Abstract
Chronic obstructive pulmonary disease (COPD) and asthma are chronic inflammatory diseases of the airways. Galectin-13 has recently been forwarded as a biomarker for airway eosinophilic inflammation in asthma. However, the association between galectin-13 and COPD remains unknown. To examine the changes in galectin-13 expression in acute exacerbations of COPD (AECOPD) and the stable phase of COPD and unveil the association between galectin-13 expression and eosinophilic inflammation in COPD, we measured plasma galectin-13 expression in different phases of COPD patients (n = 60, 44 AECOPD patients, and 16 stable COPD patients) and healthy controls (n = 15). Plasma levels of galectin-13 in 60 COPD patients were further analyzed and compared to systemic inflammation, airway eosinophilic inflammation, and lung function. The plasma galectin-13 level was markedly increased in subjects with AECOPD compared to stable COPD patients and healthy controls. Plasma galectin-13 levels in COPD subjects were positively correlated with serum CRP (rs = 0.46, p = 0.0003), peripheral blood eosinophilia count (rs = 0.57, p<0.0001), and FeNO (rs = 0.46, p = 0.0002). In addition, the level of galectin-13 was negatively correlated with FEV1 (rs = −0.43, p = 0.0001), FEV1 pred (%) (rs = −0.544, p<0.0001), as well as FEV1/FVC (rs = −0.46, p<0.0001). Multiple linear regression analysis suggested that plasma galectin-13 levels were affected by FEV1 pred (%), peripheral blood eosinophilia count, and FeNO. We concluded that galectin-13 levels were increased in COPD patients, and elevated galectin-13 expressions related to airway eosinophilic inflammation. Galectin-13 may facilitate the identification of COPD endotypes and may become a potential therapeutic target.
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death globally. It is a heterogeneous disease characterized by persistent airflow limitation associated with chronic airway inflammation. Acute exacerbation of COPD (AECOPD) is responsible for the mortality and economic burden on patients and societies. Studies have shown that COPD has a range of phenotypes and endotypes, and they display differences in mechanisms, clinical outcomes, and responses to treatment [Citation1, Citation2]. A growing body of evidence has demonstrated that increased blood eosinophils play important roles in COPD [Citation3, Citation4]. Peripheral blood eosinophil count and fractional exhaled nitric oxide (FeNO) level have been implicated as biomarkers for airway eosinophilia in COPD patients [Citation5, Citation6].
Galectins are a family of fifteen lectins expressed by diverse cell types, including monocytes, DCs, macrophages, MCs, B cells, and T cells [Citation7]. They are a group of proteins that bind β-galactosidase, which are implicated in immune response, inflammation, cell division, motility, and death [Citation8]. In these galectins, systemic galectin-3 increased during exacerbations in smokers with COPD and chronic bronchitis and was considered a biomarker for AECOPD [Citation9, Citation10]. Galectin-9 plays an important role in pulmonary inflammation and is altered in emphysema by inhibiting the infiltration of neutrophils and decreasing matrix metalloproteinase levels [Citation11]. COPD patients showed elevated levels of galectin-8 and galectin-8 induced interleukin (IL)-6 release in bronchial epithelial cells via PI3Kα signaling [Citation12].
Galectin-13 is a member of the galectin family of proteins, which were first isolated from the human placenta. Its ability to induce apoptosis in activated T cells and to regulate the activity of neutrophils suggests important immune functions [Citation13]. In our previous study, we found that galectin-13 is a novel marker for airway eosinophilia in asthma and may contribute to allergic airway eosinophilic inflammation by regulating the expression of MCP-1 and eotaxin-1 [Citation14]. Therefore, we hypothesized that galectin-13 might contribute to airway inflammation in COPD, especially eosinophilic inflammation.
To test our hypothesis, we detected the plasma galectin-13 expression in subjects with COPD and healthy controls. Correlation assays between plasma galectin-13 expression, systemic inflammation indicators, eosinophilic inflammation and airflow obstruction were performed.
Method
Study population
We recruited 60 subjects with COPD (44 AECOPD patients, 16 stable COPD patients), and 15 healthy control subjects. We recruited COPD subjects from the outpatient and inpatient departments of Respiratory and Critical Care Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. Control subjects were recruited from the health screening center of our hospital. Informed consent was signed by all study participants, and all experiments were approved by the ethics committee of Tongji Hospital, Huazhong University of Science and Technology.
Inclusion and exclusion criteria
Patients with COPD were diagnosed by experienced physicians based on the clinical presentation and the results of pulmonary function tests, according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations [Citation2]. AECOPD patients had increased chronic respiratory symptoms, such as breathlessness, cough frequency or severity, and increased sputum volume beyond normal day-to-day variations, leading to a change in medication.
The exclusion criteria were as follows: i) patients with additional lung disease other than COPD (e.g., asthma-COPD overlap, asthma, pulmonary fibrosis, bronchitis); ii) allergic diseases (e.g., allergic rhinitis, atopic dermatitis); or iii) other serious diseases (e.g., chronic kidney disease, diabetes, malignant tumor).
Measurements of clinical parameters
We recorded detailed clinical and demographic data. Smoking history was shown by the number of pack years. The exacerbation frequency in the previous two years and detailed medication information were obtained from COPD patients. Laboratory measurements, including total leukocyte count, neutrophil count, eosinophil count, serum C-reaction protein (CRP), and procalcitonin (PCT), were performed at the clinical laboratory in our hospital. Chest CT and fractional exhaled nitric oxide (FeNO) were performed for each patient at admission.
Pulmonary function tests
Patients underwent pulmonary function tests according to the American Thoracic Society guidelines using an appropriately calibrated spirometer (Jaeger, Wurzburg, Germany) by a professional. Each patient underwent three technically acceptable measurements, and the highest value was selected to be presented. A reversibility assessment was performed 20 min after salbutamol inhalation.
Sample acquisition
The venous blood samples were collected from the subjects at admission and after treatment for 7 to 10 days in the hospital. These blood samples were centrifuged at 1,000 rpm at 4 °C for 15 min, and the plasma was collected and stored at −80 °C until analysis.
Measurements of galectin-13
Human galectin-13 (BioVendor, Czech) was determined using commercially available ELISA sets. ELISA was performed according to the manufacturer’s instructions. All samples and standards were measured in duplicate.
Statistical analysis
Prism version 6 (GraphPad Software, USA) and SPSS version 23 (SPSS Inc., USA) were used to analyze the data. For normally distributed data, we calculated means ± standard deviation (SD) and used parametric tests (one-way analysis of variance with Tukey correction or unpaired t-test) to compare across groups. For non-normally distributed data, we calculated medians (with interquartile ranges) and used non-parametric tests (Kruskal-Wallis test with Dunn intergroup comparison or Mann-Whitney test). We analyzed correlations using Spearman Rank Order Correlation.
Results
Subject characteristics and laboratory measurements
A total of 44 AECOPD patients (44 males; mean age 64.5, 58.7–70 years), 16 stable COPD patients (16 males; mean age 65, 58–69 years), and 15 healthy controls (15 males; mean age 65, 60.5–68 years) were recruited in this study. Subject characteristics and laboratory measurements are summarized in . Compared with healthy controls, subjects with COPD (both AECOPD and stable COPD patients) had lower FEV1, FVC, FEV1/FVC (%), and FEV1 pred (%). There was no significant difference in lung function between AECOPD and stable COPD patients. Blood leukocyte count, neutrophil count, serum C-reactive protein (CRP), and PCT in the AECOPD group were significantly higher than those in the stable COPD group and healthy controls. The plasma level of galectin-13 in COPD (both AECOPD and stable COPD) patients was higher than in healthy controls. Compared with the acute exacerbation stage, galectin-13 expression was lower in the stable phase of COPD (). To assess galectin-13 expression in the airways, induced sputum samples from subjects with COPD and controls were detected by ELISA. Consistent with our findings in plasma, the levels of galectin-13 in sputum from subjects with COPD were greater than healthy controls (). Furthermore, sputum galectin-13 levels were positively correlated with plasma galectin-13 levels in COPD patients ().
Figure 1. Levels of galectin-13 in plasma and sputum. (A) The plasma galectin-13 levels in AECOPD patients (n = 44), stable COPD (n = 16), and healthy controls (n = 15). (B) The induced sputum galectin-13 levels in AECOPD patients (n = 18), stable COPD patients (n = 9), and healthy controls (n = 8). (C) The correlation between plasma and induced sputum galectin-13 expression.
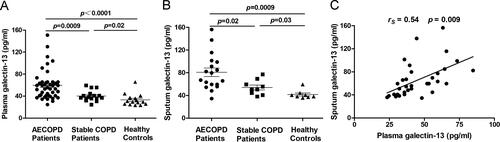
Table 1. Clinical characteristics and laboratory parameters of study subjects.
The association between plasma galectin-13 expression and systemic inflammation factors in COPD
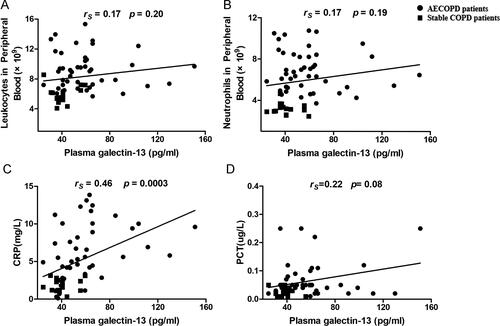
To investigate the association between galectin-13 expression and inflammatory factors in COPD, we analyzed the correlation between plasma galectin-13 levels and leukocyte counts, as well as the neutrophil counts, serum CRP, and PCT in blood from the COPD patients. We found that there was a weak but significant correlation between plasma galectin-13 levels and serum CRP (rs = 0.46, p = 0.0003, n = 60 for all COPD subjects; rs = 0.35, p = 0.02, n = 44 for AECOPD subjects). However, no statistically significant correlation was detected between plasma galectin-13 levels and leukocyte counts (rs = 0.17, p = 0.20, n = 60 for all COPD subjects; rs = −0.05, p = 0.75, n = 44 for AECOPD subjects), neutrophil counts (rs = 0.17, p = 0.19, n = 60 for all COPD subjects; rs = −0.11, p = 0.48, n = 44 for AECOPD subjects) and PCT (rs = 0.22, p = 0.08, n = 60 for all COPD subjects; rs = 0.15, p = 0.35, n = 44 for AECOPD subjects) ().
Increased plasma galectin-13 expression associates with eosinophilic inflammation in COPD
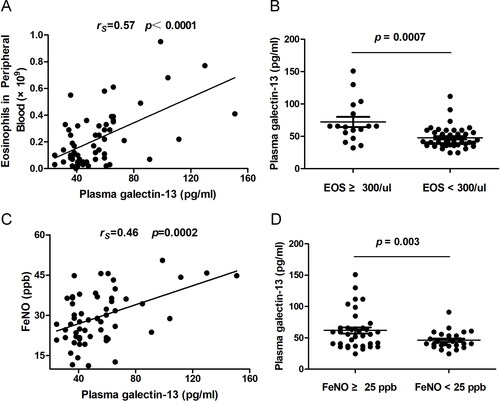
We next examined whether the galectin-13 expression is associated with eosinophilic inflammation in COPD patients. The correlation between galectin-13 expression and the percentage of peripheral blood eosinophilia count and FeNO was analyzed. We found that galectin-13 expression was significantly correlated with the percentage of peripheral blood eosinophilia count (rs = 0.57, p<0.0001) and FeNO (rs = 0.46, p = 0.0002). Moreover, when blood eosinophil status and bronchial eosinophil status were classified to evaluate the association between eosinophilic inflammation and plasma galectin-13 expression in COPD patients, the data indicate that plasma galectin-13 is associated with airway eosinophilic inflammation in COPD ().
Figure 3. Increased plasma galectin-13 expression is associated with airway eosinophilic inflammation in COPD. (A) The association between plasma galectin-13 levels and the percentage of eosinophils in peripheral blood. (B) Plasma galectin-13 levels in COPD subjects with different eosinophil counts in peripheral blood. (C) The association between epithelial galectin-13 levels and FeNO. (D) Plasma galectin-13 levels in COPD subjects with different levels of FeNO.
Correlation between plasma galectin-13 expression and pulmonary function
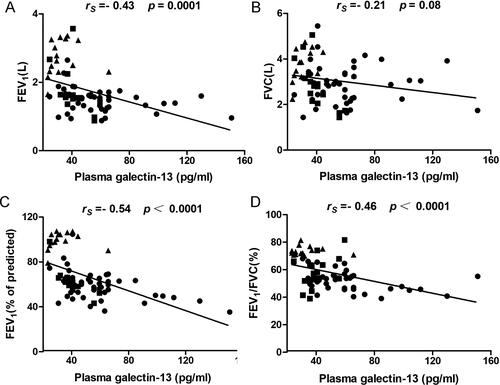
The pulmonary function test is an important method for diagnosing and evaluating COPD patients. The associations between plasma galectin-13 expression and FEV1, FVC, FEV1 pred (%), and FEV1/FVC (%) were shown in . The results showed that the galectin-13 level was negatively correlated with FEV1 (rs = −0.43, p = 0.0001, n = 75 for all subjects; rs = −0.33, p = 0.01, n = 60 for all COPD subjects), FEV1 pred (%) (rs = −0.54, p<0.0001, n = 75 for all subjects; rs = −0.48, p<0.0001, n = 60 for all COPD subjects), and FEV1/FVC (%) (rs = −0.46, p<0.0001, n = 75 for all subjects; rs = −0.32, p = 0.01, n = 60 for all COPD subjects). However, no correlation was found between the level of galectin-13 and FVC (rs = −0.21, p = 0.08, n = 75 for all subjects; rs = −0.12, p = 0.35, n = 60 for all COPD subjects) ().
Multiple linear regression analysis
To explore the factors affecting the expression of galectin-13, we conducted a multiple linear regression analysis of age, BMI, smoking history, lung function, FeNO, leukocyte count, neutrophil count, eosinophil count, CRP, and PCT. The main results are shown in . The results showed that FEV1 pred (%) (β = −0.85, p<0.0001), peripheral blood eosinophil count (β = 41.13, p = 0.004), and FENO (β = 0.56, p = 0.049) were the major influence factors affecting galectin-13 levels, whereas age, BMI, smoking history, and systemic inflammation factors did not appear to be significant factors influencing galectin-13 expression.
Table 2. Multiple linear regression analysis.
Plasma galectin-13 level in acute exacerbation and convalescence phase of COPD
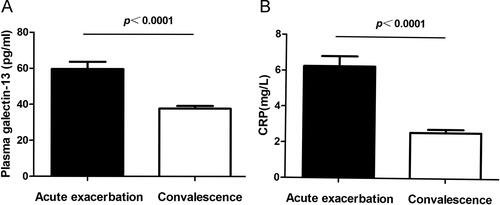
Patients with AECOPD were recollected blood samples when they recovered to the convalescence phase after our treatment. The therapy for COPD exacerbation included antibiotics, systemic corticosteroids, and bronchodilators. We retested the systemic inflammatory indicator serum CRP and plasma galectin-13 expression. Compared with the acute exacerbation stage, galectin-13 decreased statistically in the convalescence phase. Consistent with galectin-13, serum CRP displayed a decreased level during the convalescence phase ().
Discussion
In the present study, we found that plasma galectin-13 expression was increased in both the stable COPD and AECOPD groups compared to healthy controls. Plasma galectin-13 expression was significantly correlated with systemic inflammation, and the levels of galectin-13 in COPD subjects may reflect eosinophilic inflammation. Correlation analysis showed that galectin-13 was significantly negatively correlated with FEV1, FEV1 pred (%), and FEV1/FVC. Multiple linear regression analysis showed that FEV1 pred (%), peripheral blood eosinophil count, and serum CRP were the major factors affecting galectin-13 levels.
Galectins are a family of β-galactoside-binding lectins that participate in diverse biological processes, such as cell growth, cell activation, adhesion, migration, phagocytosis, and apoptosis [Citation15, Citation16]. Today, we know of 15 members of the galectin family. Among them, galectin-3, -8, and -9 are involved in COPD or emphysema. Galectin-13, a member of the galectin family, is the proposed homology structure of the human eosinophil Charcot-Leyden crystal protein/galectin-10 [Citation17]. In our previous study, we demonstrated that galectin-13 was located in airway epithelial cells and submucosal gland cells in asthma subjects and that galectin-13 may play an important role in eosinophilic airway inflammation [Citation14].
In our study, we revealed that plasma galectin-13 was increased in the COPD subjects, especially in the exacerbation phase of COPD, which suggests that galectin-13 may be involved in COPD exacerbation attacks. Respiratory infection is the most common trigger for exacerbations of COPD. Inflammatory biomarkers such as CRP and PCT are promising tools for evaluating COPD exacerbations [Citation18–20]. In our study, the plasma galectin-13, plasma leukocytes and neutrophils count, serum CRP, and PCT levels were all increased in AECOPD patients. Among them, the serum CRP level was positively correlated with the plasma galectin-13 level. This finding suggests that galectin-13 may play a potential role in the pathogenesis of inflammation in COPD.
Over the past decade, many studies have investigated the heterogeneity of COPD inflammation [Citation4, Citation21]. Eosinophilic inflammation in COPD has been proposed as a potential treatment target for inhaled corticosteroid (ICS) therapy [Citation22, Citation23]. The latest Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) 2020 report suggests using blood eosinophil counts to guide treatment in COPD patients [Citation2]. The percentage of peripheral blood eosinophilia was identified as a biomarker for reflecting sputum eosinophilia in COPD, and the FeNO level was also regarded as a good surrogate marker of airway eosinophilia in COPD [Citation6, Citation24]. In our study, all included COPD patients were without any asthmatic features, and the results demonstrated that galectin-13 expression was significantly correlated with the percentage of blood eosinophil counts and FeNO. Both COPD and asthma patients have increased plasma galectin-13 levels, and there is a relationship between galectin-13 levels and eosinophilic airway inflammation [Citation14], but whether they act differently is unknown. Different phenotypes have different impacts on COPD clinical outcomes, and our findings show promise that galectin-13 may facilitate the identification of COPD endotypes and may contribute to the development of precise management of different phenotypes.
Airflow obstruction is the main characteristic of COPD. Correlation analysis showed that galectin-13 was negatively correlated with FEV1, FEV1 pred (%), and FEV1/FVC. Additional multiple linear regression confirmed that FEV1 pred (%) significantly negatively affected galectin-13. These findings suggest that galectin-13 is negatively correlated with lung function. However, the mechanism by which galectin-13 participates in chronic airway inflammatory disease is still unknown. We expect that future studies will provide more insight into the role of galectin-13 in the lungs.
We compared plasma galectin-13 levels in the acute exacerbation and convalescence phases of COPD. Elevated plasma galectin-13 level, blood leukocyte counts, and serum CRP were decreased when the patients came to the convalescence phase after our treatment. These results suggest that galectin-13 may be involved in AECOPD. However, whether the level of systemic galectin-13 can predict COPD patients developing exacerbations remains unknown.
Our study has some limitations. First, the sample size is relatively small. Future studies with larger sample sizes in different centers should be performed to confirm these results. Second, the statistical significance of our study is the level of association. The mechanism of galectin-13 that participates in systemic and airway inflammation in COPD needs to be explored. Despite these limitations, this study provides preliminary information about changes in galectin-13 levels in COPD.
In summary, our findings indicate that galectin-13 is increased in COPD patients and differs significantly in the acute exacerbation phase and the stable phase. Elevated galectin-13 expressions may predict systemic inflammation, airway eosinophilic inflammation, and poor pulmonary function.
Author contributions
L.Y. and S.Z. conceptualized the study design. L.Y., Y.F., D.C. and S.Z. collected demographic, clinical, and laboratory data. L.Y. and Y.J. analyzed the data. L.Y. and Y.J. interpreted the results. L.Y. and S.Z. wrote the manuscript with all authors providing feedback for revision. The authors read and approved the final report.
| Abbreviations: | ||
| COPD | = | Chronic obstructive pulmonary disease |
| AECOPD | = | Acute exacerbation of chronic obstructive pulmonary disease |
| FeNO | = | fractional exhaled nitric oxide |
| CRP | = | C-reaction protein |
| PCT | = | procalcitonin |
| FEV1 | = | forced expiratory volume in 1 s |
| FVC | = | forced vital capacity |
Acknowledgements
We thank the patients who volunteered for this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Agustí A, Celli B, Faner R. What does endotyping mean for treatment in chronic obstructive pulmonary disease? Lancet. 2017;390(10098):980–987. DOI:10.1016/S0140-6736(17)32136-0
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of Chronic Obstructive Pulmonary Disease: updated 2020. Available from: http://www.goldcopd.org.
- Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27. DOI:10.1016/j.jaci.2016.05.011
- Miravitlles M, Soler-Cataluña JJ, Soriano JB, et al. Determinants of blood eosinophil levels in the general population and patients with COPD: a population-based, epidemiological study. Respir Res. 2022;23(1):49. DOI:10.1186/s12931-022-01965-3
- Kostikas K, Brindicci C, Patalano F. Blood eosinophils as biomarkers to drive treatment choices in asthma and COPD. Curr Drug Targets. 2018;19(16):1882–1896. DOI:10.2174/1389450119666180212120012
- Antus B, Paska C, Barta I. Predictive value of exhaled nitric oxide and blood eosinophil count in the assessment of airway eosinophilia in COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:2025–2035. DOI:10.2147/COPD.S257965
- Salazar F, Sewell HF, Shakib F, et al. The role of lectins in allergic sensitization and allergic disease. J Allergy Clin Immunol. 2013;132(1):27–36. DOI:10.1016/j.jaci.2013.02.001
- Porębska N, Poźniak M, Matynia A, et al. Galectins as modulators of receptor tyrosine kinases signaling in health and disease. Cytokine Growth Factor Rev. 2021;60:89–106. DOI:10.1016/j.cytogfr.2021.03.004
- Feng W, Wu X, Li S, et al. Association of serum galectin-3 with the acute exacerbation of chronic obstructive pulmonary disease. Med Sci Monit. 2017;23:4612–4618. DOI:10.12659/msm.903472
- Sundqvist M, Andelid K, Ekberg-Jansson A, et al. Systemic galectin-3 in smokers with chronic obstructive pulmonary disease and chronic bronchitis: the impact of exacerbations. Int J Chron Obstruct Pulmon Dis. 2021;16:367–377. DOI:10.2147/COPD.S283372
- Horio Y, Ichiyasu H, Kojima K, et al. Protective effect of galectin-9 in murine model of lung emphysema: involvement of neutrophil migration and MMP-9 production. PLoS One. 2017;12(7):e0180742. DOI:10.1371/journal.pone.0180742
- Kono Y, Colley T, To M, et al. Cigarette smoke-induced impairment of autophagy in macrophages increases galectin-8 and inflammation. Sci Rep. 2021;11(1):335. DOI:10.1038/s41598-020-79848-0
- Than NG, Balogh A, Romero R, et al. Placental protein 13 (PP13) – a placental immunoregulatory galectin protecting pregnancy. Front Immunol. 2014;5:348. DOI:10.3389/fimmu.2014.00348
- Yi L, Zhang S, Feng Y, et al. Increased epithelial galectin-13 expression associates with eosinophilic airway inflammation in asthma. Clin Exp Allergy. 2021;51(12):1566–1576. DOI DOI:10.1111/cea.13961
- Compagno D, Tiraboschi C, Garcia JD, et al. Galectins as checkpoints of the immune system in cancers, their clinical relevance, and implication in clinical trials. Biomolecules. 2020;10(5):750. DOI:10.3390/biom10050
- Nio-Kobayashi J. Tissue- and cell-specific localization of galectins, β-galactose-binding animal lectins, and their potential functions in health and disease. Anat Sci Int. 2017;92(1):25–36. DOI:10.1007/s12565-016-0366-6
- Visegrády B, Than NG, Kilár F, et al. Homology modelling and molecular dynamics studies of human placental tissue protein 13 (galectin-13). Protein Eng. 2001;14(11):875–880. DOI:10.1093/protein/14.11.875
- Kawamatawong T, Apiwattanaporn A, Siricharoonwong W. Serum inflammatory biomarkers and clinical outcomes of COPD exacerbation caused by different pathogens. Int J Chron Obstruct Pulmon Dis. 2017;12:1625–1630. DOI:10.2147/COPD.S132132
- Zhou W, Tan J. The expression and the clinical significance of eosinophils, PCT and CRP in patients with acute exacerbation of chronic obstructive pulmonary disease complicated with pulmonary infection. Am J Transl Res. 2021;13(4):3451–3458.
- Song W, Wang Y, Tian F, et al. Clinical significance of procalcitonin, C-Reactive protein, and interleukin-6 in helping guide the antibiotic use for patients with acute exacerbations of chronic obstructive pulmonary disease. Dis Markers. 2021;2021:8879401. DOI:10.1155/2021/8879401
- Su L, Qiao Y, Luo J, et al. Characteristics of the sputum microbiome in COPD exacerbations and correlations between clinical indices. J Transl Med. 2022;20(1):76. DOI:10.1186/s12967-022-03278-x
- Weissler JC, Adams TN. Eosinophilic chronic obstructive pulmonary disease. Lung. 2021;199(6):589–595. DOI:10.1007/s00408-021-00492-0
- Li A, Chan HP, Gan PXL, et al. Eosinophilic endotype of chronic obstructive pulmonary disease: similarities and differences from asthma. Korean J Intern Med. 2021;36(6):1305–1319. DOI:10.3904/kjim.2021.180
- Siva R, Green RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;29(5):906–913. DOI:10.1183/09031936.00146306