Abstract
Previous research has identified unexpectedly strong associations between dyspnea and pain, but the reasons remain unclear. Ascertaining the underlying biological and psychological mechanisms might enhance the understanding of the experience of both conditions, and suggest novel treatments. We sought to elucidate whether demographic factors, disease severity, psychological symptoms and biomarkers might account for the association between pain and dyspnea in individuals with COPD. We analyzed data from 301 patients with COPD who were followed in a prospective longitudinal observational study over 2 years. Measures included self-reported dyspnea and pain, pulmonary function tests, serum levels of inflammatory cytokines, measures of physical deconditioning, and scales for depression and anxiety. Analyses involved cross-sectional and longitudinal linear regression models. Pain and dyspnea were strongly correlated cross-sectionally (r = 0.77, 95% CI 0.72–0.82) and simultaneously across time (r = 0.42, 95% CI 0.28–0.56). Accounting for any of the other health factors only slightly mitigated the associations. Symptoms of pain and dyspnea thus may be fundamentally linked in COPD, rather than being mediated by common biological, psychological, or functional factors. From the patient’s perspective, pain and dyspnea may be part of the same essential experience. It is possible that treatments for one condition would improve the other.
Introduction
Dyspnea has been defined as the “subjective experience of breathing discomfort” [Citation1] It is a cardinal symptom of chronic obstructive pulmonary disease (COPD) and also occurs commonly with advancing age. Between 70% and 80% of patients with COPD report dyspnea, with prevalence as high as 98% in the last year of life [Citation2–5], and dyspnea is associated with decreased functional status, worse quality of life, and higher levels of disability [Citation6,Citation7].
Most research into the causes of dyspnea have focused on cardiovascular health conditions [Citation1]. However, there is emerging evidence that pain has strong associations with dyspnea. In a large Medicare sample, pain was much or common among those with dyspnea, and pain and dyspnea appeared to occur, develop, and resolve concurrently [Citation8]. Other studies have also found that the two symptoms co-occur and may even trigger each other [Citation9–12]. Similarly, in patients with COPD, pain was significantly associated with higher dyspnea scores [Citation13,Citation14]. In a qualitative study of COPD patients, while patients linked dyspnea and pain, they did not report the direction of cause and effect [Citation15]. The same types of medications, in particular opioids, mitigate both symptoms [Citation16].
The association between pain and dyspnea, and any underlying mechanism connecting the two, have been incompletely investigated. Experimental research has indicated that the same neurologic processes occur in both dyspnea and pain [Citation9,Citation10], that dyspnea is an inherently noxious sensation [Citation17], and that pain increases respiratory drive and thus also the sensation of dyspnea [Citation11]. Observational research led to the proposal that the same individuals might experience both symptoms, even if they do not share the same causal pathway [Citation8]. Certain comorbidities, in particular depression or anxiety, might influence the experience or reporting of somatic symptoms of all sorts. Indeed, there is strong evidence that dyspnea and pain both have important affective components [Citation17,Citation18]. Some research has indicated that physical deconditioning [Citation8] or serotonergic function may be key modulators of the relationship [Citation13,Citation19,Citation20]. Still others have proposed that airflow limitations and decreased ventilation inherent to COPD cause increased work of breathing that may result in fatigue, exhaustion, and pain [Citation14].
There are several key areas of uncertainty. First, the relationship between impaired lung function, dyspnea, and pain has not been fully elucidated. One meta-analysis did indicate that worse pulmonary function status, measured by FEV1, involved a lower prevalence of pain [Citation21]. Second, various cytokines have been implicated in the experience of pain [Citation22], and also in lung disease [Citation23], but exploration of their relationship to dyspnea has thus far been limited [Citation24]. Such factors might provide evidence for common physiological pathways. Third, although skeletal muscle function and exercise have known associations with dyspnea and chronic pain [Citation25–27], the role of physical deconditioning as a mediator has never been examined.
Modeling the mediating factors in the experience of dyspnea and pain could lead to better understanding of the pathophysiology of COPD, and possibly to treatment approaches that would minimize the unpleasant experience of both. We therefore sought to identify potential mechanisms that would explain the co-occurrence of dyspnea and pain in COPD. We hypothesized that pulmonary function, inflammatory cytokines, physical deconditioning, and/or the presence of comorbid depression or anxiety would mediate the association. Because many of these factors show wide distributions, or do not have absolute thresholds for different degrees of intensity, we focused on individual-level changes across time as predictors of varying association between dyspnea and pain.
Methods
Data source
We analyzed data from the COPD Activity: Serotonin Transporter, Cytokines, and Depression (CASCADE) study, a two-year longitudinal observational study of 301 persons with COPD enrolled in Seattle, Washington and San Antonio, Texas [Citation28]. Participants were recruited from queries of electronic medical records and pulmonary function tests, three medical centers with chest clinics, a research database maintained by the investigators, Better Breather support groups, pulmonary rehabilitation programs, community pulmonology practices, advertisements, the study website, and other referrals. Participants had a clinical diagnosis of COPD, age 40 years or older, current or past cigarette smoking (>10 pack years), stable disease with no exacerbations in the last 4 weeks, and were English speaking. COPD was confirmed by spirometry, using a cutoff of FEV1/FVC ratio < 0.70 and an FEV1% predicted < 80%. Patients were excluded if they had any of the following conditions: other lung diseases, non-COPD chronic inflammatory disease (e.g. chronic antibiotic use, ongoing infection, or auto-immune disease), lung or metastatic cancer, severe chronic kidney disease, HIV/AIDS, chronic oral prednisone use, bipolar disease, psychotic disorders, and dementia. Participants were assessed at baseline, one year, and two years, using self-report questionnaire measures, spirometry, 6-minute walk test, handgrip test, physical activity measured by accelerometer, and serum cytokine levels. The inclusion and exclusion criteria of the original CASCADE study were designed to allow for the examination of COPD-specific inflammation and depression in patients with COPD. Approvals were obtained by institutional review boards at the three clinical sites: University of Washington, VA Puget Sound Health Care System, and University of Texas Health Science Center-San Antonio.
Measures
The primary outcome was pain which was assessed using two items from the SF-36, “How much bodily pain have you had in the last 4 weeks?”, and “During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?” Although the first pain item is customarily rated from one to six, the sixth and most severe level was omitted from the version completed in this study. A raw score was calculated based on these two questions and converted to a 100-point scale [Citation29,Citation30].
Dyspnea, the main predictor of interest, was quantified using the University of California, San Diego Shortness of Breath Questionnaire (SOBQ), a 24-item self-report scale [Citation31]. Twenty-one items ask about shortness of breath during specific activities of daily living; if a participant does not normally carry out that activity, the question asks about estimated degree of anticipated shortness of breath. Three other items ask about limitations due to shortness of breath. Each item is rated from zero to five, for a total of 120 points. For ease of interpretation relative to the above pain scale, this was converted to a 100-point scale. To be consistent with the Dyspnea score, the Pain scores were reverse scored so that the higher scores indicated more pain.
We examined other factors that might be associated with dyspnea or pain, or which might alter the reporting of subjective symptoms. The PHQ-9 depression scale asks about the nine hallmark symptoms of depression during a two-week period, each scaled as zero to four. The Hospital Anxiety and Depression Questionnaire (HAD)-Anxiety subscale was used to assess anxiety symptoms [Citation32]. These were categorized by severity for descriptive statistics (see for cutoff values) and included as continuous variables for regression analyses. Obstructive sleep apnea (OSA) was assessed using the STOP-BANG questionnaire, with a cutoff of 4 or higher indicating high risk of OSA [Citation33]. The predicted forced expiratory volume (FEV1) was measured using spirometry. The percent predicted values were those included in the spirometer software, based on sex, age, and height. Although we measured both pre- and post-bronchodilator spirometry, we used the pre-bronchodilator FEV1 in our analyses in order to examine lung function at its worst.
Table 1. Demographic characteristics and outcome measures of participants.
We used two measures to capture different aspects of physical deconditioning. First, hand grip strength was used to assess skeletal muscle function. This was measured using a Jamar dynamometer (Fabrication Enterprises, White Plains, NY). While standing, participants were instructed to squeeze the grip handle as hard as they were able using their dominant hand with a 90-degree flexion at the elbow. The maximum value from 3 attempts was used. Second, distance walked during a 6-minute walk test was used to evaluate exercise capacity. Both of these measures have previously been used to assess patients with COPD [Citation34–38].
In an effort to ascertain whether humoral factors mediate the relationship between dyspnea and pain, serum levels of the following biomarkers were included in our analysis: C-reactive protein (CRP), interferon (INF), granulocyte macrophage-colony stimulating factor (GM-CSF), tumor necrosis factor (TNF-α), Interleukin (IL-1), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, and IL-13. Additional technical laboratory details can be found in a prior study published using the same CASCADE data set [Citation28].
We adjusted for demographic and health variables that could confound the association between dyspnea and pain. These included sex, age, race, education, marital status, smoking, and body mass index (BMI). The number of comorbidities was totaled, using the Charlson Comorbidity Index. We also adjusted by COPD disease severity, using the Global Initiative for Chronic Obstructive Lung Disease (GOLD) categories. Marital status was dichotomized as married or not. Race involved yes/no questions about different categories (Asian; Hawaiian Native or Pacific Islander; African American; Native American or Alaskan Native; Caucasian; Hispanic/Latino; and Other), and participants could answer as many as applied. Each was included in the models. Given our hypothesis that inflammation may mediate the relationship between pain and dyspnea, we elected to adjust for current smoking status rather than total pack-years. Our reasoning being that the former is more likely to impact inflammation than the latter [Citation39]. This was assessed by a single question asking whether the participant had used any cigarettes in the past week.
Analyses
We first characterized the sample at baseline by examining the distributions of many variables including sex, level of education, race/ethnicity, married/partner-status, as well as the other potential co-factors outlined above. We produced scatterplots of the cross-sectional ratings of the dyspnea and pain items, and the one-year changes in these (). We then developed linear regression models to quantify the degree to which degree of pain (or change in pain) was associated with degree of dyspnea (or change in dyspnea), controlling for the factors listed above. The first model was unadjusted; the second adjusted for demographic and health status factors (age, sex, race, marital status, education, BMI, GOLD category, comorbidities); the third adjusted also for other predictors (depression, anxiety, predicted FEV1, sleep apnea [Citation40], hand grip strength, walking distance); and the fourth adjusted for all the previous variables, as well as each of the biomarkers (cited above). One set of models estimated cross-sectional associations, including all three of the time points, using baseline predictors. The other set of models estimated changes in the coincident yearly change in the variables across both transition points (baseline to year one, and year one to year two). We examined the coefficients and p-values to identify which factors were associated with pain and changes in pain. Given the multiple comparisons, we used a standard of p < 0.01 to signify statistical significance.
Some of these variables measure similar constructs, such as hand grip strength and walking distance, and depression and anxiety. Including collinear elements in the same regression models increases the risk of Type II error for those predictors. We therefore examined each predictor independently, controlling for other factors that were fundamentally different (i.e. demographics, biomarkers, self-report scales, and measured functioning). For the grouped potential confounders, including collinear elements would not diminish the significance of the variables that were tested separately. We assumed that the models would identify significant relationships even if the relationships did not have the same variance across the range of predictors. Because this was an exploratory investigation, we did not apply a formal test for homoscedasticity.
Results
Characteristics of the sample are seen in . Data on pain and dyspnea were available for 301 participants.
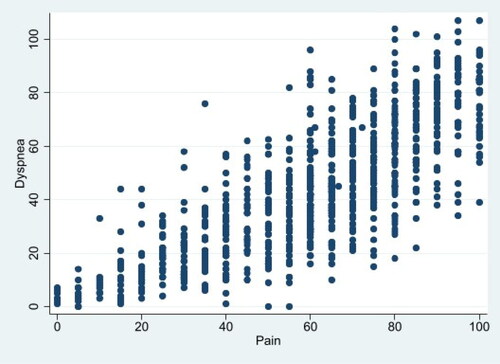
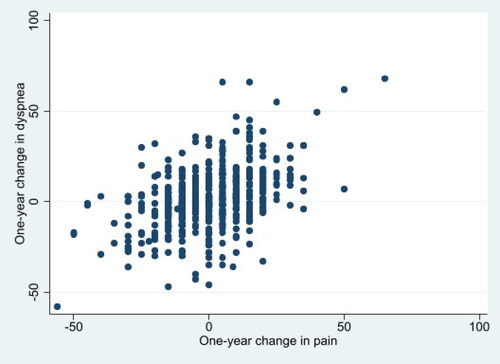
shows the cross-sectional associations between pain and dyspnea, each measured on a 100-point scale. shows longitudinal associations between the two.
shows the results of the regression model for dyspnea as a predictor of pain. The coefficient represents the increase in pain (0–100 scale) associated with each 1-point increase in dyspnea (0–100 scale). The first row is the cross-sectional association, and the second row involves the year-to-year changes in dyspnea and pain.
Table 2. Regression models for dyspnea as a predictor of pain.
In the unadjusted cross-sectional model, a 1-point increase in dyspnea is associated with a 0.78-point increase in pain (p < 0.001), and was similar after adjusting for demographic characteristics, BMI, COPD severity and comorbidity (β = 0.79, p < 0.001). After additionally adjusting for FEV1, sleep apnea, hand grip strength, 6-minute walk distance, anxiety, and depression, a 1-point increase in dyspnea was associated with a 0.57-point increase in pain score (p < 0.001). Inflammatory biomarkers did not predict pain scores, and did not change the strength of the association between dyspnea and pain (β = 0.77, p < 0.001).
In models examining whether a 1-year change in dyspnea was associated with a 1-year change in pain, a 1-point increase in dyspnea was associated with a 0.41-point increase in pain (). In model 3, adjusting for demographics, BMI, GOLD category, comorbidity and other predictors, worse dyspnea over 1 year continued to be associated with worse pain over 1 year (β = 0.36, p < 0.001).
shows the results of analyses of predictors that were found to be significantly associated with pain at a p < 0.01 level in models after adjusting for dyspnea. There were four variables associated with pain after adjustment for dyspnea in cross-sectional analyses: FEV1, depression, anxiety and 6-minute walk distance. Of these four factors, depression and 6-MWT were still associated with pain in Model 3 after adjustment for dyspnea, demographics, GOLD category, comorbidity and other predictors. For example, patients with a 1-point worse depression score had a 0.68-point increased pain score (p < 0.012) and participants who walked 100 fewer feet (30.5 meters) had a 1.40-point increased pain score (p < 0.001).
Table 3. Significant predictors of pain in regression models that include dyspnea.
In models to examine the change in variables with change in pain scores over a 1-year period, there were three variables associated with pain after adjustment for dyspnea: FEV1, depression and BMI. Of these only increasing BMI was associated with increasing pain scores after adjustment for dyspnea and other factors in Model 3 (β 2.33, p = 0.005). The inclusion of biomarkers had very little effect on the coefficients.
The magnitude of the coefficients was rather small in comparison to the coefficients for dyspnea, when considering the scale. For instance, three points worse on the 100-point dyspnea scale had a roughly similar association with pain as did walking an additional 100 feet (30.5 meters) in a 6-minute walk test (about 10% of the average distance).
In analyses to examine whether the results differed using post-bronchodilator FEV1 instead of pre-bronchodilator FEV1, the coefficients for post-bronchodilator FEV1 in the same models differed by less than 10% from those for pre-bronchodilator FEV1 (with a correlation coefficient of 0.947).
Discussion
We examined factors implicated in the experience and reporting of dyspnea and pain including physical status (lung functioning and physical performance), markers of inflammation, comorbidities, and/or mental health issues. Contrary to our hypothesis, we did not find that adjusting for these other factors greatly changed the estimated association between dyspnea and pain in either cross-sectional or in changes over one year. The coefficients in remained mainly stable, with a high degree of statistical significance, regardless of the covariates entered into the models. To our knowledge, no research has yet quantitatively explored the role of these potential mediating factors.
Our primary finding was that the relationship between dyspnea and pain was not altered by adjusting for other predictors. This suggests that the association between dyspnea and pain may be fundamental, and less dependent on intermediary factors. This is unexpected, because the two symptoms do not formally overlap in terms of clinical findings, except insofar as both are unpleasant. Although we were not able to control for all the potential intermediary factors, we examined a large number, including COPD disease severity, demographic factors, psychological distress, BMI, comorbidities, lung function, and inflammatory cytokines. Our research indicates that although lung function, BMI, depressive symptoms are associated with pain independently of dyspnea, they do not alter the strong relationship between the report of dyspnea and the report of pain. Taken together, our findings imply that pain and dyspnea may be two sides of the same experience.
One important possibility is that pain and dyspnea are inherently linked through the neurologic structures or networks involved in processing perceptions of both. Multiple studies have indicated that the insular cortex, in particular, may play a crucial role as a central modulator. For instance, Schön et. al. found that individuals with lesions to the right insular cortex were less sensitive to experimentally-induced pain and dyspnea, compared to matched healthy controls [Citation41]. And Leupoldt et. al. found that patients with asthma report less pain and dyspnea in the affective unpleasantness dimension with accompanying functional magnetic resonance imaging data indicating altered activity in both the insular cortex and the periaqueductal gray [Citation42]. This raises the possibility that therapeutics aimed at these neurologic processes may relieve symptoms. As an extreme example, targeted destruction of brain structures has already been used to relieve dyspnea and pain in patients with mesothelioma [Citation43].
Of the covariates, the only ones that remained highly statistically significant as predictors of pain in the setting of dyspnea in fully controlled models were less distance walked (for cross-sectional associations) and change in BMI (with increasing BMI associated with more pain). One possibility is that pain ratings may be primarily related to musculoskeletal or large joint pathology. This observation makes it even more surprising that dyspnea would be strongly associated with pain complaints. In addition, the distance walked in six minutes is a measure of functional capacity, and is a different dimension of disease severity in COPD than lung function, suggesting that COPD disease severity may be associated with pain [Citation44]. Anxiety, depression, and impaired pulmonary functioning were associated with pain in unadjusted models, and may constitute a constellation of symptoms in those with trouble breathing, but these effects did not hold after controlling for other factors.
The absence of any apparent effect of cytokines is perhaps not surprising in these analyses, because cytokines are not well-known markers for pain in COPD. But the lack of any statistically significant findings across multiple analyses (14 cytokines used in two models) argues that the subjective experiences of dyspnea and pain are most likely not altered substantially by the degree of inflammation. It would also argue against attempting to manage pain-related dyspnea with systematic anti-inflammatory agents.
In our analysis, we elected to use pre-bronchodilator spirometry rather than post-bronchodilator values. We assumed (because it does not seem to have been investigated scientifically) that if there were a contemporaneous association between obstruction and pain, participants would likely recall the worst moments of each symptom. These would most plausibly occur before bronchodilator use. Notably, our pre- and post-bronchodilator values were highly correlated with a correlation coefficient of 0.947, thus this selection was unlikely to alter our analysis.
There are several limitations to this research. First, because all participants had COPD, it is unclear if the associations we identified can be extended to other populations. Second, the degree of racial/ethnic variation was minimal. Third, our measure of pain is designed to broadly capture bodily pain and pain interference, but not pain to specific areas of the body. We therefore could not completely exclude the possibility that certain kinds of pain (e.g. low back pain vs. hip pain) were more or less closely associated with dyspnea. In addition, we did not track pain medication usage among patients, so we are unable to assess its role as a potential confounding variable. Fourth, although our study tracked a large number of variables, there may be certain unmeasured physical or psychological factors that impact both dyspnea and pain. Fifth, the percent predicted FEV1 was computed by the spirometers used in the study. Sixth, it is possible that we were not able to detect a small effect size for some variables to predict pain due to the sample size. Seventh, we did not evaluate any potential impact of respiratory medications in our analysis. Eighth, because of human error the sixth pain intensity rating of “severe” normally included as part of the SF-36 pain subscale was omitted, leaving only five responses. We do not feel that this impacts our results because the scaling was consistent across all participants and we were interested in the relative rather than the absolute effects on the experience of pain. Finally, we built models based on the face value of the predictors, but did not formally test for complex relationships or collinearity, which increases the risk of Type II errors.
Conclusion
Symptoms of pain and dyspnea are closely related in COPD, but the relationship does not appear to be mediated by various biological, psychological, or functional factors. From the patient’s perspective, they may be part of the same essential experience. By extension, it is possible that treatments for one symptom would improve the other.
Declaration of interests
The authors report no conflict of interest. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data are not available.
Additional information
Funding
References
- Parshall MB, Schwartzstein RM, Adams L, American Thoracic Society Committee on Dyspnea, et al. An official American thoracic society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–452. DOI:10.1164/rccm.201111-2042ST
- Carette H, Zysman M, Morelot-Panzini C, Initiatives BPCO (Bronchopneumopathie Chronique Obstructive) Scientific Committee and Investigators, et al. Prevalence and management of chronic breathlessness in COPD in a tertiary care center. BMC Pulm Med. 2019;19(1):95. DOI:10.1186/s12890-019-0851-5
- Elkington H, White P, Addington-Hall J, et al. The healthcare needs of chronic obstructive pulmonary disease patients in the last year of life. Palliat Med. 2005;19(6):485–491. DOI:10.1191/0269216305pm1056oa
- Rennard S, Decramer M, Calverley PM, et al. Impact of COPD in North America and Europe in 2000: subjects’ perspective of confronting COPD international survey. Eur Respir J. 2002;20(4):799–805. DOI:10.1183/09031936.02.03242002
- Uzaslan E, Mahboub B, Beji M, et al. The burden of chronic obstructive pulmonary disease in the Middle East and North Africa: results of the BREATHE study. Respir Med. 2012;106(Suppl 2):S45–S59. DOI:10.1016/S0954-6111(12)70014-8
- American Thoracic Society. Dyspnea: mechanisms, assessment, and management: a consensus statement. Am J Respir Crit Care Med. 1999;159(1):321–340.
- Smith AK, Currow DC, Abernethy AP, et al. Prevalence and outcomes of breathlessness in older adults: a national population study. J Am Geriatr Soc. 2016;64(10):2035–2041. DOI:10.1111/jgs.14313
- Clark N, Fan VS, Slatore CG, et al. Dyspnea and pain frequently co-occur among medicare managed care recipients. Ann Am Thorac Soc. 2014;11(6):890–897. DOI:10.1513/AnnalsATS.201310-369OC
- Schon D, Dahme B, von Leupoldt A. Associations between the perception of dyspnea, pain, and negative affect. Psychophysiology. 2008;45(6):1064–1067. DOI:10.1111/j.1469-8986.2008.00704.x
- von Leupoldt A, Sommer T, Kegat S, et al. Dyspnea and pain share emotion-related brain network. Neuroimage. 2009;48(1):200–206. DOI:10.1016/j.neuroimage.2009.06.015
- Nishino T, Shimoyama N, Ide T, et al. Experimental pain augments experimental dyspnea, but not vice versa in human volunteers. Anesthesiology. 1999;91(6):1633–1638. DOI:10.1097/00000542-199912000-00014
- Desbiens NA, Mueller-Rizner N, Connors AF, et al. The relationship of nausea and dyspnea to pain in seriously ill patients. Pain. 1997;71(2):149–156. DOI:10.1016/s0304-3959(97)03353-8
- Borge CR, Wahl AK, Moum T. Association of breathlessness with multiple symptoms in chronic obstructive pulmonary disease differences in subjective and objective respiratory parameters in patients with chronic obstructive pulmonary disease with and without pain. J Adv Nurs. 2010;66(12):2688–2700. DOI:10.1111/j.1365-2648.2010.05447.x
- Bentsen SB, Rustoen T, Miaskowski C. Differences in subjective and objective respiratory parameters in patients with chronic obstructive pulmonary disease with and without pain. COPD. 2012;7:137–143. DOI:10.2147/COPD.S28994
- Lohne V, Heer HC, Andersen M, et al. Qualitative study of pain of patients with chronic obstructive pulmonary disease. Heart Lung. 2010;39(3):226–234. DOI:10.1016/j.hrtlng.2009.08.002
- Davis MP, Behm B, Balachandran D. Looking both ways before crossing the street: assessing the benefits and risk of opioids in treating patients at risk of sleep-disordered breathing for pain and dyspnea. J Opioid Manag. 2017;13(3):183–196. DOI:10.5055/jom.2017.0385
- Morélot-Panzini C, Gilet H, Aguilaniu B, et al. Real-life assessment of the multidimensional nature of dyspnoea in COPD outpatients. Eur Respir J. 2016;47(6):1668–1679. DOI:10.1183/13993003.01998-2015
- Gracely RH. Affective dimensions of pain: how many and how measured? APS J. 1992;1(4):243–247.
- IsHak WW, Wen RY, Naghdechi L, et al. Pain and depression: a systematic review. Harv Rev Psychiatry. 2018;26(6):352–363. DOI:10.1097/HRP.0000000000000198
- Smoller JW, Pollack MH, Systrom D, et al. Sertraline effects on dyspnea in patients with obstructive airways disease. Psychosomatics. 1998;39(1):24–29. DOI:10.1016/S0033-3182(98)71377-5
- van Dam van Isselt EF, Groenewegen-Sipkema KH, Spruit-van Eijk M, et al. Pain in patients with COPD: a systematic review and meta-analysis. BMJ Open. 2014;4(9):e005898. DOI:10.1136/bmjopen-2014-005898
- Cook AD, Christensen AD, Tewari D, et al. Immune cytokines and their receptors in inflammatory pain. Trends Immunol. 2018;39(3):240–255. DOI:10.1016/j.it.2017.12.003
- Pinkerton JW, Kim RY, Robertson AAB, et al. Inflammasomes in the lung. Mol Immunol. 2017;86:44–55. DOI:10.1016/j.molimm.2017.01.014
- Ryan R, Spathis A, Clow A, et al. Breathlessness and inflammation: potential relationships and implications. Curr Opin Support Palliat Care. 2016;10(3):242–248. DOI:10.1097/SPC.0000000000000229
- O’Donnell DE, James MD, Milne KM, et al. The pathophysiology of dyspnea and exercise intolerance in chronic obstructive pulmonary disease. Clin Chest Med. 2019;40(2):343–366. DOI:10.1016/j.ccm.2019.02.007
- Park SM, Kim GU, Kim HJ, et al. Low handgrip strength is closely associated with chronic low back pain among women aged 50 years or older: a cross-sectional study using a national health survey. PLoS One. 2018;13(11):e0207759. DOI:10.1371/journal.pone.0207759
- Park SM, Kim GU, Kim HJ, et al. Walking more than 90 minutes/week was associated with a lower risk of self-reported low back pain in persons over 50years of age: a cross-sectional study using the Korean national health and nutrition examination surveys. Spine J. 2019;19(5):846–852. DOI:10.1016/j.spinee.2018.11.007
- Nguyen HQ, Herting JR, Pike KC, et al. Symptom profiles and inflammatory markers in moderate to severe COPD. BMC Pulm Med. 2016;16(1):173. DOI:10.1186/s12890-016-0330-1
- Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), Short-Form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res. 2011;63(Suppl 11):S240–S252. DOI:10.1002/acr.20543
- WareJrJE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. DOI:10.1097/00005650-199206000-00002
- Eakin EG, Resnikoff PM, Prewitt LM, et al. Validation of a new dyspnea measure: the UCSD shortness of breath questionnaire. University of California, San Diego. Chest. 1998;113(3):619–624. DOI:10.1378/chest.113.3.619
- Bjelland I, Dahl AA, Haug TT, et al. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. DOI:10.1016/s0022-3999(01)00296-3
- Nagappa M, Liao P, Wong J, et al. Validation of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis. PLoS One. 2015;10(12):e0143697. DOI:10.1371/journal.pone.0143697
- Redelmeier DA, Bayoumi AM, Goldstein RS, et al. Interpreting small differences in functional status: the six minute walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155(4):1278–1282. DOI:10.1164/ajrccm.155.4.9105067
- Puhan MA, Mador MJ, Held U, et al. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J. 2008;32(3):637–643. DOI:10.1183/09031936.00140507
- Puhan MA, Chandra D, Mosenifar Z, National Emphysema Treatment Trial (NETT) Research Group, et al. The minimal important difference of exercise tests in severe COPD. Eur Respir J. 2011;37(4):784–790. DOI:10.1183/09031936.00063810
- Holland AE, Hill CJ, Rasekaba T, et al. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91(2):221–225. DOI:10.1016/j.apmr.2009.10.017
- Nyberg A, Saey D, Maltais F. Why and how limb muscle mass and function should be measured in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12(9):1269–1277. DOI:10.1513/AnnalsATS.201505-278PS
- Çolak Y, Afzal S, Lange P, et al. Smoking, systemic inflammation, and airflow limitation: a mendelian randomization analysis of 98 085 individuals from the general population. Nicotine Tob Res. 2019;21(8):1036–1044. DOI:10.1093/ntr/nty077
- Rizzi M, Palma P, Andreoli A, et al. Prevalence and clinical feature of the “overlap syndrome”, obstructive sleep apnea (OSA) and chronic obstructive pulmonary disease (COPD), in OSA population. Sleep Breath. 1997;2(3):68–72. DOI:10.1007/BF03038868
- Schön D, Rosenkranz M, Regelsberger J, et al. Reduced perception of dyspnea and pain after right insular cortex lesions. Am J Respir Crit Care Med. 2008;178(11):1173–1179. DOI:10.1164/rccm.200805-731OC
- von Leupoldt A, Sommer T, Kegat S, et al. Down-regulation of insular cortex responses to dyspnea and pain in asthma. Am J Respir Crit Care Med. 2009;180(3):232–238. DOI:10.1164/rccm.200902-0300OC
- Pereira EA, Paranathala M, Hyam JA, et al. Anterior cingulotomy improves malignant mesothelioma pain and dyspnoea. Br J Neurosurg. 2014;28(4):471–474. DOI:10.3109/02688697.2013.857006
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. DOI:10.1056/NEJMoa021322