Abstract
Our previous study suggested that hypomethylation of perforin promoter of CD4 + T cells might be involved in the pathogenesis of autoimmune emphysema of rats. Whether transfer of this kind of cells hypomethylated in vitro into naive immunocompetent rats also results in emphysema is unknown yet. To test the hypothesis above, thirty Sprague Dawley (SD) rats were randomly divided into three groups: a model group (n = 10), a normal control group (n = 10) and a sham operation group (n = 10). In the model group, spleen-derived CD4 + T cells of normal rats were treated with 5-azacytidine (5-Aza), complete Freund’s adjuvant and Phosphate Buffered Saline (PBS), then transferred into naive immunocompetent rats. The normal control group was injected with CD4 + T lymphocytes from spleens of normal rats and the same amount of adjuvant and PBS as above. In sham operation group, normal rats were injected intraperitoneally with complete Freund’s adjuvant and PBS. Histopathological evaluations (mean linear Intercept (MLI) and mean alveolar numbers (MAN)), anti-endothelial cell antibodies (AECA) in serum and bronchoalveolar lavage fluid (BALF), lung vascular endothelial growth factor (VEGF)), the apoptotic index (AI) of alveolar septal cells and the methylation levels of perforin promoter of CD4 + T cells were investigated. The levels of the methylation above and MAN were lower in the model group than in the control and the sham operation group, while the AECA in serum and BALF, VEGF, MLI and the AI were greater (all p < 0.05). The methylation levels of perforin promoter were positively correlated with the MAN (r = 0.747, p < 0.05) and negatively correlated with AI, AECA, MLI, and VEGF (r was −0.789, −0.746, −0.743, −0.660, respectively, all p < 0.05). This study suggests that transfer of invitro CD4 + T cells with hypomethylation of perforin promoter into rats causes autoimmune emphysema, possibly by increasing expression of VEGF and promoting alveolar septal cell apoptosis.
Introduction
Pulmonary emphysema is a major component of chronic obstructive pulmonary disease (COPD). The exact mechanisms that prompt the development and progression of this disease are not clear yet. Cigarette smoking is a major risk factor for the development of COPD, which results in pulmonary chronic inflammation, oxidative stress, and protease/antiprotease imbalance, thereby causing lung injury and emphysema [Citation1].
But nonsmokers may also develop chronic airflow restriction [Citation2]. It is believed that less than 50% of heavy smokers suffer from COPD [Citation3], and the alveolar destruction associated with airspace enlargement usually progresses in severe COPD patients, although they have quitted smoking for many years [Citation4]. Previous studies have demonstrated that autoimmunity has been suspected to relate to the development of COPD [Citation5] and that cellular immunity induced by T lymphocytes is involved in the pathogenesis of this disease [Citation6, Citation7], suggesting the possible role of T cells in COPD -emphysema.
Vascular endothelial growth factor(VEGF), an obligatory endothelial cell (EC) survival factor abundantly expressed in lung tissues, serves as a critical lung structure maintenance factor [Citation8] and anti-endothelial cell antibodies (AECA) is a heterogeneous group of autoimmune antibody. Related literatures have showed that the abnormal expression of VEGF and AECA plays pathogenic roles in vascular endothelial cells activation and induction of cell apoptosis [Citation9, Citation10]. In recent years, previous studies have found that the imbalance of apoptosis and proliferation of alveolar septal cells including alveolar epithelial cells and vascular endothelial cells also results in the damage of lung tissues and the formation of emphysema. Therefore, some scholars even call it the fourth pathogenesis of COPD-emphysema [Citation11, Citation12]. Since one study showed intraperitoneal injection of human umbilical vein endothelial cells into immunocompetent rats caused AECA humoral response, apoptosis of alveolar septal cells and emphysema and that adoptive transfer of CD4+ T spleen cells isolated from the emphysema rats above into naive immunocompetent rats resulted in emphysema, too [Citation8]. We think CD4 + T cell–dependent mechanisms are sufficient to trigger the development of emphysema. Based on our previous study indicating that hypomethylation of perforin gene promoter in CD4+ T cells and the apoptosis of alveolar septal cells might be involved in the pathogenesis of autoimmune emphysema in rats [Citation13], we speculate that hypomethylation of perforin promoter in CD4+ T cells may induce increase of AECA and apoptosis of alveolar septal cells associated with the development of experimental autoimmune emphysema.
5-azacytidine (5-Aza) is a DNA methyltransferase inhibitor. Treating CD4+ T cell clones, as well as polyclonal CD4+ T cells in vitro, with DNA methylation inhibitors caused changes of its reactivity and function. Since transfer of pathogenic CD4 + T cells caused autoimmune emphysema [Citation8], we hypothesize that transfer of CD4 + T cells hypomethylated may induce autoimmune emphysema of rats.
In this study, we used 5-Aza to treat CD4 + T cells of rats in vitro, and then injected normal naive immunocompetent rats intraperitoneally with them to further investigate the role of hypomethylated CD4 + T cells in experimental autoimmune emphysema. Our study showed transfer of the cells above into rats caused autoimmune emphysema to cast light on the possible immunological pathogenesis of this kind of disease.
Methods
CD4+T cells culture and 5-Aza intervention
Experiments were approved by the Animal Ethics Committee of Guizhou Provincial People’s Hospital (ethics IRB numbers: 2015011). Spleen-derived CD4 + T lymphocytes from normal naive immunocompetent Sprague Dawley (SD) rats were isolated by MACS1 magnetic beads as previously described[Citation8]. The purity of enriched CD4+T cell isolates was evaluated by flow cytometry and generally more than 94%. Then, part of them were incubated in cell culture medium containing 5-Aza for 72 h (5-Aza concentration was 10 μmol/l). Those cells (Cell group 1, n = 10) were used to establish emphysema models. Other spleen-derived CD4 + T lymphocytes, not treated by 5-Aza (Cell group 2, n = 10) were also incubated for 72 h.
DNA extraction and bisulfite amplicon sequencing in cell group 1 and cell group 2
In Cell group 1 and Cell group 2, DNA extraction of rats’ spleens was performed as described previously [Citation14], and then bisulfite next-generation sequencing (NGS) was carried out to investigate the perforin gene promotor methylation [Citation15]. The degree of methylation is expressed as the percentage of total cytosines methylated at individual CpG sites. For most CpG sites, hundreds of reads per sample were used for each percentage methylation determination. In all cases, at least 10 reads/CpG site/samples were used.
Animals and CD4 + T lymphocytes hypomethylated
Fifty male SD rats were purchased from Chongqing Tengxin Technology Co., Ltd. (weight range between 180 and 200 g, 6 weeks old). Thirty rats were randomly divided into a model group (n = 10), a normal control group (n = 10) and a sham operation group (n = 10). The model group was injected intraperitoneally with cells (1 × 107) from cell group 1, complete Freund’s adjuvant (1 ml) and PBS (1 ml). The normal control group was injected intraperitoneally with cells (1 × 107) from cell group 2, and the same amount of adjuvant and PBS as above. The sham operation group was also injected intraperitoneally with the same amount of adjuvant and PBS as above.
Morphological assessment of lung tissues
At day 21 of the experiment, all rats were anesthetized with chloral hydrate (3 ml/kg) intraperitoneally. Then the rats were killed. The right lungs were filled with 4% paraformaldehyde for half an hour before fixed in paraformaldehyde for twenty-four hours and then stained with hematoxylin-eosin (HE). The pathological changes were observed under a light microscope. The mean linear intercept (MLI) and the mean alveolar numbers (MAN) of lung tissues were determined as described previously [Citation13, Citation16]. MLI, as a measure of interalveolar wall distance, was determined by light microscopy at a total magnification of ×100. It was calculated for each sample based on 15 random fields using a cross-line. The total length of the cross-line divided by the numbers of the alveolar walls intersecting the test lines was defined as MLI. MAN, an indicator of alveolar density, was calculated for each sample based on 15 random fields by counting the numbers of alveoli in each field and dividing this number by the area of the field.
Bronchoalveolar lavage and serum analysis
Bronchoalveolar lavage fluid (BALF) and serum were prepared as described previously [Citation16]. BAL was performed as follows: 3 ml aliquots of normal saline were slowly infused in the lungs through tracheostomy, at a constant pressure of 25 cm H2O, and then withdrawn gently. The lavage was repeated three times using the same syringe. BALF and serum were collected and centrifuged at 1000 rpm for 5 min. Then, the supernatants were stored at −70 °C. The levels of AECA were determined using enzyme linked immunosorbent assay (ELISA) according to the manufacturer’s protocol.(Abbkine Scientific Co., Ltd, USA).
Immunohistochemistry
Expression of VEGF in rat lung was detected by immunohistochemistry according to the manufacturer’s protocol. (Servicebio, WuHan, China). Image Pro Plus 6.0 (Media Cybernetics Company) was used to determine the average optical density(OD) value.
Quantitative analysis of apoptosis
Alveolar septal cell apoptosis was detected by Terminal deoxynucleotidyl transferase- mediated dUTP nick end labeling (TUNEL) technique (In Situ Apoptosis Detection Kit; Roche, Basel, Switzerland). The total alveolar septal cells and the number of positive alveolar septal cells were counted by one observer. The AI was calculated as described previously [Citation16]. The percentage of positive alveolar septal cells of the total number of alveolar septal cells was calculated for each image and a mean value was obtained for each rat. The results were expressed as the apoptotic index (AI).
DNA extraction and bisulfite amplicon sequencing in three groups
Spleen-derived CD4 + T lymphocytes from the model group, the normal control group and the sham operation group were isolated by MACS1 magnetic beads as previously described [Citation8]. The purity of enriched CD4+ T cell isolates was evaluated by flow cytometry and generally more than 94%. Then the DNA extraction and bisulfite amplicon sequencing were performed as above.
Statistical analysis
SPSS 22.0 statistical software (IBM, USA) was used for data analysis. The data were expressed as the Mean ± standard deviation. Differences were evaluated for statistical significance using analysis of variance and Student’s t test. Pearson correlation was used. P values < 0.05 were considered to be statistically significant.
Results
Methylation status of perforin gene promoter region in Cell group 1 and Cell group 2
The degree of purity of CD4+ T cell isolated was more than 94% as tested by flow cytometry. The methylation levels of the perforin gene promoters of CD4 + T lymphocytes were shown in . The methylation levels were lower in Cell group 1 than in the Cell group 2 ((0.8186 ± 0.0482) of Cell group 1 vs (0.9316 ± 0.0234) of Cell group 2, p < 0.05, ).
Table 1. The methylation levels of the perforin gene promoter in CD4 + T cells of the two Cell groups.
Histopathological alteration
The lung tissues were normal in the normal control group () and the sham operation group (), while the model group had enlarged airspace and a loss of alveolar septa showing noticeable emphysema (). The MLI was significantly greater while the MAN was lower in the model group than in the control group (MLI: (47.79 ± 16.83)×10−6m of model group vs (34.75 ± 6.90)×10−6m of control group; MAN: (322.31 ± 90.05)×106/m2 of model group vs(467.28 ± 59.97)×106/m2 of control group) and sham operation group (MLI:(47.79 ± 16.83)×10−6m of model group vs (32.69 ± 6.65)×10−6m of sham operation group; MAN:(322.31 ± 90.05)×106/m2 of model group vs (470.36 ± 60.06)× 106/m2 of sham operation group, p < 0.05, ).
Figure 1. Histology of lung tissue. Notes: Lung tissue section was stained with HE. (A) Control rats receiving normal CD4 + T cells, adjuvant and PBS; (B) Rats receiving adjuvant and PBS; (C) Rats receiving CD4 + T cells hypomethylated, adjuvant and PBS. Data presented were one representative image data. Magnification: 400×. Abbreviations: HE, hematoxylin-eosin; PBS, phosphate buffered saline.
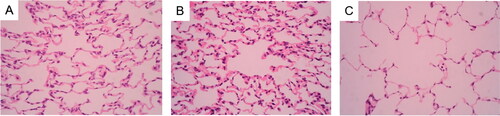
Figure 2. Expression of VEGF in rat lungs Notes: Expression of VEGF in rat lungs was detected by immunohistochemistry. (A) Control rats receiving normal CD4 + T cells, adjuvant and PBS; (B) Rats receiving adjuvant and PBS; (C) Rats receiving CD4 + T cells hypomethylated, adjuvant and PBS. Data presented were one representative image data. Magnification: 400×. Abbreviations: VEGF, Vascular endothelial growth factor; PBS, phosphate buffered saline.
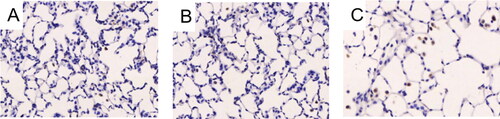
Table 2. The difference of MLI and MAN in the three groups.
Comparison of AECA levels
The levels of AECA were higher both in serum and BALF in the model group than in the control group and the sham operation group(AECA in serum: (60.93 ± 12.58) ng/ml of model group vs (27.27 ± 6.41)ng/ml of control group; (60.93 ± 12.58) ng/ml of model group vs (23.82 ± 6.25) ng/ml of sham operation group; AECA in BALF: (41.57 ± 13.49) ng/ml of model group vs (19.52 ± 9.80) ng/ml of control group; (41.57 ± 13.49) ng/ml of model group vs (16.11 ± 7.83) ng/ml, p < 0.05, ).
Table 3. The difference of AECA in the three groups.
Comparison of VEGF levels
The mean optical density of VEGF in lung tissues was significantly greater in the model group than in the control group and the sham operation group (VEGF: (20.43 ± 5.90) of model group vs (14.09 ± 2.67) of control group; (20.43 ± 5.90) of model group vs (13.99 ± 3.47) of sham operation group, p < 0.05, , ), indicating a high level of expression of VEGF.
Table 4. The difference of VEGF OD in lung tissues in the three groups.
Quantitative analysis of cell apoptosis in alveolar septa
The apoptosis of alveolar septal cells was detected by TUNEL in the lung tissues of the three groups. In the model group, there were more stained cells, namely apoptotic cells in the alveolar septa () while fewer cells were stained in the control group and the sham operation group (). The AI was higher in the model group than in the control group and the sham operation group. ((21.91 ± 8.83)% of model group vs (8.62 ± 3.56)% of control group; (21.91 ± 8.83)% of model group vs (7.82 ± 2.80)% of sham operation group, p < 0.05, ).
Figure 3. Apoptosis of alveolar septal cells in rat lungs. Notes: Apoptosis of alveolar septal cells in rat lungs was detected by immunohistochemistry. (A) Rats receiving CD4 + T cells hypomethylated, adjuvant and PBS; (B) Control rats receiving normal CD4 + T cells, adjuvant and PBS; (C) Rats receiving adjuvant and PBS. Data presented were one representative image data. Magnification: 400×. PBS, phosphate buffered saline.
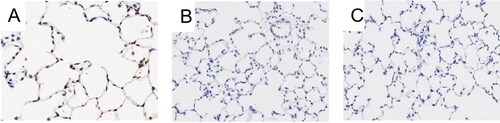
Table 5. The difference of AI of alveolar septal cells in the three groups.
Methylation status of perforin gene promoter region in the three groups
The degree of purity of CD4+ T cell isolated was more than 94% as tested by flow cytometry. The methylation levels of the perforin gene promoters of CD4 + T lymphocytes were shown in . The methylation levels of the model group were lower than those of the normal group and the sham operation group ((0.8610 ± 0.0243) of model group vs (0.9346 ± 0.0192) of control group; (0.8610 ± 0.0243) of model group vs (0.9364 ± 0.0217) of sham operation group, p < 0.05, ).
Table 6. The methylation levels of the perforin gene promoter in CD4 + T cells in the three groups.
Correlation analysis
In the model group, the methylation levels of perforin gene promoter in CD4+ T cells were positively correlated with the MAN(r = 0.747, p < 0.05) but negatively correlated with the AI(r = −0.789, p < 0.05), the AECA levels in serum and BALF (r = −0.746, −0.661, respectively, p < 0.05)), the MLI (r = −0.743, p < 0.05) and the VEGF OD (r = −0.66, p < 0.05).
Discussion
As we know, the inflammation in COPD patients remains to be persistent and progressive even if they have stopped smoking. Some related literatures suggested that autoimmune response might be involved in the development of COPD-emphysema [Citation17], but the details are not clear yet and further study is needed.
Taraseviciene-Stewart et al. [Citation8] established an autoimmune emphysema rat model by intraperitoneal injection of xenogeneic endothelial cells in the early twenty first century for the first time. They also found adoptive transfer of pathogenic spleen-derived CD4+ T cells into naive immunocompetent rats resulted in emphysema with increased concentration of AECA in vivo, which suggests CD4 + T cell–dependent mechanisms are sufficient to trigger the development of autoimmune emphysema in rats.
DNA methylation is an epigenetic mechanism involving the transfer of a methyl group onto the C5 position of the cytosine to form 5-methylcytosine. It plays an important role in normal development in many higher organisms, usually occurring in the CpG islands, a CG-rich region, upstream of the promoter region. As a powerful methylation inhibitor, 5-Aza can inhibit the growth and proliferation of tumor cells, induce cells’ differentiation, and change the responsiveness and function of T cells [Citation18, Citation19]. Related studies also showed that treated with 5-Aza in vitro, CD4 + T lymphocytes were hypomethylated and could be made autoreactive [Citation20]. Since transfer of pathogenic CD4+ T cells into naive immunocompetent rats caused emphysema [Citation8], we speculate that CD4+ T cells with hypomethylation of perforin promoter may result in autoimmune mechanisms associated with autoimmune diseases.
Our previous study showed that the promoter of perforin gene in spleen-derived CD4 + T lymphocytes was hypomethylated in autoimmune emphysema in rats [Citation13], indicating that the gene hypomethylation was involved in the pathogenesis of this disease in rats. So, we hypothesize that transfer of this kind of cells hypomethylated in vitro into normal rats may cause emphysema too.
In order to test the hypothesis above, we first treated spleen-derived CD4 + T cells with 5-Aza in vitro and showed that the methylation levels of perforin promoter in spleen-derived CD4 + T cells were lower in the cell group 1 than in the cell group 2, which confirmed the effect of 5-Aza on methylation of perforin gene promoter in CD4 + T cells. Then, we injected normal rats intraperitoneally with the hypomethylated CD4+ T cells and found that the AECA and the MLI were greater in the model group than in the normal group and the sham operation group, while the MAN and the perforin promoter methylation in CD4 + T cells became lower, which showed transfer of those 5-Aza-treated cells was sufficient to induce autoimmune response in rats. Our results further suggested the hypomethylation of perforin promoter in CD4 + T cells was one of important mechanisms to trigger autoimmune emphysema in rats, thus providing new insights into this disease.
Apoptosis, a type of cell death mechanism, is controlled by the interactions between several molecules and responsible for the elimination of unwanted cells from the body [Citation21]. It is wildly accepted that the imbalance of apoptosis and proliferation of alveolar septal cells is implicated in the pathogenesis of emphysema [Citation22], which has captured more attention from all over the world.
VEGF is abundantly expressed in the normal lung, which plays a critical role in the development, growth, and survival of blood vessels. Some of previous literatures have showed that the expression of VEGF is decreased, which induces the apoptosis of alveolar septal cells [Citation23, Citation24] associated with COPD-emphysema, but related studies demonstrated that it was also involved in the process of small airway remodeling in emphysema rats and emphysema patients [Citation24, Citation25]. As a result, some scholars even speculated that VEGF might play different roles in different stages of emphysema [Citation26]. At the early stage, VEGF increases to promote the formation of airway epithelia and vascular endothelial cells, while at the late stage, it decreases due to the serious destruction of lungs.
This study showed the expression of VEGF in lung tissues and AI of alveolar septal cells were significantly increased in the model group as compared with the normal group and that the methylation levels of perforin promoter were positively correlated with the MAN and negatively correlated with AI, AECA, MLI and VEGF . So, we speculate that there is a compensatory increase of VEGF to repair the injury at the early stage of the disease induced by adoptive transfer of hypomethylated CD4 + T cells and that increased apoptosis of alveolar septal cells may also contribute to the pathogenesis of autoimmune emphysema in rats.
However, further clinical trials are needed to investigate the role of hypomethylation of perforin promoter in CD4 + T cells in emphysema and the effect of methyl donors on this disease to provide a potential new therapeutic option for the prevention of this disease in humans.
Conclusion
Transfer of invitro CD4 + T cells with hypomethylation of perforin promoter into rats’ abdomens causes autoimmune emphysema, possibly by increasing expression of VEGF and promoting alveolar septal cell apoptosis.
| Abbreviations: | ||
| SD | = | Sprague Dawley |
| 5-Aza | = | 5-azacytidine |
| PBS | = | Phosphate buffered saline |
| MLI | = | Mean linear Intercept |
| MAN | = | Mean alveolar numbers |
| AECA | = | Antiendothelial cell antibodies |
| BALF | = | Bronchoalveolar lavage fluid |
| VEGF | = | Vascular endothelial growth factor |
| AI | = | Apoptotic index |
| COPD | = | Chronic obstructive pulmonary disease |
| EC | = | Endothelial cell |
| HE | = | Hematoxylin-eosin |
| TUNEL | = | Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling |
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Huang X, Zhu Z, Guo X, et al. The roles of microRNAs in the pathogenesis of chronic obstructive pulmonary disease. Int Immunopharmacol. 2019;67:335–347. DOI:10.1016/j.intimp.2018.12.013
- Lamprecht B, McBurnie MA, Vollmer WM, BOLD Collaborative Research Group, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139(4):752–763. DOI:10.1378/chest.10-1253
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary Disease (2020 report)[EB/OL]. https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19WMV.pdf
- Sze MA, Dimitriu PA, Suzuki M, et al. Host response to the lung microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(4):438–445. DOI:10.1164/rccm.201502-0223OC
- Caramori G, Ruggeri P, Di Stefano A, et al. Autoimmunity and COPD: Clinical implications. Chest. 2018;153(6):1424–1431. DOI:10.1016/j.chest.2017.10.033
- Sileikiene V, Laurinaviciene A, Lesciute-Krilaviciene D, et al. Levels of CD4+ CD25 + T regulatory cells in bronchial mucosa and peripheral blood of chronic obstructive pulmonary disease indicate involvement of autoimmunity mechanisms. Adv Respir Med. 2019;87(3):159–166. DOI:10.5603/ARM.2019.0023
- Jin Y, Wan Y, Chen G, et al. Treg/IL-17 ratio and treg differentiation in patients with COPD. PLoS One. 2014;9(10):e111044. DOI:10.1371/journal.pone.0111044
- Taraseviciene-Stewart L, Scerbavicius R, Choe KH, et al. An animal model of autoimmune emphysema. Am J Respir Crit Care Med. 2005;171(7):734–742. DOI:10.1164/rccm.200409-1275OC
- Laddha AP, Kulkarni YA. VEGF and FGF-2: Promising targets for the treatment of respiratory disorders. Respir Med. 2019;156:33–46. DOI:10.1016/j.rmed.2019.08.003
- Michalska-Jakubus M, Kowal M, Adamczyk M, et al. Anti-endothelial cell antibodies do not correlate with disease activity in systemic sclerosis. Postepy Dermatol Alergol. 2018;35(2):185–191. DOI:10.5114/ada.2018.75241
- Lee JH, Hanaoka M, Kitaguchi Y, et al. Imbalance of apoptosis and cell proliferation contributes to the development and persistence of emphysema. Lung. 2012;190(1):69–82. DOI:10.1007/s00408-011-9326-z
- Zhao YL, Li F, Liu YW, et al. Adiponectin attenuates endoplasmi c reticulum stres s and alveolar epithelial apoptosis in COPD rats. Eur Rev Med Pharmacol Sci. 2017;21(21):4999–5007.
- Zhang C, Yan MY, Lu P, et al. Hypomethylation of perforin regulatory elements in CD4+ T cells from rat spleens contributes to the development of autoimmune emphysema. Respirology. 2014;19(3):376–381. DOI:10.1111/resp.12240
- Wilmot B, Fry R, Smeester L, et al. Methylomic analysis of salivary DNA in childhood ADHD identifies altered DNA methylation in VIPR2. J Child Psychol Psychiatry. 2016;57(2):152–160. DOI:10.1111/jcpp.12457
- Gilbert KM, Blossom SJ, Erickson SW, et al. Chronic exposure to trichloroethylene increases DNA methylation of the IFNG promoter in CD4+ T cells. Toxicol Lett. 2016;260:1–7. DOI:10.1016/j.toxlet.2016.08.017
- Zhang XY, Zhang C, Sun QY, et al. Infliximab protects against pulmonary emphysema in smoking rats. Chin Med J (Engl). 2011;124(16):2502–2506.
- Karayama M, Inui N, Suda T, et al. Antiendothelial cell antibodies in patients with COPD. Chest. 2010;138(6):1303–1308. DOI:10.1378/chest.10-0863
- Jia Y, Chang Y, Guo Z, et al. Transcription factor Tbx5 promotes cardiomyogenic differentiation of cardiac fibroblasts treated with 5-azacytidine. J Cell Biochem. 2019;120(10):16503–16515. DOI:10.1002/jcb.28885
- Li W, Wu D, Niu Z, et al. 5-Azacytidine suppresses EC9706 cell proliferation and metastasis by upregulating the expression of SOX17 and CDH1. Int J Mol Med. 2016;38(4):1047–1054. DOI:10.3892/ijmm.2016.2704
- Lu Q, Wu A, Ray D, et al. DNA methylation and chromatin structure regulate T cell perforin gene expression. J Immunol. 2003;170(10):5124–5132. DOI:10.4049/jimmunol.170.10.5124
- Kiraz Y, Adan A, Kartal Yandim M, et al. Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biol. 2016;37(7):8471–8486. DOI:10.1007/s13277-016-5035-9
- Demedts IK, Demoor T, Bracke KR, et al. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 2006;7(1):53. DOI:10.1186/1465-9921-7-53
- Gong J, Zhao H, Liu T, et al. Cigarette smoke reduces fatty acid catabolism, leading to apoptosis in lung endothelial cells: Implication for pathogenesis of COPD. Front Pharmacol. 2019;10:941.
- Wang L, Xu Z, Chen B, et al. The role of vascular endothelial growth factor in small-airway remodelling in a rat model of chronic obstructive pulmonary disease. Sci Rep. 2017;7:41202. DOI:10.1038/srep41202
- Kranenburg AR, de Boer WI, Alagappan VK, et al. Enhanced bronchial expression of vascular endothelial growth factor and receptors (flk-1 and flt-1) in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(2):106–113. DOI:10.1136/thx.2004.023986
- Chetta A, Olivieri D. Role of inhaled steroids in vascular airway remodelling in asthma and COPD. Int J Endocrinol. 2012;2012:397693. DOI:10.1155/2012/397693
