?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We aimed to explore the role of antithrombin III (AT-III) activity in diagnosing patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) and chronic bronchitis, and its relationship with all-cause mortality of AECOPD patients. We performed univariate and multivariate Cox regression analyses of the factors determining all-cause mortality. We recruited 279 patients with AECOPD and 91 with chronic bronchitis. On admission, patients with AECOPD had lower AT-III activity (80.7 vs. 86.35%, p = 0.002) and higher neutrophil percentages (70.12 vs. 66.40%, p = 0.02) than those with chronic bronchitis. The patients who died were older (78 vs. 73 years, p < 0.001); had higher CRP (39.05 vs. 5.65 mg/L, p < 0.001), D-dimer (1.72 vs. 0.46 mg/L, p < 0.001), FIB (3.56 vs. 3.05 g/L, p = 0.01) levels; and exhibited lower AT-III activity (71.29 vs. 82.94%, p < 0.001) than the survivors. The AT-III area under the receiver operating characteristic curve for predicting COPD all-cause mortality was 0.75 (p < 0.001), optimal cutoff point 79.75%, sensitivity 86.8%, and specificity 57.1%. Multivariate Cox regression analyses showed that increased levels of CRP (HR = 1.005, p = 0.02), D-dimer (HR = 1.17, p = 0.01), WBC count (HR = 1.11, p = 0.002), and reduced AT-III activity (HR = 0.97, p = 0.02) were independent prognostic factors for all-cause mortality. Patients with AT-III ≤ 79.75% were 4.52 times (p = 0.001) more likely to die than those with AT-III > 79.75%. AT-III activity was lower in patients with AECOPD than in those with chronic bronchitis and is potentially useful as an independent predictor of all-cause mortality in patients with AECOPD: reduced AT-III activity and increased CRP and D-dimer levels indicate a higher risk of all-cause mortality.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable, heterogeneous disease characterized by irreversible airway obstruction. COPD is of worldwide concern because of its high prevalence, morbidity, and mortality, posing a severe challenge to healthcare systems [Citation1]. As COPD progresses, severe and very severe patients often undergo repeated hospitalizations due to deterioration and comorbidities, eventually developing respiratory failure; many people die prematurely from it or its complications, which severely affect quality of life and increase health care-related costs [Citation2].
COPD is characterized by both local and systemic inflammation. Patients with COPD have also been reported to experience coagulation system abnormalities [Citation3], particularly those with acute exacerbation. Activation of the coagulation system contributes to the development of concurrent diseases. This potentially results in adverse outcomes (readmission or death). Elevated fibrinogen levels are associated with greater deterioration, faster decline in lung function, and higher mortality [Citation4–6]. Other clotting-system activation biomarkers, including D-dimer, prothrombin, and the thrombin–antithrombin (TAT) complex, have also been associated with increased COPD mortality [Citation5,Citation7–9]. Furthermore, certain studies have demonstrated that antithrombin III (AT-III) activity is considerably decreased in COPD [Citation10,Citation11]. AT-III is the primary physiological inhibitor affecting thrombin’s coagulation activity [Citation12]. It combines with thrombin to form a TAT complex, whose purpose is to prevent and mediate clot formation, thus preventing thrombosis and vascular inflammation that may lead to thrombosis [Citation13]. AT also possesses anti-inflammatory properties [Citation14]. According to research, it can reduce acute inflammation and diffuse intravascular coagulation caused by ischemia/reperfusion injury, endotoxicosis, and sepsis [Citation12,Citation15,Citation16]. AT-III is correlated with the severity of coronary stenosis in patients with acute coronary syndrome [Citation17] and is also associated with the severity and survival of patients with sepsis, as AT-III potentially inhibits thrombin when microcirculation vessels are damaged during infection [Citation18], but not independently associated with hospital mortality among critically ill patients with suspected sepsis [Citation19]. A meta-analysis showed no statistically significant effect of AT-III on mortality of critical illness patients with a total of 3882 participants (RR = 0.95 (0.88 − 1.03), I2 statistic = 0%), and the included trials were mostly small [Citation20]. However, the role of AT-III in COPD remains under explored; therefore, we aimed to explore the role of AT-III activity in the diagnosis of AECOPD and its association with post-admission mortality in patients with AECOPD.
Materials and methods
Study design and patients
All study participants signed informed consent forms, and the Institutional Review Board of Liyuan Hospital, Tongji Medical College, Huazhong University of Science and Technology, approved the study design(IRB ID: [2021] IEC [A014]). Moreover, patient data were anonymized during the study, and the study adhered to the principles of the Declaration of Helsinki.
The StatBox software suite was used to calculate the sample size required at a significance level of α = 0.05, test efficiency of 1-β = 0.85, and ratio of 3:1. The minimum sample size was 264 patients in the experimental group and 88 in the control group [Citation21]. We collected the data of patients hospitalized with AECOPD (N = 279) between January 1, 2017, and January 1, 2021, and compared them with the data of age- and sex-matched patients experiencing acute episodes of chronic bronchitis (N = 91) during the same period at a ratio of 3:1.
The inclusion criteria were as follows: all patients >40 years of age with no history of asthma prior to this age and presence of chronic bronchitis, defined as the presence of chronic cough and phlegm for at least 3 months each year for 2 consecutive years, and FEV1/FVC 70%. An acute attack of chronic bronchitis was defined as experiencing an exacerbation of the respiratory symptoms mentioned above, changes in sputum volume, or color, and requirement for additional antibiotics.The criterion used to define COPD was a post-bronchodilator FEV1/FVC <70%.
The exclusion criteria were as follows: (1)other respiratory diseases requiring intervention, e.g. asthma, tuberculosis, bronchiectasis, pneumoconiosis, and other restrictive ventilation dysfunction; (2)patients with severe heart, liver, and kidney diseases or in the acute stage of such diseases as well as those with malignant tumors; (3)acute onset of allergic diseases; and (4)diseases or drugs affecting coagulation, e.g. atrial fibrillation, acute cerebrovascular accident, thrombosis, and anticoagulant drugs. Our endpoint was all-cause mortality between the first day of admission to the 1 year of follow-up. One-year mortality in AECOPD patients was defined as death from all causes within 1 year after discharge.
The patients with frequent exacerbations were defined as those with COPD who had experienced at least two or more exacerbations in the previous year. AECOPD was defined as acute changes in the respiratory symptoms of a patient such as dyspnea; changes in sputum production, volume, or color; requirement for additional antibiotic or systemic steroid treatments or hospitalization [Citation22]. This definition was followed by the the hospital service staff. Our study did not include patients with AECOPD hospitalized for COVID-19 because a real-time reverse transcription polymerase chain reaction was used for qualitative detection of SARS-CoV-2 RNA in nasopharyngeal swabs prior to admission in COPD patients requiring hospitalization. Only COVID-19-negative patients were admitted to our hospital, while positive patients were admitted to the specialized infectious disease facility for isolation treatment.
Data acquisition
Data regarding patients diagnosed with AECOPD and chronic bronchitis between January 1, 2017, and January 1, 2021, were collected from our hospital. Data collected from the patients’ electronic medical records included age, sex, disease classification, laboratory findings, and lung function. Blood samples were collected from patients on the day they were admitted to the hospital and before they received any medication to determine D-dimer levels, fibrin degradation products (FDP), fibrinogen (FIB), c-reactive protein (CRP), AT-III, red blood cell counts, white blood cell (WBC) counts, platelet counts, neutrophil counts, neutrophil percentages, lymphocyte counts, lymphocyte percentages, hemoglobin levels, and blood gases. All laboratory parameters were assessed at the hospital laboratory. Readmission and death in patients with AECOPD at 4 weeks, 3 months, 6 months, and 12 months after discharge were recorded.
The Pro-BNP level was assessed using the Elecsys proBNP II detection kit (electrochemiluminescence method, Roche Diagnostics Products GmbH, Mannheim, Germany). The AT-III level was assessed using the Berichrom Antithrombin III(A) test kit, (Hair color substrate method, Simens Healthcare Diagnostics Products GmbH, Marburg, Germany) (the reference range is 80%–120%). The FIB level was determined using the Dade Thrombin Reagent test kit (Clauss method, Simens Healthcare Diagnostics Products GmbH), and the reference range is 1.8–3.5 g/L. The D-dimer level was determined using the INNOVANCE® D-Dimer test kit (immunoturbidimetry method, Simens Healthcare Diagnostics Products GmbH), and the reference range is 0–0.55 mg/L. The CRP level was quantified by latex immunoturbidimetry (reference range, 0–6.0 mg/L). The levels of FDP were measured using the Latex Test BL-2-P-FDP test kit (immunoturbidimetry method, BIOLINKS CO., LTD, Yokohama-shi Kanagawa, Japan) (the reference is less than 0.5 ug/mL), and the solutions were prepared according to the manufacturer’s instructions.
Statistical analysis
The single-sample Kolmogorov–Smirnov test was used to test the normal distribution of continuous variables. Normally distributed continuous variables are expressed as the mean ± standard deviation. Non-normally distributed variables are represented as the median (quartile range). Categorical variables are expressed as percentages. Normally distributed data were analyzed using ANOVA and the chi-square (χ2) test, and the nonparametric Mann–Whitney U test was used for the statistical analysis of non-normally distributed data. Categorical variables were analyzed using the χ2 test or Fisher’s exact test.
In addition, we developed a receiver operating characteristic (ROC) curve to assess the value of the newly measured AT-III in predicting mortality. The Youden index [Citation23] was used to determine the optimal cutoff for AT-III that can predict all-cause mortality with maximum sensitivity and specificity. Univariate Cox regression analysis was used to identify variables associated with all-cause mortality. In addition, multivariate Cox regression analysis was used to explain the contribution of AT-III in controlling confounding factors (p ≤ 0.1 in univariate Cox regression). Kaplan–Meier estimation was used to compare mortality between the AT-III groups with and without reduction. Survival curves were compared using a logarithmic rank test. All analyses were performed using the Statistical Products and Services Solutions (SPSS) software (version 22.0; IBM® SPSS® Statistics Inc., Armonk, NY, USA). Two-sided P-values < 0.05 were considered statistically significant.
Results
Patient characteristics
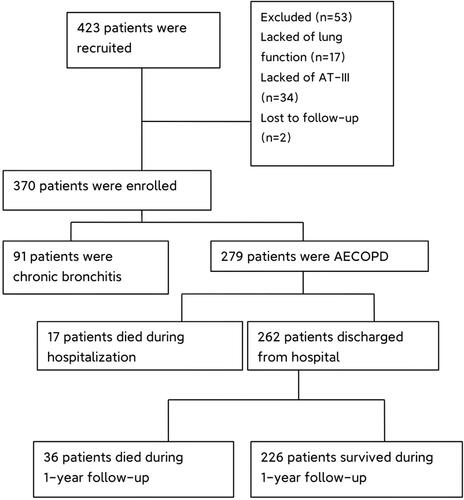
A total of 423 patients were recruited for this study; 91 had acute attacks of chronic bronchitis, while 279 were diagnosed with AECOPD and followed up (). Our results () illustrate that patients with COPD exhibited higher rates of neutrophil percentage (70.12 vs. 66.40%, p = 0.02), as well as lower AT-III activity (80.70 vs. 86.35%, p = 0.002) and lymphocyte percentages (19.39 vs. 23.40%, p = 0.002) than those with chronic bronchitis. Additionally, patients of COPD were more likely to smoke (55.20% vs. 36.70%, p = 0.002), while current smokers were not statistically different between the two groups (26.20% vs. 21.10%, p = 0.41).
Table 1. Baseline characteristics of AECOPD and chronic bronchitis patients.
To further understand the factors affecting all-cause mortality of patients with AECOPD, we divided the 279 patients with COPD into two groups (survivors and non-survivors) according to whether they had died or not. The demographic and baseline clinical characteristics of the patients in both groups are shown in . Seventeen patients died during hospitalization, nine (52.94%) died from respiratory failure complicated by severe pneumonia or septic shock, and eight (47.06%) died from respiratory failure. And 36 patients died during follow-up, including two (5.55%) who died of the novel coronavirus pneumonia, two (5.55%) of deep venous thrombosis (DVT), two (5.55%) of acute myocardial infarction, six (16.67%) of sepsis, five (13.89%) of respiratory failure with acute heart failure and nineteen (52.78%) of respiratory-related diseases. Patients who died were older (78 vs. 73 years, p < 0.001); had a higher rate of exacerbations (during preceding year ≥ two occasions; 67.90% vs. 18.60%, p < 0.001) and chronic heart failure (CHF) (26.40% vs. 9.30%,p = 0.001); experienced a more severe inflammatory response, had higher WBC counts (8.58 vs. 6.40 × 109/L, p = 0.02), neutrophil percentages (76.61 vs. 68.72%, p < 0.001) and CRP (39.05 vs. 5.65 mg/L, p < 0.001), exhibited higher levels of D–dimer (1.72 vs. 0.46 mg/L, p < 0.001), FDP(7.11 vs. 2.18 ug/mL, p < 0.001), FIB (3.56 vs. 3.05 g/L, p = 0.01), Pa(CO2) (44.90 vs. 39.70 mmHg, p = 0.02); and had lower AT-III activity (71.29 vs. 82.94%, p < 0.001) and lymphocyte percentages (13.71 vs. 20.62%, p < 0.001) than survivors. The prothrombin time (PT) (12.40 vs. 11.90 s, p = 0.02) of dead patients were longer than that of survivor patients, but within the normal reference range. There were no statistical differences in sex, Pa (O2), PLT, history of CHD, hypertension and diabetes between the two groups.
Table 2. The demographic and baseline clinical characteristics of Non-survivors group and survivors group.
presents the demographic and baseline clinical characteristics of hospitalized deaths and deaths within 1-year. Patients who died in hospital experienced a more severe inflammatory response, had higher WBC counts (9.99 vs. 6.19 × 109/L,p = 0.003) and neutrophil percentages(85.00 vs. 74.80%, p = 0.03), and D- dimer (2.06 vs. 1.22 mg/L,p = 0.03) than those who died within 1-year; however, there were no statistical differences in age, sex, smoking, lung function, pH, Pa(CO2), Pa(O2), CRP, AT-III, coagulation function, and history of deterioration, CHF, CHD and diabetes.
Table 3. The demographic and baseline clinical characteristics of 1-year of deaths group and in-hospital deaths group.
Comparisons of at-III activity, D-dimer, CRP, and FIB in COPD all-cause mortality
Spearman correlation testing demonstrated that all-cause mortality was significantly positively correlated with D-dimer (r = 0.40, p < 0.001), CRP (r = 0.25, p < 0.001), FDP (r = 0.39, p < 0.001), FIB(r = 0.15, p = 0.01), and age (r = 0.19, p = 0.001) and negatively correlated with AT-III (r=–0.33, p < 0.001).
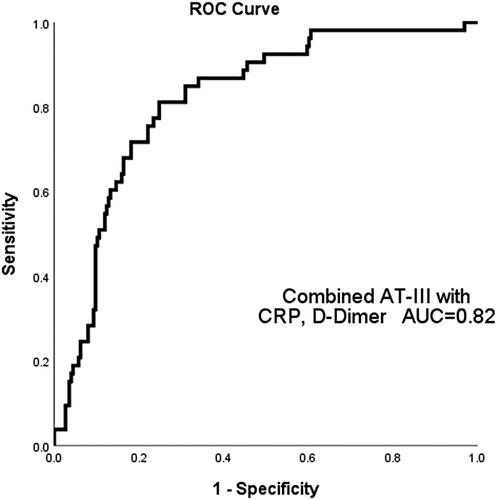
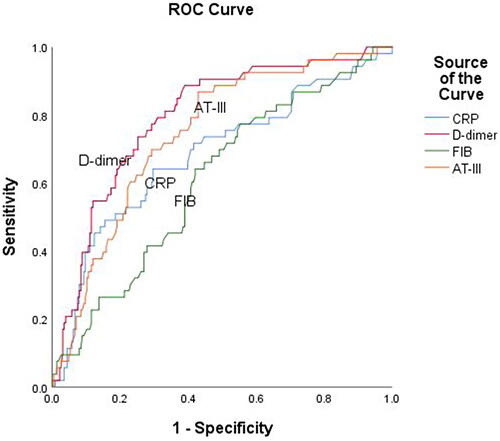
We performed ROC curve analysis () to determine the efficacy of AT-III, D-dimer, CRP, and FIB in predicting all-cause mortality. The analysis yielded the following area under the curve (AUC) values: 0.61 (95% confidence interval [CI]: 0.53–0.69, p = 0.01) for FIB, 0.75 (95% CI: 0.68–0.81, p < 0.001) for AT-III, 0.68 (95% CI: 0.60–0.77, p < 0.001) for CRP, and 0.79 (95% CI: 0.73–0.86, p < 0.001) for D-dimer. According to the ROC curves, the optimal AT-III cutoff point for predicting all-cause mortality was 79.75%, sensitivity 86.8%, and specificity 57.1%, respectively. When using a combined marker approach involving AT-III, CRP, and D-dimer, an AUC of 0.82 was observed (95% CI: 0.76–0.88, p < 0.001), at a sensitivity of 81.10% and specificity of 75.00% ().
Figure 2. ROC curve analysis to determine the efficacy of AT-III, D-dimer, CRP, and FIB in predicting all-cause mortality of AECOPD patients. The area under ROC curve (AUC) of AT-III was 0.75 (95% CI: 0.68–0.81, p < 0.001), FIB was 0.61 (95% CI: 0.53–0.69, p = 0.01), CRP was 0.68 (95% CI: 0.60–0.77, p < 0.001) and D-dimer was 0.79 (95% CI: 0.73–0.86, p < 0.001).
Factors linked to all-cause mortality
Univariate Cox regression analysis was performed for each variable to determine the independent factors for total mortality (). We found that P-values associated with age (hazard ratio [HR] = 1.57, 95% CI: 1.16-2.13, p = 0.004), frequent exacerbations (HR = 6.74,95% CI: 3.78-12.01, p < 0.001), current smoker (HR = 0.41, 95% CI: 0.18-0.92, p = 0.03), CHF(HR = 3.30, 95% CI: 1.79-6.08, p < 0.003), COPD stage (HR = 1.68, 95% CI:1.21-2.34, p = 0.002), PH < 7.35 (HR = 3.46, 95% CI: 1.71-7.00, p = 0.001), PaCO2≥50mmHg (HR= 2.72, 95% CI:1.57-4.72, p < 0.001), PaO2<60 mmHg (HR = 2.51, 95% CI: 1.13-5.56, p = 0.02), WBC (HR = 1.11, 95% CI: 1.04-1.18, p = 0.001), CRP(HR = 1.006, 95% CI: 1.003-1.009, p < 0.001), D-dimer (HR = 1.23, 95% CI: 1.14-1.34, p < 0.001), and AT-III (HR = 0.94, 95% CI: 0.91-0.96, p < 0.001) were less than 0.1. Multivariate Cox regression analysis ()was performed to control for other potential confounding variables, and it showed that frequent exacerbations(HR = 5.06, 95% CI: 2.60-9.83, p < 0.001), CRP(HR = 1.005, 95% CI: 1.001-1.009, p = 0.02), D-dimer (HR = 1.17, 95% CI: 1.04-1.31, p = 0.01), WBC count (HR= 1.11, 95% CI: 1.04-1.19, p = 0.002), and AT-III (HR= 0.97, 95% CI: 0.95-0.995, p = 0.02) were significantly associated with all-cause mortality. AT-III ≤ 79.75% with all-cause mortality () revealed that patients with AT-III ≤ 79.75% were 4.52 times(95% CI: 1.80–11.34, p = 0.001) more likely to die than those with AT-III > 79.75%, after adjusting for history of frequent exacerbations, age, current smoker, CHF, COPD stage, PH < 7.35, Pa(CO2), Pa(O2), CRP, WBC and D-dimer. Kaplan–Meier estimation revealed a difference in all-cause mortality between the AT-III ≤79.75% and >79.75% groups. Patient mean survival time was 356.68 ± 4.18 (95I% CI: 348.49-364.88) and 292.72 ± 11.11 (95I% CI: 269.94-313.51) days in the AT-III > 79.75% and AT-III ≤ 79.75% groups, respectively. The log-rank test showed that the two survival curves were significantly different, and the survival rate of the AT-III > 79.75% group was higher than that of the AT-III ≤79.75% group ().
Figure 4. Kaplan–Meier survival curves evaluating the time to death in days for patients with AT-III >79.75% and AT-III ≤ 79.75%.
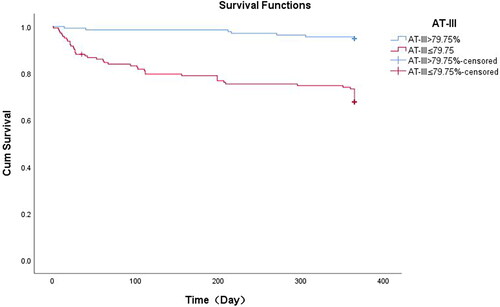
Table 4. Univariate and multivariatecox regression analyses of factors for all-causemortality.
Table 5. Univariate and multivariatecox regression analyses of factors for all-causemortality.
Factors associated with mortality during hospitalization
We assessed the factors associated with in-hospital mortality using all available baseline variables from the entire cohort in univariate Cox regression analysis. Table S1 shows that frequent exacerbations (p = 0.006), COPD stage(p = 0.05), PH <7.35(p < 0.001), Pa(CO2)≥50 mmHg (p = 0.01), Pa(O2)<60 mmHg (p = 0.06), increased WBC counts (p < 0.001), D-dimer (p < 0.001), CRP(p = 0.001), and AT-III ≤ 79.75% (p = 0.007) were risk factors for death during hospitalization. Multiple logistic regression analysis revealed that after controlling for related covariates AT-III ≤ 79.75% (HR = 10.46, 95% CI: 1.25-87.89, p = 0.03), WBC (HR = 1.21, 95% CI: 1.08-1.35, p = 0.001), D-dimer level (HR = 1.24, 95% CI: 1.04-1.49, p = 0.02) and PH < 7.35 (HR = 5.11, 95% CI: 1.02-25.65, p = 0.047)were independent factors associated with in-hospital mortality (Table S1).
Factors linked to mortality at one-year follow-up
We found that age(HR= 1.90, 95% CI: 1.28-2.80, p = 0.001), history of frequent exacerbations(HR = 8.95, 95% CI: 4.31-18.57, p = 0.001), Current smoker(HR = 0.38, 95% CI: 0.13-1.12, p = 0.08), CHF (HR = 2.50, 95% CI: 1.09-5.70, p = 0.03), COPD stage (HR = 1.62, 95% CI: 1.09-2.43, p = 0.02), D-dimer(HR = 1.17, 95% CI: 1.05-1.32, p = 0.006), CRP (HR = 1.005, 95% CI: 1.001-1.01, p = 0.02)and AT-III ≤ 79.75%(HR = 5.98, 95% CI: 2.49-14.37, p = 0.001) were associated with P-values ≤0.1 in univariate Cox regression analysis. Multinomial Cox regression analysis revealed that age (HR = 1.72, 95% CI: 1.08-2.74, p = 0.02), history of frequent exacerbations (HR = 5.89, 95% CI: 2.61-13.29, p < 0.001), CRP (HR = 1.006, 95% CI: 1.001-1.01, p = 0.01) and AT-III ≤ 79.75% (HR = 2.68, 95% CI: 1.07-6.72, p = 0.036) were independent factors associated with 1-year mortality (Table S2).
Discussion
Chronic bronchitis and COPD are complex conditions in which the airways and alveoli become abnormal, predominantly owing to high exposure to toxic particles or gases. Chronic bronchitis reflects the stage preceding the development of COPD. Not all patients with chronic bronchitis progress to COPD, possibly due to a specific predisposition determined by genetic factors or other risk factors [Citation24]. Chronic infiltration of various inflammatory cells in patients with COPD leads to structural changes in airway epithelial, stromal and parenchymal cells [Citation25], which are more serious than those in patients with chronic bronchitis. Given that the acute onset of clinical symptoms in chronic bronchitis and COPD is very similar, distinguishing the two groups of patients is challenging and mainly relies on lung function and imaging examinations. Therefore, we set out to compare the differences in the levels of circulatory biomarkers between the two groups, to determine if any could be used to guide early intervention. In our study, patients with AECOPD exhibited lower AT-III activity (80.70 ± 14.89 vs. 86.35 ± 16.07%, p = 0.002) and were more likely to smoke (55.20% vs. 36.70%, p = 0.002) than those with chronic bronchitis; nonetheless, no statistical differences in age, sex, comorbidities, WBC count and CRP, FDP, and FIB levels were identified. In addition, in patients who were smokers, there was no statistically significant difference in AT-III levels between patients with chronic bronchitis and those with AECOPD (80.36 ± 13.87 vs. 84.38 ± 17.39%, p = 0.15). This suggests that AT-III may be a potential blood biomarker for early diagnosis or differentiation of the two pathologies, regardless of smoking status. Additionally, the decrease in the AT-III level on the first day of admission was not associated with the severity of AECOPD and smoking status. The median levels of AT-III in mild, moderate, severe, and very severe AECOPD were 84.50 ± 12.49%, 82.63 ± 15.16%, 78.56 ± 15.11%, and 78.80 ± 14.11%, respectively (p = 0.09) and in smokers and nonsmokers of AECOPD were 80.25 ± 16.01 and 81.34 ± 13.88%, p = 0.54.
Some studies have shown that stable patients with COPD may also exhibit a degree of coagulation system activation [Citation7]. High circulating D-dimer levels have been associated with stable COPD [Citation7,Citation26]. Husebø [Citation7] and colleagues confirmed that elevated D-dimer levels in the stable state predict mortality in patients with COPD compared with those in the healthy control group. Furthermore, D-dimer [Citation7,Citation26] and TAT [Citation7] levels were lower in stable patients with COPD than in patients with AECOPD. Husebø and colleagues considered TAT to represent the “bad thrombin” captured by AT in the process of tissue injury; thus, a decrease in AT maybe related to the consumption of TAT formation. However, we did not compare stable patients with COPD in our study; therefore, we could not determine whether the reduction in baseline AT-III levels in patients with AECOPD was due to chronically low levels caused by the disease itself or the reduced consumption of acute episodes.
AT-III possesses anti-inflammatory properties, predominantly related to its inhibition of neutrophil activity [Citation27]. Bergantini and colleagues reported that in patients with idiopathic pulmonary fibrosis, the association between AT-III activity and neutrophils is independent of coagulation cascade regulation. because AT-III interacts with cellular inflammatory mediators produced primarily by neutrophils [Citation28]. Zuo and colleagues reported that recombinant human AT-III therapy could reduce nitrosative stress, airway obstruction, and pulmonary parenchymal edema in an animal model of smoke-mediated acute lung injury based on AT-III’s anti-inflammatory effect of inhibiting neutrophil activation [Citation12]. In addition, AT-III plays a role in the pathogenesis of burn and smoke inhalation lung injuries. Following such injuries, patients develop a hypercoagulable state, and the concentration of AT-III decreases to 50% in the first 24 h [Citation29]. The same result was obtained in our study, as AT-III was negatively correlated with the percentage of neutrophils (r=-0.13, p = 0.03). In addition, AT-III was negatively correlated with D-dimer (r=-0.26, p < 0.001) and FDP levels (r=-0.25, p < 0.001), suggesting that the reduction of AT-III activity may be mainly related to the interaction of cellular inflammatory mediators produced by neutrophils and the hypercoagulable state in vivo.
Strong evidence has been generated suggesting that COPD is not only a disease confined to the lungs but also involves inflammatory mechanisms that affect the whole body. CRP is often used as a marker of systemic inflammatory responses in patients with AECOPD [Citation30]. The inclusion of 15 systematic analyses of late mortality in patients with COPD revealed that increased baseline CRP levels were significantly associated with higher mortality (HR 1.53, 95% CI: 1.32–1.77, I2=68.7%, p < 0.001) [Citation31]. Patients with COPD reportedly exhibit higher circulating levels of coagulation markers, including FIB [Citation4,Citation32], TAT [Citation7,Citation9], prothrombin [Citation33], and D-dimer [Citation34]. D-dimer is a downstream product of coagulation system activation. It is generally considered a hypercoagulable state in the body; therefore, it is widely used in clinical practice as a marker of pulmonary embolism. Elevated circulating D-dimer levels are often associated with mortality in patients with AECOPD [Citation8,Citation34]. Another plasma biomarker associated with mortality in patients with AECOPD is fibrinogen, an acute phase reactive protein derived primarily from the liver and a marker of systemic inflammatory response in the body. Concurrent elevated fibrinogen levels have also been reported to be associated with a faster decline in lung function and frequent deterioration in patients with COPD [Citation4,Citation6,Citation32]. Consistent with these observations, we found higher plasma levels of D-dimer, FIB, WBC, and CRP as well as lower AT-III activity in patients who died than in those who survived. However, in our study, for every 1-mg/L and 1 × 109/L increase in CRP level and WBC count, patients exhibited 1.005-fold (p = 0.009) and 1.11-fold (p = 0.002) increases in all-cause mortality, respectively, compared with survivors, and for every 1-mg/L increase in D-dimer level, patients exhibited a 1.13-fold increase in all-cause mortality (p = 0.04). In addition, we also found that AT-III (r=–0.33, p < 0.001) was negatively associated with all-cause mortality and had a favorable predictive value (AUC = 0.75, (95% CI: 0.68–0.81, p < 0.001), higher to CRP 0.68 (95% CI: 0.60–0.77, p < 0.001) and FIB 0.61 (95% CI: 0.53–0.69, p = 0.01); further, patients with AT-III ≤ 79.75% were 4.52 times (p = 0.001) more likely to die than those with AT-III > 79.75% after controlling for history of frequent exacerbations, age, current smoker, CHF, COPD stage, PH, Pa(CO2), Pa(O2), CRP, D-dimer, and WBC. AT-III, CRP, and D-dimer levels were robust, independent predictors of all-cause mortality. As such, we used a combined markers approach involving AT-III, CRP, and D-dimer levels. Compared with the results for AT-III only, although the AUC value and sensitivity did not change significantly, the specificity increased from 57.1% to 75.00% in the combined approach. These findings suggest that AECOPD patients with reduced AT-III activity and increased CRP and D-dimer levels have a higher risk of all-cause mortality.
We found that patients with PH < 7.35 (HR = 5.11, 95% CI: 1.02-25.65, p = 0.047), increased WBC (HR = 1.21, 95% CI: 1.08-1.35, p = 0.001) and D-dimer (HR = 1.24, 95% CI: 1.04-1.49, p = 0.02), and AT-III ≤ 79.75% (HR = 10.46, 95% CI: 1.25-87.89, p = 0.03) were more likely to die during hospitalization. However, the patients who died at one year of follow-up were more associated with age(HR = 1.72, 95% CI: 1.08-2.74, p = 0.02), history of frequent exacerbations (HR = 5.89, 95% CI: 2.61-13.29, p < 0.001), increased CRP (HR = 1.006, 95% CI: 1.001-1.01, p = 0.01), and AT-III ≤ 79.75% (HR = 2.68, 95% CI: 1.07-6.72, p = 0.036). In our study, 52.94%% of in-hospital deaths from respiratory failure complicated by severe pneumonia or septic shock, and the patients who died during hospitalization were associated with higher WBC counts (9.99 vs. 6.19 × 109/L, p = 0.003) and neutrophil percentages (85 vs. 74.80%, p = 0.03) than deaths within 1-year, a correlation that was statistically significant. This suggests that in-hospital deaths were associated with a heavier inflammatory response and acid respiratory failure on admission, reflecting the severity of acute exacerbations [Citation35], and both factors have been reported to be significant predictors of in-hospital mortality [Citation36]. However, compared with patients who died in-hospital, patients who died after discharge had a less inflammatory reaction at admission; therefore, the survival rate of patients discharged from hospital with symptomatic relief was higher through self-defense response and drug-assisted therapy, while age, frequency of deterioration in the previous year, systemic inflammatory response (CRP level), and other indicators reflect the overall condition of patients and were associated with late mortality.
19.44% of the 1-year deaths were due to cardiovascular events, and the proportion of patients with death complicated with heart failure (26.40 vs. 9.30%, p = 0.001) was higher than that of patients with survival. The impact of age and exacerbation history on COPD mortality has been well documented in previous studies [Citation37]. Mortality and morbidity in patients with COPD with heart failure are higher than in each segment alone, and the two diseases share risk factors such as advanced age, smoking, and systemic inflammatory responses [Citation38]. In a cohort study of 20,296 subjects, Mannino and colleagues showed that co-existence of diabetes, arterial hypertension, and cardiovascular disease in patients with COPD led to a higher risk of death and hospitalization [Citation39]. The impact of obstructive airway disease increases the risk of a coronary event by 30%, for every 10% decrease in FEV1, cardiovascular mortality increases by 28% [Citation40]. Therefore, appropriate comorbid management can minimize the impact of mortality from non-COPD related causes [Citation41]. In our study, among those who died within a year, 5.55% (2/36) died of DVT and 16.67% (6/36) of sepsis. Many studies have confirmed the role of AT-III in sepsis among patients who died within one year [Citation18,Citation27]. Although previous studies found statistical differences in AT-III activity between patients with COPD and those in the healthy control group [Citation10,Citation11], Kunter [Citation10] and colleagues demonstrated that AT-III was significantly lower in patients with AECOPD than in healthy controls(76.68 ± 29.35 vs.93.30 ± 14.81,p < 0.05). Ashitani [Citation42] and colleagues showed that the TAT III complex level significantly increased in patients with AECOPD (2.90 ± 1.60 vs.1.80 ± 0.80, p < 0.05), suggesting AT-III consumption. However, these studies were exclusively designed to illustrate the differences between the two groups of laboratory tests, and they did not investigate the prognosis. AT deficiency has been shown to increase the risk of venous thrombosis and recurrence, and in a study cohort, patients with mild AT deficiency (AT activity, 70–80%) had a 2.4-fold (95%CI: 1.50–3.80) increased risk of venous thrombosis recurrence compared with patients with normal AT levels (>80%) [Citation43]. In another cohort of 2,357 patients with first venous thromboembolism, AT activity <70% was associated with a 3.7-fold risk (95% CI: 1.40–9.90) [Citation44]. Among patients who were admitted with AECOPD, DVT was diagnosed in 10.70% (6/56) and pulmonary embolism (PE) in 8.90% (5/56) of the cases [Citation45]. In a recent meta-analysis of 880 patients with unexplained AECOPD, PE prevalence was 16.10% (95% CI: 8.30%–25.80%) [Citation46]. Therefore, it is hypothesized that reduced AT-III activity in some patients with COPD may play a role in thrombosis. However, in our study, the baseline AT-III activity of patients who died in-hospital and those who died within 1 year was decreased, and there was no statistical difference (71.00% vs. 72.10%, p = 0.89). The main cause of death in hospitalized patients was respiratory failure combined with severe pneumonia or sepsis. This may be because thrombosis involves a complex pathophysiological process. The formation of thrombi may mean the extreme consumption of AT-III and other anticoagulant indicators (such as protein C or protein S), and the shedding of thrombi leads to acute pulmonary embolism, which may result in death in patients with COPD. In this study, patients who died in-hospital had a severe inflammatory response and developed complications with type II respiratory failure; thus, they may have died of other causes in the process of thrombosis or shedding. However, the sample size and, accordingly, the number of thrombotic events in this study were limited. In addition, the levels of thrombin and TAT can help evaluate the coagulation and anticoagulation status in patients with AECOPD and also affect the activity of AT-III; unfortunately, we were unable to determine the levels of thrombin and TAT owing to the limited volume of serum available. As such, further evidence is warranted in order to confirm whether the reduction of AT-III activity is related to the development of thrombosis in patients with COPD.
Conclusion
AT-III activity in patients with AECOPD was significantly lower than that in patients with chronic bronchitis. AT-III may play a role in the pathogenesis and progression of AECOPD and can be used as a biomarker to distinguish AECOPD from acute attacks of chronic bronchitis. AT-III activity was not affected by smoking and COPD severity and was an independent predictor of all-cause mortality. The risk of all-cause mortality in patients with AECOPD was positively correlated with age and D-dimer, CRP, FDP, and FIB levels and negatively correlated with the AT-III level. Patients with reduced AT-III activity and increased CRP and D-dimer levels had a higher risk of all-cause mortality. Finally, AT-III was also an independent predictor of in-hospital and 1-year mortality.
To the best of our knowledge, this study is the first to evaluate the relationship between AT-III activity and mortality in patients with COPD. This laboratory parameter can be a simple, economical, and useful biomarker of prognosis in patients with AECOPD and can be used as an important index for assessing short-term and 1-year mortality.
Research limitations
Notwithstanding, our study has certain limitations:
The duration of exacerbation before admission varied, which might have affected the results of hematological parameters measured on the first day of admission.
The number of included subjects was limited; therefore, more prospective or cohort studies are required to assess the impact of AT-III activity on patients with COPD.
Some studies have shown that stable patients with COPD may also exhibit a degree of coagulation system activation. It would be more helpful to test AT-III levels regularly of stable COPD when patients left the hospital or after discharge. However, this study did not include stable patients as a control. Therefore, we were unable to compare AT-III levels with levels of acute and stable COPD expression. Our future studies will further verify these directions.
Supplemental Material
Download Zip (209.3 KB)Data availability statement
Data for this study are available from the corresponding author.
Declaration of interest
The authors report that there are no competing interests to declare.
Additional information
Funding
References
- López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23. Epub 2015/10/24. DOI:10.1111/resp.12660
- Løkke A, Hilberg O, Tønnesen P, et al. Direct and indirect economic and health consequences of COPD in Denmark: a national register-based study: 1998–2010. BMJ Open. 2014;2014/;4(1):e004069. Epub 01/08. DOI:10.1136/bmjopen-2013-004069 PubMed PMID: 24394800; PubMed Central PMCID: PMCPMC3902350.
- Liu M, Hu R, Jiang X, et al. Coagulation dysfunction in patients with AECOPD and its relation to infection and hypercapnia. J Clin Lab Anal. 2021;35(4):e23733. Epub 2021/03/26. DOI:10.1002/jcla.23733 PubMed PMID: 33764623; PubMed Central PMCID: PMCPMC8059715.
- DAHL M, TYBJOERG-HANSEN ANNE, VESTBO J, et al. Elevated plasma fibrinogen associated with reduced pulmonary function and increased risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(6):1008–1011. Epub 2001/10/06. doi:10.1164/ajrccm.164.6.2010067.
- Duvoix A, Dickens J, Haq I, et al. Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax. 2013;68(7):670–676. Epub 2012/06/30. DOI:10.1136/thoraxjnl-2012-201871 PubMed PMID: 22744884; PubMed Central PMCID: PMCPMC3711372.
- Serban KA, Pratte KA, Bowler RP. Protein biomarkers for COPD outcomes. Chest. 2021;159(6):2244–2253. Epub 2021/01/13. DOI:10.1016/j.chest.2021.01.004 PubMed PMID: 33434499; PubMed Central PMCID: PMCPMC8213963.
- Husebø GR, Gabazza EC, D’Alessandro Gabazza C, et al. Coagulation markers as predictors for clinical events in COPD. Respirology. 2021;26(4):342–351. Epub 2020/11/10. DOI:10.1111/resp.13971
- Fruchter O, Yigla M, Kramer MR. D-dimer as a prognostic biomarker for mortality in chronic obstructive pulmonary disease exacerbation. Am J Med Sci. 2015;349(1):29–35. Epub 2014/09/19. DOI:10.1097/maj.0000000000000332
- Vaidyula VR, Criner GJ, Grabianowski C, et al. Circulating tissue factor procoagulant activity is elevated in stable moderate to severe chronic obstructive pulmonary disease. Thrombosis Research. 2009;124(3):259–261. Epub 2009/01/24. PubMed PMID: 19162305; PubMed Central PMCID: PMCPMC2877030. DOI:10.1016/j.thromres.2008.12.030
- Kunter E, Ilvan A, Ozmen N, et al. Effect of corticosteroids on hemostasis and pulmonary arterial pressure during chronic obstructive pulmonary disease exacerbation. Respiration. 2008;75(2):145–154. Epub 2006/12/05. DOI:10.1159/000097748
- Mariĭnov K, Petrova D, Kamenov V, et al. Anticoagulants and changes in the coagulation status of patients with chronic obstructive lung disease and various degrees of respiratory failure. Vutreshni Bolesti. 1988;27(1):86–93. Epub 1988/01/01.
- Zuo XJ, Nicolaidou E, Okada Y, et al. Antithrombin III inhibits lymphocyte proliferation, immunoglobulin production and mRNA expression of lymphocyte growth factors (IL-2, gamma-IFN and IL-4) in vitro. Transpl Immunol. 2001;9(1):1–6. Epub 2001/10/30. DOI:10.1016/s0966-3274(01)00042-9
- Umerah CO, Momodu II. Anticoagulation. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; 2022.
- Di Stefano A, Turato G, Maestrelli P, et al. Airflow limitation in chronic bronchitis is associated with T-lymphocyte and macrophage infiltration of the bronchial mucosa. Am J Respir Crit Care Med. 1996;153(2):629–632. Epub 1996/02/01. DOI:10.1164/ajrccm.153.2.8564109
- Ma Y, Wang J, Gao J, et al. Antithrombin up-regulates AMP-activated protein kinase signalling during myocardial ischaemia/reperfusion injury. Thromb Haemost. 2015;113(2):338–349. Epub 2014/09/19. DOI:10.1160/th14-04-0360 PubMed PMID: 25230600; PubMed Central PMCID: PMCPMC4308562.
- Aytekin FO, Tekin K, Kabay B, et al. Antithrombin III attenuates pulmonary tissue injury caused by mesenteric ischemia-reperfusion. Am J Surg. 2005;189(2):161–166. Epub 2005/02/22. DOI:10.1016/j.amjsurg.2004.11.003
- Lu J, Niu D, Zheng D, et al. Predictive value of combining the level of lipoprotein-associated phospholipase A2 and antithrombin III for acute coronary syndrome risk. Biom Rep. 2018;9(6):517–522. Epub 2018/12/14. PubMed PMID: 30546880; PubMed Central PMCID: PMCPMC6256189. DOI:10.3892/br.2018.1162
- Lestari IN, Yoel C, Lubis M. The association between the level of antithrombin III and mortality in children with sepsis. Open Access Maced J Med Sci. 2019;7(6):959–961. Epub 2019/04/13. DOI:10.3889/oamjms.2019.211 PubMed PMID: 30976340; PubMed Central PMCID: PMCPMC6454173.
- Pettilä V, Pentti J, Pettilä M, et al. Predictive value of antithrombin III and serum C-reactive protein concentration in critically ill patients with suspected sepsis. Crit Care Med. 2002;30(2):271–275. Epub 2002/03/13. DOI:10.1097/00003246-200202000-00001
- Allingstrup M, Wetterslev J, Ravn FB, et al. Antithrombin III for critically ill patients. Cochrane Database Syst Rev. 2016;2(2):Cd005370. // DOI:10.1002/14651858.CD005370.pub3
- StatBox: A Online Statistical Computing System. Biostatistics Team of CMT. https://www.biostats.cn/statbox/.
- GOLD Executive Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (Updated 2017). Available from: https://goldcopd.org/.
- Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. Epub 1950/01/01. DOI:10.1002/1097-0142(1950)3:1 < 32::aid-cncr2820030106 > 3.0.co;2-3
- Wirtz HR. [Chronic bronchitis, COPD]. Internist (Berl). 2005;46(2):175–191. quiz 92-3. Epub 2005/01/19. DOI:10.1007/s00108-004-1335-z
- Wang Y, Xu J, Meng Y, et al. Role of inflammatory cells in airway remodeling in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3341–3348. Epub 2018/10/24. DOI:10.2147/copd.S176122 PubMed PMID: 30349237; PubMed Central PMCID: PMCPMC6190811.
- Polosa R, Malerba M, Cacciola RR, et al. Effect of acute exacerbations on circulating endothelial, clotting and fibrinolytic markers in COPD patients. Intern Emerg Med. 2013;8(7):567–574. Epub 2011/06/11. DOI:10.1007/s11739-011-0636-1
- Sungurlu S, Kuppy J, Balk RA. Role of antithrombin III and tissue factor pathway in the pathogenesis of sepsis. Crit Care Clin. 2020;36(2):255–265. Epub 2020/03/17. DOI:10.1016/j.ccc.2019.12.002
- Bergantini L, d’Alessandro M, Cameli P, et al. Antithrombin III as predictive indicator of survival in idiopathic pulmonary fibrosis (IPF) patients treated with nintedanib: a preliminary study. Intern Med J. 2021;51(5):705–711. Epub 2020/02/11. DOI:10.1111/imj.14768
- Rehberg S, Yamamoto Y, Sousse LE, et al. Antithrombin attenuates vascular leakage via inhibiting neutrophil activation in acute lung injury. Crit Care Med. 2013;41(12):e439-46. Epub 2013/10/11. DOI:10.1097/CCM.0b013e318298ad3aPubMed PMID: 24107637; PubMed Central PMCID: PMCPMC4063122.
- Zemans RL, Jacobson S, Keene J, et al. Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir Res. 2017;18(1):117. Epub 2017/06/15. DOI:10.1186/s12931-017-0597-7 PubMed PMID: 28610627; PubMed Central PMCID: PMCPMC5470282.
- Leuzzi G, Galeone C, Taverna F, et al. C-reactive protein level predicts mortality in COPD: a systematic review and meta-analysis. Eur Respir Rev. 2017;26(143):160070. Epub 2017/02/02. DOI:10.1183/16000617.0070-2016
- Mannino DM, Tal-Singer R, Lomas DA, et al. Plasma fibrinogen as a biomarker for mortality and hospitalized exacerbations in people with COPD. Chron Obstr Pulm Dis. 2015;2(1):23–34. Epub 2015/02/17. DOI:10.15326/jcopdf.2.1.2014.0138 PubMed PMID: 25685850; PubMed Central PMCID: PMCPMC4326107.
- Undas A, Jankowski M, Kaczmarek P, et al. Thrombin generation in chronic obstructive pulmonary disease: dependence on plasma factor composition. Thrombosis Res. 2011;128(4):e24-8–e28. Epub 2011/06/01. PubMed PMID: 21624643; PubMed Central PMCID: PMCPMC3183323. DOI:10.1016/j.thromres.2011.05.004
- Hu G, Wu Y, Zhou Y, et al. Prognostic role of D-dimer for in-hospital and 1-year mortality in exacerbations of COPD. Int J Chron Obstructive Pulm Dis. 2016;11:2729–2736. Epub 2016/11/16. PubMed PMID: 27843309; PubMed Central PMCID: PMCPMC5098517. DOI:10.2147/COPD.S112882
- Hartl S, Lopez-Campos JL, Pozo-Rodriguez F, et al. Risk of death and readmission of hospital-admitted COPD exacerbations: European COPD audit. Eur Respir J. 2016;47(1):113–121. Epub 2015/10/24. DOI:10.1183/13993003.01391-2014
- Morasert T, Jantarapootirat M, Phinyo P, et al. Prognostic indicators for in-hospital mortality in COPD with acute exacerbation in Thailand: a retrospective cohort study. BMJ Open Resp Res. 2020;7(1):e000488. Epub 2020/05/30. PubMed PMID: 32467292; PubMed Central PMCID: PMCPMC7259855. DOI:10.1136/bmjresp-2019-000488
- Moll M, Qiao D, Regan EA, et al. Machine learning and prediction of all-cause mortality in COPD. Chest. 2020;158(3):952–964. Epub 2020/05/01. PubMed PMID: 32353417; PubMed Central PMCID: PMCPMC7478228. DOI:10.1016/j.chest.2020.02.079
- Horodinschi RN, Bratu OG, Dediu GN, et al. Heart failure and chronic obstructive pulmonary disease: a review. Acta Cardiol. 2020;75(2):97–104. Epub 2019/01/17. DOI:10.1080/00015385.2018.1559485
- Mannino DM, Thorn D, Swensen A, et al. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–969. Epub 2008/06/27. doi:10.1183/09031936.00012408.
- Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev. 2013;22(130):454–475. Epub 2013/12/03. DOI:10.1183/09059180.00008612
- Kostikas K, Clemens A, Patalano F. Prediction and prevention of exacerbations and mortality in patients with COPD. Expert Rev Respir Med. 2016;10(7):739–753. Epub 2016/05/03. DOI:10.1080/17476348.2016.1185371
- Ashitani J, Mukae H, Arimura Y, et al. Elevated plasma procoagulant and fibrinolytic markers in patients with chronic obstructive pulmonary disease. Intern Med. 2002;41(3):181–185. Epub 2002/04/04. DOI:10.2169/internalmedicine.41.181
- Di Minno MN, Dentali F, Lupoli R, et al. Mild antithrombin deficiency and risk of recurrent venous thromboembolism: a prospective cohort study. Circulation. 2014;129(4):497–503. Epub 2013/10/23. DOI:10.1161/circulationaha.113.003756
- Sokol J, Timp JF, Cessie S, et al. Mild antithrombin deficiency and risk of recurrent venous thromboembolism: results from the MEGA follow-up study. J Thromb Haemost. 2018;16(4):680–688. Epub 2018/01/30. DOI:10.1111/jth.13960
- Erelel M, Cuhadaroğlu C, Ece T, et al. The frequency of deep venous thrombosis and pulmonary embolus in acute exacerbation of chronic obstructive pulmonary disease. Respir Med. 2002;96(7):515–518. Epub 2002/08/27. DOI:10.1053/rmed.2002.1313
- Aleva FE, Voets L, Simons SO, et al. Prevalence and localization of pulmonary embolism in unexplained acute exacerbations of COPD: a systematic review and Meta-analysis. Chest. 2017;151(3):544–554. Epub 2016/08/16. DOI:10.1016/j.chest.2016.07.034