Abstract
Approximately, half of COPD patients die from cardiovascular diseases. A prolongation of cardiac repolarization (measured as QTc interval) is associated with cardiovascular events or cardiovascular deaths in populations of older adults and COPD. One way to reduce the QTc could be to increase physical activity (PA). We investigated whether QTc can be reduced by an increase in PA in patients with severe COPD. This is a secondary outcome analysis from a randomized controlled trial investigating the effects of a 3 months pedometer based program to improve PA. 12-lead ECG was assessed at baseline and after 3 months. We measured PA using a validated triaxial accelerometer. Data were analyzed from 59 participants. Multiple regression modeling, including adjustment for baseline QTc, sex, QT prolonging medications, BMI, smoking status and FEV1%, showed no evidence for an association between an improvement of ≥15% PA and QTc reduction. A 15% improvement in PA according to step counts over 3 months seems not to reduce QTc interval by its MCID of 20 ms in patients with severe to very severe COPD.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) and cardiovascular disease (CVD) are among the top three diseases that cause the most deaths globally [Citation1]. Both diseases impose a large burden of morbidity and mortality [Citation2,Citation3]. Impaired lung function and thus COPD are associated with CVD [Citation4–8]. Cardiovascular mortality increases by 28% for every 10% decrease in Forced Expiratory Volume in 1 s (FEV1) [Citation9]. In general, half of COPD patients die from cardiovascular diseases [Citation10]. One method for early detection of cardiovascular pathologies is the surface electrocardiography (ECG) [Citation11,Citation12].
A prolongation of the heart rate corrected QT interval (QTc) generated from the ECG is associated with increased age [Citation13]. It is established that a prolonged QTc is associated with cardiovascular events and cardiovascular death in populations of older adults [Citation14–16]. Further, Armstrong et al. [Citation17] showed in a general population an association between lower lung function and longer QTc in men but not in women. In COPD patients, the evidence for an increased incidence of prolonged QTc is still arguable. Sievi et al. [Citation18], Tukek et al. [Citation19] and Yldiz et al. [Citation20] showed associations of COPD with prolongation of QTc. In contrast, Lahousse et al. [Citation21] and Zupanic et al. [Citation22] could not detect any difference in QTc between COPD patients and patients without COPD. One factor influencing these different results is certainly the different methods of calculation and measurement of QTc hampering the comparison. However, it remains recognized that QTc prolongation on an individual level should be prevented to reduce the cardiovascular risk.
Some studies have demonstrated a positive effect of increased physical activity (PA) on QTc in various populations [Citation23–25]. In COPD patients, it was shown that PA positively affects endothelial function, a determinant of CVD [Citation5,Citation26,Citation27].
To the best of our knowledge, there are no data on the effect of PA enhancement on QTc duration in patients with COPD.
To investigate if PA is a modifying factor of QTc in COPD we aimed to explore the effect of changes in daily PA on changes in QTc in patients with severe to very severe COPD.
Materials
Study subjects
This study addressed the analysis of a secondary outcome from a randomized controlled trial (RCT) investigating the effects of a combined PA counseling and pedometer-based feedback intervention compared to usual care in severe and very severe COPD. The intervention group received motivational support (Three telephone audits aimed at helping participants comply with the protocol), an activity diary with an individual step count goal and a pedometer. Individual step count goal was set at an increase of ≥ 15% from baseline since this improvement is reached after pulmonary rehabilitations [Citation28]. Further information is published elsewhere [Citation29]. 74 patients between 40 years or older with diagnosed severe and very severe COPD (i.e. FEV1 <50% pred.) according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD)-classification [Citation30] were assessed in the pulmonary outpatient clinic at the University Hospital Zurich, Switzerland between May 2017 and May 2020. Patients with diagnosed mental or physical disability precluding informed consent or compliance with the study protocol, experiencing an exacerbation of COPD within the last 6 weeks, attending pulmonary rehabilitation within the last 6 months and pregnant patients were not included.
Data from patients who had an available ECG and PA data at baseline and after 3 months were analyzed for this study.
The study was conducted in accordance with the declaration of Helsinki and all subjects provided written informed consent. The Ethics Committee of the Canton of Zurich approved the study (EK-ZH-NR: 2016-00151), and the study is registered on www.ClinicalTrials.gov, NCT03114241.
Method
Electrocardiography
On study visit days patients were asked to abstain from alcohol, tobacco, or caffeine. Care has been taken to ensure that the room temperature and lighting were set at the same level for all measurements. At the beginning of the ECG measurement, patients rested for 5 minutes in supine position. For all ECG recordings, a commercially available 12-lead ECG (AT 104 PC, Schiller-Reomed AG, Switzerland) was used and set at 25-mm/s paper speed and 10-mm/mV amplitude. The analysis of the ECG was performed with dedicated ECG analysis software (DatInf® Measure 2.1d, DatInf GmbH, Tübingen, Germany) by one investigator who was blinded to the patient’s data and randomization. Normally, the longest QT time is found in lead II, V2, V3, V5 or V6 [Citation31–34]. Each of the twelve leads were measured cursory. Subsequently the lead with the longest QT time was measured during three consecutive heart cycles. Measures of QT interval were determined as indicated in . Then the QT interval was converted to QTc interval using Bazett’s formula [Citation35].
Figure 1. The length of the QT interval was obtained by identifying the QRS onset and the point at which the downward slope of the T wave returns to baseline.
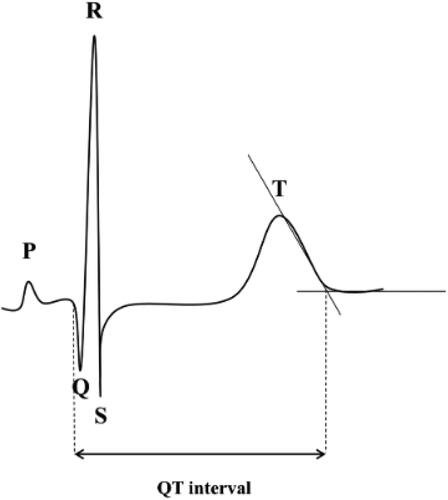
QT interval was defined as starting point at earliest onset of the QRS complex to the end of the T wave. The end point of the T wave was defined as the cutting point of the tangent to the downward slope of the T-wave and the isoelectric line [Citation36]. QTc prolongation was considered when the QTc interval was longer than 450 ms in males and 460 ms in females [Citation32,Citation37,Citation38].
Daily physical activity
PA was assessed by the number of steps per day. The steps were measured by a triaxial accelerometer of a multisensory activity monitor (SenseWear Pro™; Bodymedia Inc., Pittsburgh, PA, USA), validated for the use in COPD populations [Citation39]. The device was worn on the left upper arm at baseline and after three months for seven consecutive days each time. As a minimum, the device had to be worn for 4 days with a daily exposure time of at least 22.5 h for the data to be considered valid [Citation40].
Respiratory variables
All patients were examined with standard pulmonary function testing according to American Thoracic Society and European Respiratory Society (ATS/ERS) technical statement to measure forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) [Citation41].
Analysis
All results are shown as mean values and standard deviation (SD) unless otherwise stated. A two-sided p-value of < 0.05 was considered statistically significant. Analysis was performed using R version 4.0.3 (R Core Team 2020, R Foundation for Statistical Computing, Vienna, Austria). A multivariable regression modeling containing change in QTc between baseline and 3 months follow up as the dependent variable, and grouping variable (step improvement of ≥15% count compared to baseline vs. step improvement of <15% count) as the independent variable was performed. The model was adjusted for predefined, possible modifying factors such as age, baseline QTc, smoking status, body mass index (BMI), sex, FEV1% pred., and QT-prolonging medications (e.g. Antihistamines or psychotropic drugs; see crediblemeds.org). Residual analysis of the final model was performed to check the regression assumptions.
To detect a clinically relevant mean (SD) difference in QTc of 20 (15) ms [Citation42] with a two-sided significance level of 0.5 and a power of 80%, a sample size of 10 participants per group would be required.
Results
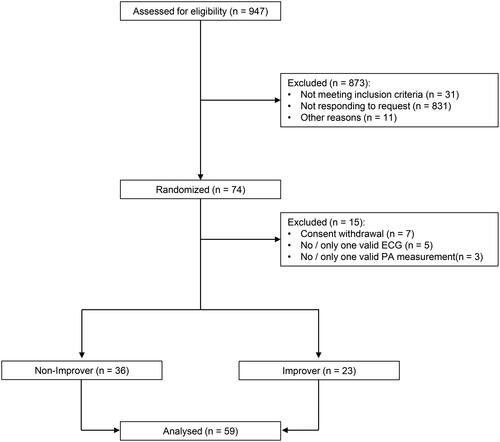
Out of the 74 COPD patients who were included in the original study 59 patients attended the 3-month follow- up with a valid ECG and a valid PA measurement and were included for analysis (). The time point after three months was chosen for follow-up because in the primary study the intervention lasted three months.
Figure 3. Boxplots showing QTc at baseline and 3 month follow-up visit in Non-Improver (difference from baseline to end of study -13 ms) and Improver (difference from baseline to end of study -27 ms), p-value for effect adjusted baseline QTc = 0.16. Data are mean (SD). Solid gray line: mean.
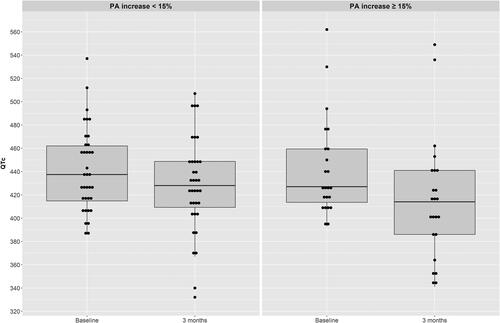
For the analysis, patients were grouped into improvers (IG = 23), who were able to increase PA by the number of steps at least by 15% after 3 months, and non-improvers (NG= 36), who did not achieve the desired 15% increase PA in steps. Mean (SD) age in the IG was 63 (10) and 68 (8) years in the NG. Both groups included predominantly males (NG: 69%, and IG: 70%). Physician prescribed drugs with a known prolonging effect on the QTc were taken by 13 (36%) patients in the NG and by 15 (65%) patients in the IG. Most frequent substance classes were antidepressants followed by proton pump inhibitors. The mean (SD) baseline QTc in the IG was 442 (42) ms and in the NG 441 (36) ms. Twenty-one patients of the NG and 10 of the IG showed a long QTc at baseline. Patients’ characteristics are shown in . After 3 months of intervention, QTc in the NG group decreased by 13 (39) ms to 428 (41) ms. In the IG group QTc decreased by 27 (33) ms to 416 (53) ms (). The mean group difference between NG and IG showed no evidence of a difference between the two groups (-14 ms, 95% CI: −33 to −6, p > 0.05) (). Correcting the QTc value for the baseline value, there was again no evidence of a difference between NG and IG in mean group difference (-15 ms, 95% CI: −33 to 6, p > 0.05). The further adjustment of possible effect modifiers (i.e. age, sex, FEV1% pred., current smoker, BMI, QTc prolonging drugs, and QTc at baseline) confirmed that there is no evidence for an influence of PA enhancement on QTc change (Coef. (95% CI) of 12 (7/33), p = 0.248).
Table 1. Patients’ characteristics.
Table 2. Changes of QTc (Barzett) stratified according to the course of PA.
Discussion
This study investigated the effect of enhancement in PA on changes in QTc interval in a sample of severe to very severe COPD patients. With an improvement of at least 15% steps over 3 months, there was no evidence of a clinically relevant and statistically significant reduction in QTc time after adjustment for known modifying factors. The difference in QTc between IG and NG was 14 ms, which does not correspond to clinically relevant reduction (MCID of 20 ms) [Citation42].
There are a number of large cross-sectional studies with divergent results on whether PA has an effect on QTc time. Ma et al. [Citation23] examined general Chinese population over the age of 35 years and showed that high PA reduced the risk of prolonged QTc. In addition, Michishita et al. [Citation43] found in their study including 586 older adults that a low PA level leads to a prolongation of the QTc in male by 57 ms and female by 47 ms. In contrast Zhang et al. [Citation44] examined 7795 random subjects with mean age of 56 years. They could not find any association between PA and QTc. All these mentioned cross-sectional studies assessed PA with a questionnaire. This way of collecting PA is time and money saving but often inaccurate compared to an accelerometer. In addition, also the way QTc was calculated and measured varied across the studies. Different correction formulas up to no corrections were used in the different studies. Some studies calculate means out of all leads. Others only take one specific lead or take the lead with the longest QT time. These major differences in study design, measurement of QTc, and measurement of PA between studies affect the incorporation of our results into the existing literature.
Furthermore, data from several sources found that the increased QTc is associated with obesity [Citation45], smoking status [Citation46,Citation47], age [Citation13], FEV1 [Citation17] and sex [Citation24,Citation25]. With respect to this knowledge, we have corrected our model for these variables and have not found any effect.
The main reason for this difference compared to our study is certainly the study design. All previous studies are cross-sectional studies.
There is an intervention study by Schuit et al. [Citation24] investigating the effect of PA enhancing in 229 healthy elderly. They showed a variation of QTc reduction according to sex. For women, mean QTc interval was significantly but not clinically relevant reduced by 7 ms in the group, which had trained three to four times a week over 6 months. In the male participants, training intervention of the study had no effect on QTc. In contrast, Current study showed no statistically significant and clinical relevant influence by sex on the association of QTc and PA improvement. This difference could be explained by differences in study population and intervention
There is one more study from Zupanic et al. [Citation22] that examined 18 participants with COPD, who did a rehabilitation program for 4 weeks. They found a decrease of QTc time by 6 ms after rehabilitation program. This is like in our study neither statistically significant nor clinically relevant. The difference to our study was that they only studied a period of 1 month, had a smaller sample and calculated the QTc using Framingham’s formula. But interestingly, they also found a difference in QTc time. In our study we looked at 3 months and found a difference in QTc time of 13 ms. The study from Kohlbrenner et al. (2020) is encouraging [Citation5]. It shows strong evidence that PA elevation improves endothelial function in COPD. With a longer intervention time or with a larger step improvement, one could perhaps also find evidence that PA improvement reduces QTc time in COPD.
This study has some limitations. We only examined patients with severe or very severe COPD. Our data are therefore not generalizable to the general COPD population. Further studies are needed to verify whether our observations are reproducible in advanced COPD.
Conclusion
In conclusion, at least a 15% improvement in PA according to step counts over 3 months has no clinical impact on the duration of QTc in patients with severe to very severe COPD. Future research should possibly increase the step improvement over 3 months or a longer time period, which is kept rather low in our study, to possibly see an effect on the QTc interval.
Declaration of interest
C.F. Clarenbach reports personal fees from Roche, Novartis, Boehringer Ingelheim, GSK, Astra Zeneca, Sanofi, Vifor, Mundipharma outside the submitted work.
Funding
This work was supported by Lunge Zurich.
References
- World Health Organization. Global health estimates. Disease Burden by Cause, Age, Sex, by Country and by Region; 2000–2019. Geneva: World Health Organization; 2020.
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–2128. DOI:10.1016/S0140-6736(12)61728-0
- Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1736–1788.
- Kurl S, Jae SY, Kauhanen J, et al. Impaired pulmonary function is a risk predictor for sudden cardiac death in men. Ann Med. 2015;47(5):381–385. DOI:10.3109/07853890.2015.1036111
- Kohlbrenner D, Clarenbach CF, Thiel S, et al. A few more steps lead to improvements in endothelial function in severe and very severe COPD. Respir Med. 2021;176:106246. DOI:10.1016/j.rmed.2020.106246
- Schünemann HJ, Dorn J, Grant BJ, et al. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the buffalo health study. Chest. 2000;118(3):656–664. DOI:10.1378/chest.118.3.656
- Persson C, Bengtsson C, Lapidus L, et al. Peak expiratory flow and risk of cardiovascular disease and death. A 12-year follow-up of participants in the population study of women in gothenburg, Sweden. Am J Epidemiol. 1986;124(6):942–948. DOI:10.1093/oxfordjournals.aje.a114483
- Rodman DM, Lowenstein SR, Rodman T. The electrocardiogram in chronic obstructive pulmonary disease. J Emerg Med. 1990;8(5):607–615. DOI:10.1016/0736-4679(90)90458-8
- Sin DD, Man SF. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc. 2005;2(1):8–11. DOI:10.1513/pats.200404-032MS
- Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128(4):2640–2646. DOI:10.1378/chest.128.4.2640
- Bednar MM, Harrigan EP, Anziano RJ, et al. The QT interval. Prog Cardiovasc Dis. 2001;43(5):1–45. DOI:10.1016/S0033-0620(01)00027-5
- Elming H, Brendorp B, Kober L, et al. QTc interval in the assessment of cardiac risk. Card Electrophysiol Rev. 2002;6(3):289–294.
- Rabkin SW, Cheng X-BJ, Thompson D. Detailed analysis of the impact of age on the QT interval. J Geriatr Cardiol. 2016;13(9):740.
- Straus SM, Kors JA, De Bruin ML, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47(2):362–367. DOI:10.1016/j.jacc.2005.08.067
- Schouten EG, Dekker JM, Meppelink P, et al. QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation. 1991;84(4):1516–1523. DOI:10.1161/01.cir.84.4.1516
- de Bruyne MC, Hoes AW, Kors JA, et al. Prolonged QT interval predicts cardiac and all-cause mortality in the elderly. Eur Heart J. 1999;20(4):278–284. DOI:10.1053/euhj.1998.1276
- Armstrong HF, Lovasi GS, Soliman EZ, et al. Lung function, percent emphysema, and QT duration: the multi-ethnic study of atherosclerosis (MESA) lung study. Respir Med. 2017;123:1–7. DOI:10.1016/j.rmed.2016.12.003
- Sievi NA, Clarenbach CF, Camen G, et al. High prevalence of altered cardiac repolarization in patients with COPD. BMC Pulm Med. 2014;14(1):55. DOI:10.1186/1471-2466-14-55
- Tükek T, Yildiz P, Atilgan D, et al. Effect of diurnal variability of heart rate on development of arrhythmia in patients with chronic obstructive pulmonary disease. Int J Cardiol. 2003;88(2-3):199–206. DOI:10.1016/S0167-5273(02)00402-3
- Yildiz P, Tükek T, Akkaya V, et al. Ventricular arrhythmias in patients with COPD are associated with QT dispersion. Chest. 2002;122(6):2055–2061. DOI:10.1378/chest.122.6.2055
- Lahousse L, Niemeijer MN, van den Berg ME, et al. Chronic obstructive pulmonary disease and sudden cardiac death: the rotterdam study. Eur Heart J. 2015;36(27):1754–1761. DOI:10.1093/eurheartj/ehv121
- Zupanic E, Zivanovic I, Kalisnik JM, et al. The effect of 4-week rehabilitation on heart rate variability and QTc interval in patients with chronic obstructive pulmonary disease. COPD. 2014;11(6):659–669. DOI:10.3109/15412555.2014.898046
- Ma Q, Li Z, Guo X, et al. Prevalence and risk factors of prolonged corrected QT interval in general chinese population. BMC Cardiovasc Disord. 2019;19(1):276. DOI:10.1186/s12872-019-1244-7
- Schuit AJ, Dekker JM, de Vegt F, et al. Effect of physical training on QTc interval in elderly people. J Electrocardiol. 1998;31(2):111–116. DOI:10.1016/S0022-0736(98)90041-3
- Genovesi S, Zaccaria D, Rossi E, et al. Effects of exercise training on heart rate and QT interval in healthy young individuals: are there gender differences? EP. Europace. 2007;9(1):55–60. DOI:10.1093/europace/eul145
- Clarenbach CF, Senn O, Sievi NA, et al. Determinants of endothelial function in patients with COPD. Eur Respir J. 2013;42(5):1194–1204. DOI:10.1183/09031936.00144612
- Bernardi E, Merlo C, Cogo A. Endothelial function in COPD Is in an intermediate position Between healthy subjects and coronary artery disease patients and Is related to physical activity. Lung. 2018;196(6):669–672. DOI:10.1007/s00408-018-0168-9
- Nolan CM, Maddocks M, Canavan JL, et al. Pedometer step count targets during pulmonary rehabilitation in chronic obstructive pulmonary disease. A randomized controlled trial. Am J Respir Crit Care Med. 2017;195(10):1344–1352. DOI:10.1164/rccm.201607-1372OC
- Kohlbrenner D, Sievi NA, Senn O, et al. Long-term effects of pedometer-based physical activity coaching in severe COPD: a randomized controlled trial. Int J Chron Obstruct Pulmon Dis. 2020;15:2837–2846. DOI:10.2147/COPD.S279293
- Halpin DM, Criner GJ, Papi A, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease Am J Respir Crit Care Med 2021;203(1):24–36.
- Asensio E, Acunzo R, Uribe W, et al. Recommendations for the measurement of the QT interval during the use of drugs for COVID-19 infection treatment. Updatable in accordance with the availability of new evidence. J Interv Card Electrophysiol. 2020;59(2):315–320. DOI:10.1007/s10840-020-00765-3
- Rautaharju PM, Surawicz B, Gettes LS, Heart Rhythm Society, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American heart association electrocardiography and arrhythmias committee, council on clinical cardiology; the American college of cardiology foundation; and the heart rhythm society. Endorsed by the international society for computerized electrocardiology. J Am Coll Cardiol. 2009;53(11):982–991. DOI:10.1016/j.jacc.2008.12.014
- Lepeschkin E, Surawicz BJC. The measurement of the QT interval of the electrocardiogram. Circulation 1952;6(3):378–388. DOI:10.1161/01.cir.6.3.378
- Castiglione A, Odening K. [QT interval and its Prolongation - What does It mean?]. Dtsch Med Wochenschr. 2020;145(8):536–542. DOI:10.1055/a-0969-6312
- Bazett HC. An analysis of the time relations of electrocardiograms. Heart. 1920;7:353–370.
- Pickham D, Hasanien AA. Measurement and rate correction of the QT interval. AACN Adv Crit Care. 2013;24(1):90–96. DOI:10.4037/NCI.0b013e318274ba3e
- Merri M, Benhorin J, Alberti M, et al. Electrocardiographic quantitation of ventricular repolarization. Circulation. 1989;80(5):1301–1308. DOI:10.1161/01.cir.80.5.1301
- Johnson JN, Ackerman MJ. QTc: how long is too long? Br J Sports Med. 2009;43(9):657–662. DOI:10.1136/bjsm.2008.054734
- Van Remoortel H, Raste Y, Louvaris Z, PROactive consortium, et al. Validity of six activity monitors in chronic obstructive pulmonary disease: a comparison with indirect calorimetry. PLoS One. 2012;7(6):e39198. DOI:10.1371/journal.pone.0039198
- Watz H, Pitta F, Rochester CL, et al. An official european respiratory society statement on physical activity in COPD. Eur Respir J. 2014;44(6):1521–1537. DOI:10.1183/09031936.00046814
- Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. Am J Respir Crit Care Med. 2019;200(8):e70–e88. DOI:10.1164/rccm.201908-1590ST
- Rossi VA, Stoewhas AC, Camen G, et al. The effects of continuous positive airway pressure therapy withdrawal on cardiac repolarization: data from a randomized controlled trial. Eur Heart J. 2012;33(17):2206–2212. DOI:10.1093/eurheartj/ehs073
- Michishita R, Fukae C, Mihara R, Nakagawa Study Group, et al. Association between the physical activity and heart rate corrected-QT interval in older adults. Geriatr Gerontol Int. 2015;15(7):895–901. 10.1111/ggi.12365.
- Zhang Y, Post WS, Dalal D, et al. Coffee, alcohol, smoking, physical activity and QT interval duration: Results from the third national health and nutrition examination survey. PLoS One. 2011;6(2):e17584. DOI:10.1371/journal.pone.0017584
- Waheed S, Dawn B, Gupta K. Association of corrected QT interval with body mass index, and the impact of this association on mortality: results from the third national health and nutrition examination survey. Obes Res Clin Pract. 2017;11(4):426–434. DOI:10.1016/j.orcp.2016.09.005
- Dilaveris P, Pantazis A, Gialafos E, et al. The effects of cigarette smoking on the heterogeneity of ventricular repolarization. Am Heart J. 2001;142(5):833–837. DOI:10.1067/mhj.2001.118737
- Fauchier L, Maison-Blanche P, Forhan A, et al. Association between heart rate–corrected QT interval and coronary risk factors in 2,894 healthy subjects (the DESIR study). Am J Cardiol. 2000;86(5):557–559. DOI:10.1016/s0002-9149(00)01015-8