was unavoidable to convert the reported 6MWD values to the change value due to the variability in reporting methods between studies. The heterogeneity may have risen in the efficacy outcome results due to differences in the study population, PDE-5 inhibitor doses, trial duration and COPD duration.
Further studies
Further studies on PDE-5 inhibitors in COPD may focus on patients with severe pulmonary hypertension. Ideally, any such trials should investigate whether the use of PDE-5 inhibitors can confer prognostic benefit in such patients, including delayed disease progression, reduced hospitalisation or exacerbation, prolonged survival, and improved quality of life. Moreover, further trials could compare the effects of different PDE-5 inhibitors in COPD patients to assess the significance of specific drugs in COPD which may help distinguish between adverse effect profiles and efficacy of the treatment. Overall, there is still a domineering need for novel agents for treatment in COPD patients.
Conclusions
The results of this meta-analysis indicate that the PDE-5 inhibitor therapy does not improve exercise tolerance in patients with COPD, although it may improve certain haemodynamic parameters such as mPAP. PDE-5 inhibitor use in COPD does not impair gas exchange but can cause headaches as a common side effect. The results of the study do not support the use of PDE-5 inhibitors for treating COPD.
Authors’ contribution statement
IN and LW conceptualised the study. IN and DM conducted the literature screening, data extraction and data analysis. IN and CJ wrote the original draft under the supervision of LW. All authors critically reviewed, commented and edited on all other drafts. LW is the guarantor of the work.
Supplemental Material
Download PDF (606.5 KB)Declaration of interest
No competing interest declared.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3):1700214. DOI:10.1183/13993003.00214-2017
- Soriano JB, Abajobir AA, Abate KH, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. DOI:10.1016/S2213-2600(17)30293-X
- Soriano JB, Kendrick PJ, Paulson KR, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Respir Med. 2020;8(6):585–596. DOI:10.1016/S2213-2600(20)30105-3
- Snell N, Strachan D, Hubbard R, et al. S32 epidemiology of chronic obstructive pulmonary disease (COPD) in the UK: findings from the British lung foundation’s ‘respiratory health of the nation’ project. Thorax. 2016;71(Suppl 3):A20.1–A20. DOI:10.1136/thoraxjnl-2016-209333.38
- Kanner RE, Connett JE, Williams DE, et al. Effects of randomized assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disease: the lung health study. Am J Med. 1999;106(4):410–416. DOI:10.1016/S0002-9343(99)00056-X
- Bolton CE, Bevan-Smith EF, Blakey JD, British Thoracic Society Standards of Care Committee, et al. British thoracic society guideline on pulmonary rehabilitation in adults. Thorax. 2013;68(Suppl 2):ii1–30. DOI:10.1136/thoraxjnl-2013-203808
- BTS guidelines for the management of chronic obstructive pulmonary disease. The COPD guidelines group of the standards of care committee of the BTS. Thorax. 1997;52(Suppl 5):S1–S28.
- Britton M. The burden of COPD in the U.K.: results from the confronting COPD survey. Respir Med. 2003;97:S71–S79. DOI:10.1016/S0954-6111(03)80027-6
- Llor C, Moragas A, Hernandez S, et al. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(8):716–723. DOI:10.1164/rccm.201206-0996OC
- Blanco I, Piccari L, Barbera JA. Pulmonary vasculature in COPD: the silent component. Respirology. 2016;21(6):984–994. DOI:10.1111/resp.12772
- Toward TJ, Smith N, Broadley KJ. Effect of phosphodiesterase-5 inhibitor, sildenafil (viagra), in animal models of airways disease. Am J Respir Crit Care Med. 2004;169(2):227–234. DOI:10.1164/rccm.200211-1372OC
- Urbanova A, Medvedova I, Kertys M, et al. Dose dependent effects of tadalafil and roflumilast on ovalbumin-induced airway hyperresponsiveness in guinea pigs. Exp Lung Res. 2017;43(9–10):407–416. DOI:10.1080/01902148.2017.1386735
- Zhang X, Feng Q, Cote RH. Efficacy and selectivity of phosphodiesterase-targeted drugs in inhibiting photoreceptor phosphodiesterase (PDE6) in retinal photoreceptors. Invest Ophthalmol Vis Sci. 2005;46(9):3060–3066. DOI:10.1167/iovs.05-0257
- Hao Y, Zhu Y, Mao Y, et al. Efficacy and safety of Sildenafil treatment in pulmonary hypertension caused by chronic obstructive pulmonary disease: a meta-analysis. Life Sci. 2020;257:118001. DOI:10.1016/j.lfs.2020.118001
- Henrie AM, Nawarskas JJ, Anderson JR. Clinical utility of tadalafil in the treatment of pulmonary arterial hypertension: an evidence-based review. Core Evid. 2015;10:99–109.
- Alkhayat K, Eid M. Sildenafil citrate therapy for secondary pulmonary arterial hypertension due to chronic obstructive lung disease. Egypt J Chest Dis Tuberc. 2016;65(4):805–809. DOI:10.1016/j.ejcdt.2016.05.005
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. DOI:10.1136/bmj.n71
- Higgins JP, Altman DG, Gotzsche PC, Cochrane Statistical Methods Group, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. DOI:10.1136/bmj.d5928
- Lederer DJ, Bartels MN, Schluger NW, et al. Sildenafil for chronic obstructive pulmonary disease: a randomized crossover trial. COPD. 2012;9(3):268–275. DOI:10.3109/15412555.2011.651180
- Vitulo P, Stanziola A, Confalonieri M, et al. Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: a randomized controlled multicenter clinical trial. J Heart Lung Transplant. 2017;36(2):166–174. DOI:10.1016/j.healun.2016.04.010
- Goudie AR, Lipworth BJ, Hopkinson PJ, et al. Tadalafil in patients with chronic obstructive pulmonary disease: a randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2014;2(4):293–300. DOI:10.1016/S2213-2600(14)70013-X
- Shrestha SK, Srivastava B, Karki M, et al. Effect of sildenafil citrate on pulmonary arterial systolic pressure and sub-maximal exercise capacity in chronic obstructive pulmonary disease. Kathmandu Univ Med J (KUMJ). 2017;15(60):271–278.
- Rao RS, Singh S, Sharma BB, et al. Sildenafil improves six-minute walk distance in chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Indian J Chest Dis Allied Sci. 2011;53(2):81–85.
- Blanco I, Santos S, Gea J, et al. Sildenafil to improve respiratory rehabilitation outcomes in COPD: a controlled trial. Eur Respir J. 2013;42(4):982–992. DOI:10.1183/09031936.00176312
- Holverda S, Rietema H, Bogaard HJ, et al. Acute effects of sildenafil on exercise pulmonary hemodynamics and capacity in patients with COPD. Pulm Pharmacol Ther. 2008;21(3):558–564. DOI:10.1016/j.pupt.2008.01.012
- Rafiei MR, Aghadavoudi O, Hojjat M. The effect of sildenafil on respiratory weaning of patients with chronic obstructive pulmonary diseases admitted to intensive care unit. Med Arh. 2012;66(2):104–106. DOI:10.5455/medarh.2012.66.104-106
- Salem M, Diab A, Ateya A, et al. Short term effects of sildenafil citrate therapy in secondary pulmonary hypertension. Egypt Heart J. 2014;66(1):49–53. DOI:10.1016/j.ehj.2013.03.004
- Butrous G. The role of phosphodiesterase inhibitors in the management of pulmonary vascular diseases. Glob Cardiol Sci Pract. 2014;2014(3):257–290.
- Boerrigter BG, Bogaard HJ, Trip P, et al. Ventilatory and cardiocirculatory exercise profiles in COPD: the role of pulmonary hypertension. Chest. 2012;142(5):1166–1174. DOI:10.1378/chest.11-2798
- Enright PL. The six-minute walk test. Respir Care. 2003;48(8):783–785.
- Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1447–1478. DOI:10.1183/09031936.00150414
- Blanco I, Gimeno E, Munoz PA, et al. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med. 2010;181(3):270–278. DOI:10.1164/rccm.200907-0988OC
- Barberà JA, Roger N, Roca J, et al. Worsening of pulmonary gas exchange with nitric oxide inhalation in chronic obstructive pulmonary disease. Lancet. 1996;347(8999):436–440. DOI:10.1016/S0140-6736(96)90011-2