Abstract
Refractory breathlessness is a devastating symptom in chronic obstructive pulmonary disease (COPD). Symptom-focused breathlessness services, involving palliative care teams, offer individualized support but are not yet widely available for people with nonmalignant disease among which COPD. Our primary aim was to demonstrate the feasibility of setting up a breathlessness service specifically for COPD patients within a respiratory outpatient clinic. Our secondary aims were to assess how many sessions patients need to complete the intervention; to obtain an indication of effect size (on the Chronic Respiratory Questionnaire (CRQ), subset mastery domain); and to evaluate patient and professional satisfaction. We conducted a non-randomized single-center feasibility study. Participants had COPD and refractory breathlessness. During at least one session with a respiratory nurse and a pulmonologist, and one session with a physiotherapist, patients learned non-pharmacological interventions to manage breathlessness. Of 34 screened patients, 19 were included. All completed the intervention. A median of two clinical visits and two telephone calls were needed to complete the intervention. The mean improvement of 1.55 in CRQ, mastery domain, significantly exceeded the clinically important difference of 0.5. The service was rated as excellent by the eight patients who completed the survey. The health professional team gave positive feedback on the experience of delivering the intervention. Delivery of a breathlessness service for COPD outpatients with refractory breathlessness appears feasible, easy to implement in a respiratory outpatient clinic, and has the potential to be effective. A randomized controlled clinical trial is needed to test effectiveness and cost-effectiveness in this context.
Introduction
Chronic obstructive pulmonary disease (COPD) is a highly prevalent, life-threatening lung disease. The cardinal, debilitating symptom of advanced COPD is breathlessness, which persists in most patients, even when disease management has been optimized [Citation1].
There is little correlation between physiologic parameters (such as airflow obstruction and gas exchange disturbances) and breathlessness. This is partly explained by patients’ individual cognitive and affective responses to the sensory disturbance [Citation2]. For instance, anxiety leads to rapid, shallow breathing, resulting in an increase in hyperinflation and thus, worsening of the symptom [Citation3]. Due to this anxiety-dyspnea vicious cycle, many patients with advanced COPD experience unpredictable episodic breathlessness [Citation4]. Consequently, the complexity of breathlessness in COPD patients warrants an individualized, multidisciplinary intervention [Citation5]. Patient education is vital if dyspnea crises are present, and should encompass breathing training, an action plan, and individualized use of a fan, amongst others [Citation6].
In recent years, evidence-based multidisciplinary holistic breathlessness services have been developed, mostly emerging from palliative care services in the UK and Germany [Citation7–12]. Higginson et al. [Citation10], in a study involving predominantly patients with advanced nonmalignant diseases such as COPD, heart failure, interstitial lung disease and motor neuron disease, found a significant and clinically relevant improvement in breathlessness mastery. However, evidence for the impact on mastery in people with nonmalignant disease is not consistent: a German randomized controlled trial (including patients with COPD, motor neuron disease, pulmonary hypertension, heart failure and interstitial lung disease, as well as cancer) found that mastery improved significantly, but did not reach the minimum clinically important difference [Citation11]. A Cambridge trial, involving only patients with nonmalignant disease, had a positive qualitatively identified impact, but there was no significant improvement in distress due to breathlessness [Citation12]. More recently, a Canadian breathlessness service retrospectively assessed its effectiveness for COPD patients and found no difference in breathlessness before and after the enrollment, although there was some benefit for almost half of the patients [Citation13].
Breathlessness services have not been universally adopted in all countries. Apart from the reason that the effects in trials have not been unequivocal, this may be due to the sporadic availability of ambulatory palliative care services, particularly for people with nonmalignant disease [Citation14,Citation15]. Consequently. there is a concerning gap in the care for patients with COPD and refractory breathlessness. Indeed, patients with COPD who refrain from pulmonary rehabilitation and do not visit a dedicated physiotherapist are likely to miss out on individualized breathlessness management support [Citation16].
The goal of the current study was to investigate whether it is feasible to set up a breathlessness service specifically for COPD patients, in a respiratory outpatient setting and without involvement of an expert palliative care team.
Methods
Study design
We conducted a prospective, single-center non-blinded feasibility study. The predefined criterion for assessing feasibility was 75% of the included patients completing the intervention. Secondary objectives were:
assessing how many sessions patients needed to complete the intervention.
obtaining an indication of effect size, using a Dutch translation of the Chronic Respiratory Questionnaire (CRQ). The CRQ is an interviewer led questionnaire and scores 20 items across 4 domains: dyspnea (5 items), fatigue (4 items), emotional functioning (7 items) and mastery (4 items). The mastery domain was appointed as the most relevant domain for this study, since it reflects the subjects’ feeling of control over the disease, as well as the impact the disease has on quality of life. Scale range is 1–7; high values indicate low burden. The minimum clinically important difference is 0.5 [Citation17].
assessing patient satisfaction with a designed-for-purpose questionnaire. (See the measurements paragraph.)
Subjects and screening
Outpatients with COPD (diagnosed by a pulmonologist, post-bronchodilatator FEV1/FVC below the lower limit of normal) were eligible for the study if they reported refractory dyspnea (troubled by breathlessness despite optimization of COPD treatment) with a Medical Research Council (MRC) dyspnea score of three or higher. Exclusion criteria were cognitive impairment and inability to speak Dutch. Patients were recruited by their treating pulmonologists and respiratory nurses. If patients agreed to receive information about the study, they received a letter with study information and were called by one of the investigators at least one week later.
Intervention
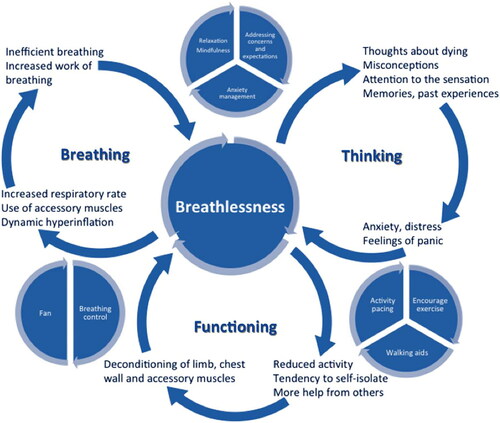
We collaborated with a well-established service, the Cambridge Breathlessness Intervention Service (CBIS), to learn components of service, and how to deliver them. CBIS has developed the breathing-thinking-functioning model (BTF), an educational tool for health professionals caring for breathless people, that is increasingly used in practice with breathless patients [Citation18]. This model increases understanding of cognitive and behavioral reactions to breathlessness that may worsen and maintain the symptom (). The aim of the intervention is to break the key vicious cycle(s), with elements of cognitive behavioral therapy (challenging misconceptions a patient may have) as well as a “toolkit” of non-pharmacological techniques. Aided by this toolkit, patient and health care provider(s) make an individual breathlessness management plan. The BTF model was used to underpin our service, called Ademen Denken Doen (ADD) in Dutch. Key elements of ADD were copied and translated from the Cambridge Breathlessness Intervention Service (CBIS). The ADD service encompasses the following:
Explanation of the interaction between ‘breathing’, ‘thinking’ and ‘functioning’.
Structured patient education on COPD.
Challenging misconceptions the patient may have.
Breathing techniques.
Relaxation techniques.
Physiotherapy session, opportunity to practise breathing techniques during exercise, opportunity to walk with a rollator.
The service uses a booklet in which key aspects of A, B, and D are explained and visualized (added as a supplement, in Dutch). For a more detailed description of intervention techniques per domain, see and the Template for Intervention Description and Replication (TIDieR) Checklist which is added as a supplement.
Table 1. Breathing domain.
Table 2. Thinking domain.
Table 3. Functioning domain.
Unlike CBIS which sits within a palliative care service, ADD is delivered by a pulmonologist, a respiratory nurse and a physiotherapist. The pulmonologist received two days’ training from CBIS, and subsequently trained the nurse and the physiotherapist.
ADD is a brief intervention: patients have at least one session with a pulmonologist and a respiratory nurse (combined), and one session with a physiotherapist who practiced the breathing techniques with them. The patients receive the booklet (with verbal explanation) and a hand-held fan (with instruction). If necessary, extra sessions are scheduled. Since there was no team experience with giving the intervention, the number of sessions for the feasibility trial was not fixed.
Measurements
At baseline, all participating patients completed the CRQ. The CRQ was repeated during the last visit.
Baseline characteristics such as pulmonary function tests were taken from the patient’s medical file.
The total number of visits to the outpatient clinic was reported.
A designed-for-purpose postal survey was sent after the intervention. The survey consisted of 12 multiple choice questions regarding components of service (e.g. “Did you find the consultation with the physiotherapist helpful?”, answer “yes/a little bit/no” with room for clarification). The postal survey was only sent once.
After the pilot study, an evaluation meeting between the team members was held, and team members’ feedback was documented.
Data analyses and statistics
We estimated that inclusion of 20 patients would be sufficient to answer our research questions. This sample size was deemed appropriate for testing acceptability of the intervention, to estimate how many sessions were needed to obtain an indication of the effect size on the CRQ (mastery domain) and to gather feedback from patients and health care providers on the strengths and weaknesses of the intervention. Because of the small sample size, only descriptive statistics were used.
Ethics, consent and permissions
Ethical approval was given by the Medical Ethics Review Committee of the Amsterdam University Medical Center (registration number 2019.199). All participating patients gave written informed consent. The trial was registered in the ISRCTN trial register, reference number: ISRCTN48274234.
Results
Patient characteristics
34 Patients were screened for the study and received study information. Of those, five patients did not meet the inclusion criteria (four in terminal phase of COPD and therefore no longer able to visit the outpatient clinic, one with severe cognitive impairment). Three patients did not want to come to the hospital, and seven refrained from participating due to other personal reasons. Consequently, 19 patients were included in the study over a 13-month period (between October 2019 and November 2020). Patients were referred by a range of pulmonologists and respiratory nurses. Baseline characteristics of the study participants are shown in . All included patients were white and born in the Netherlands.
Table 4. Baseline characteristics.
Findings
All 19 recruited patients completed the intervention. They had a median of two visits to the outpatient clinic (SD 1.07) and two telephone calls by one of the team members (SD 1.74). The mean improvement in the mastery domain of the CRQ was 1.55; 17/19 patients improved by more than the MCID of 0.5. Mean CRQ scores also improved on the other three domains (see ). Only 8/19 returned the patients satisfaction survey after the intervention. All respondents rated the service as excellent and helpful. Most patients found the ‘breathing a rectangle’ (see ) the most useful breathing technique and were enthusiastic about the hand-held fan.
Table 5. Effects on CRQ.
Implementation of the intervention within the team was relatively easy. During the evaluation meeting, the team members all described the BTF model as very helpful in both the exploration and the management of refractory breathlessness. They considered that delivering the service had raised their awareness of the symptom and given them more insights into its management. Furthermore, other colleagues were enthusiastic about the option to refer to the service, and stated that it made them more aware of refractory breathlessness, increased their knowledge on breathlessness management and increased their willingness to speak about breathlessness with their patients. Handheld fans were made available for breathless patients both in the outpatient clinic and on the ward.
Discussion
ADD has shown potential as a breathlessness service for patients with COPD. The service is delivered by a pulmonologist, a respiratory nurse and a physiotherapist, making it theoretically possible to implement this service in any pulmonary outpatient clinic. It is a brief intervention: most patients reached better symptom control after two to three sessions and two telephone calls. Recruitment for this feasibility trial was relatively easy, the service was well-received and all included patients completed the study.
Almost all patients (17 out of 19) experienced an improvement in breathlessness mastery as measured with CRQ. Although our trial was not designed to measure effectiveness, it is interesting to consider why the results may be more positive than in four previous studies focused on people with nonmalignant disease [Citation10–13]. First, patients received the service within their own outpatient clinic, from members of their usual care team. It is possible that patients prefer to learn breathlessness management strategies from their familiar healthcare team, instead of a palliative care team. Indeed, patients may have misconceptions on the word ‘palliative’ (which is often associated with the end of life) [Citation16]. Second, our patients’ baseline knowledge on breathlessness management may have been lower than in the other studies. For instance, none of our patients were familiar with the use of a handheld fan, which may have been different in the UK or German study populations. Third, this is the first study to date using the BTF model to underpin the clinical intervention and it is possible this contributed to the positive findings. For example, the ‘breathing a rectangle’-exercise, which our patients found quite helpful, is not widely used in other breathlessness services to our knowledge. Fourth, previous studies included people with a wide range of respiratory nonmalignant conditions, whereas ADD was aimed only at people with COPD; it could be argued that COPD patients are particularly likely to benefit, as presence of hyperinflation, along with associated maladaptive breathing patterns, makes them more amenable to benefit from breathing techniques than those with conditions with restrictive pathology and/or low diffusion capacity [Citation19]. Furthermore, many patients with advanced COPD experience unpredictable (untriggered) episodic breathlessness, more so than in other patient groups, which the ADD explicitly sought to manage [Citation4].
A properly powered randomized controlled trial is needed to determine whether breathlessness services embedded within respiratory care are indeed effective and also cost-effective. ADD delivers non-pharmacological breathlessness management strategies that should be offered to all COPD patients with refractory breathlessness [Citation5,Citation6,Citation14]. However, such breathlessness management strategies are not widely available to the many patients who – for various reasons such as fatigue, anxiety, transportation difficulties, limited privacy and avoiding confrontation with severely ill patients - decline pulmonary rehabilitation [Citation20]. Furthermore, health insurers must agree to reimburse the extra time it takes to deliver such a symptom-focused service. Setting up a multicenter randomized controlled trial would be helpful in increasing availability of a breathlessness service for COPD patients.
Strengths
The tested intervention builds on an existing model for non-pharmacological breathlessness management (BTF), for which research data are still scarce. We showed that the intervention was easily transposed into an outpatient setting. This intervention can be given in clinics in which a palliative care team is not yet available for outpatients. The high patient adherence is promising. It is a low-cost intervention, which has the potential to improve quality of life in patients and raise awareness of the breathlessness problem in health care providers.
Limitations
Our study has important limitations. The positive effects we found in this population should be interpreted with caution. There was no control group and the marked positive change in CRQ might partly be due to a Hawthorne effect. The fact that the service was delivered and evaluated by the team members that treated the patients’ COPD is a potential source of bias. Furthermore, only 42% of participants returned the postal survey to give feedback on the intervention, which may be partly due to low literacy skills. (In the Netherlands, almost half of COPD patients have low health literacy [Citation21]. Those who returned the questionnaire rated all parts of the intervention as ‘excellent’ without any other comments. Since 17 out of 19 patients responded to the intervention, a response bias is less likely, but the low response rate limits the validity of this result. In hindsight, qualitative interviews with participants would have provided much better insight in their experience of this intervention.
Furthermore, we did not measure how long the positive effects on breathlessness mastery lasted. Finally, the intervention was delivered in a single center only.
Conclusion
Delivering a breathlessness service, without involvement of a palliative care team, was feasible in patients with COPD in a single-center respiratory out-patient setting. Intervention adherence was high, both patient and professional participants were enthusiastic about the service, and patients’ CRQ scores improved markedly. This study suggests that it would be feasible and worthwhile to undertake a large randomized multicenter controlled clinical trial to test clinical and cost-effectiveness of such a service.
| Abbreviations: | ||
| ADD | = | Ademen Denken Doen (Dutch translation of BTF) |
| BTF | = | breathing-thinking-functioning (tool for management of breathlessness) |
| CBIS | = | Cambridge Breathlessness Intervention Service |
| COPD | = | chronic obstructive pulmonary disease |
| CRQ | = | Chronic Respiratory Questionnaire |
Declaration of Interest
The authors report there are no competing interests to declare. This was an investigator-initiated study without external funding.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Carette H, Zysman M, Morelot-Panzini C, Initiatives BPCO (bronchopneumopathie chronique obstructive) Scientific Committee and Investigators, et al. Prevalence and management of chronic breathlessness in COPD in a tertiary care center. BMC Pulm Med. 2019;19(1):95. DOI:10.1186/s12890-019-0851-5
- Marlow LL, Faull OK, Finnegan SL, et al. Breathlessness and the brain: The role of expectation. Curr Opin Support Palliat Care. 2019;13(3):200–210. DOI:10.1097/SPC.0000000000000441
- Alius MG, Pane-Farre CA, Von Leupoldt A, et al. Induction of dyspnea evokes increased anxiety and maladaptive breathing in individuals with high anxiety sensitivity and suffocation fear. Psychophysiology. 2013;50(5):488–497. DOI:10.1111/psyp.12028
- Simon ST, Higginson IJ, Benalia H, et al. Episodic and continuous breathlessness: A new categorization of breathlessness. J Pain Symptom Manage. 2013;45(6):1019–1029. DOI:10.1016/j.jpainsymman.2012.06.008
- Maddocks M, Lovell N, Booth S, et al. Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease. Lancet. 2017;390(10098):988–1002. 2DOI:10.1016/S0140-6736(17)32127-X
- Mularski RA, Reinke LF, Carrieri-Kohlman V, ATS Ad Hoc Committee on Palliative Management of Dyspnea Crisis, et al. An official American thoracic society workshop report: Assessment and palliative management of dyspnea crisis. Ann Am Thorac Soc. 2013;10(5):S98–S106. DOI:10.1513/AnnalsATS.201306-169ST
- Bausewein C, Schunk M, Schumacher P, et al. Breathlessness services as a new model of support for patients with respiratory disease. Chron Respir Dis. 2018;15(1):48–59. DOI:10.1177/1479972317721557
- Booth S, Ryan R, Spathis A. Service delivery of complex interventions for refractory breathlessness. Curr Opin Support Palliat Care. 2016;10(3):228–235. DOI:10.1097/SPC.0000000000000227
- Brighton LJ, Miller S, Farquhar M, et al. Holistic services for people with advanced disease and chronic breathlessness: A systematic review and meta-analysis. Thorax. 2019;74(3):270–281. DOI:10.1136/thoraxjnl-2018-211589
- Higginson IJ, Bausewein C, Reilly CC, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: A randomised controlled trial. Lancet Respir Med. 2014;2(12):979–987. DOI:10.1016/S2213-2600(14)70226-7
- Schunk M, Le L, Syunyaeva Z, et al. Effectiveness of a specialised breathlessness service for patients with advanced disease in Germany: A pragmatic fast track randomised controlled trial (BreathEase). ERJ. 2021;57(1):1–13.
- Farquhar MC, Prevost AT, McCrone P, et al. The clinical and cost effectiveness of a breathlessness intervention service for patients with advanced non-malignant disease and their informal carers: Mixed findings of a mixed method randomised controlled trial. Trials. 2016;17:185. DOI:10.1186/s13063-016-1304-6
- Elbehairy AF, McIsaac H, Hill E, et al. Impact of a specialized ambulatory clinic on refractory breathlessness in subjects with advanced COPD. Respir Care. 2020;65(4):444–454. DOI:10.4187/respcare.06950
- Connor S. Global atlas of palliative care at the end of life. 2nd ed. London: World Health Organization; 2020.
- Hawley P. Barriers to access to palliative care. Palliat Care. 2017;10:1–6.
- Iyer AS, Sullivan DR, Lindell KO, et al. The role of palliative care in COPD. Chest. 2021;161(5):250–1262.
- Schunemann HJ, Puhan M, Goldstein R, et al. Measurement properties and interpretability of the chronic respiratory disease questionnaire (CRQ). COPD. 2005;2(1):81–89. DOI:10.1081/copd-200050651
- Spathis A, Booth S, Moffat C, et al. The breathing, thinking, functioning clinical model: A proposal to facilitate evidence-based breathlessness management in chronic respiratory disease. NPJ Prim Care Respir Med. 2017;27(1):27.
- O’Donnell DE, Milne KM, James MD, et al. Dyspnea in COPD: New mechanistic insights and management implications. Adv Ther. 2020;37(1):41–60. DOI:10.1007/s12325-019-01128-9
- Fischer MJ, Scharloo M, Abbink JJ, et al. Participation and drop-out in pulmonary rehabilitation: A qualitative analysis of the patient’s perspective. Clin Rehabil. 2007;21(3):212–221. DOI:10.1177/0269215506070783
- Heins MJ, Spreeuwenberg P, Heijmans M. Leven met een longziekte in Nederland: cijfers en trends over de zorg- en leefsituatie van mensen met een longziekte 2018 [Living with a lung disease in the Netherlands: numbers and trends about the health care and environmental situation of people with a lung disease 2018]. Utrecht: Nivel 2019. Dutch.