Abstract
Introduction
Potential associations between Chronic Obstructive Pulmonary Disease (COPD) and osteoporosis have been studied, but areas of uncertainty remain.
Objective
This scoping review aimed to identify the published evidence on the epidemiological relationships between COPD and osteoporosis.
Methods
Experimental and observational evidence evaluating relationships between COPD and osteoporosis on epidemiology, clinical manifestations, risk factors (RFs), therapeutic management and quality of life (QoL) was searched on PubMed and Embase (until May 2023). The studies were categorized according to their objectives and characteristics. Data were analyzed using descriptive statistics.
Results
Ninety-nine studies were selected, namely 33 (33%) reporting epidemiologic measures, 11 (11%) clinical manifestations, 74 (75%) RFs (45 ones, of which body mass index [BMI; n = 22 studies], corticosteroids’ use [n = 20], and COPD severity [n = 15] were the most studied), 7 (7%) therapeutic management, and 3 (3%) QoL. Twenty-seven (27.6%) studies evaluated ≥2 domains. Most studies followed a cross-sectional design (n = 37; 37.4%). Eighty-nine studies (90%) assessed patients with COPD at baseline and studied its relationship with osteoporosis.
Conclusion
There are well-established features linking COPD and osteoporosis, including shared RFs, such as smoking, elderly, physical inactivity, or low BMI. Others deserve clarification, including the impact of COPD severity, or the use of inhaled corticosteroids on the incidence of osteoporosis and fractures, as well as the value of performing routine imaging tests, or prescribing anti-resorptive medications in COPD to prevent osteoporotic-related outcomes. QoL studies are also lacking. Investigating such issues is needed to propose clinical guidelines for managing osteoporosis in patients with COPD.
1. Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea, cough, sputum production and/ or exacerbations) due to abnormalities of the airways (bronchitis, bronchiolitis) and/ or alveoli (emphysema) that cause persistent, often progressive, airflow obstruction [Citation1]. It is the third leading cause of death worldwide, with 3,23 million deaths in 2019 [Citation2]. Disease related mortality and morbidity are estimated to increase in the next decades, mainly due to the continued exposure to risk factors [Citation3].
COPD results from the combination of several factors, namely genetic susceptibility, and environmental risk factors. One key risk factor is cigarette smoking, which includes first-hand smoking, secondhand smoke exposure, and maternal smoking. Other risk factors include biomass exposure, occupational exposure, air pollution, previous diseases (e.g., childhood asthma, childhood respiratory infections, tuberculosis), and genetic factors (e.g., α-1 antitrypsin deficiency) [Citation1,Citation3].
The diagnosis of COPD starts by characterizing patient exposure to risk factors and the presence of COPD symptoms [Citation1,Citation3]. Despite being characterized by several pulmonary manifestations, it is also associated with extrapulmonary effects and comorbidities (e.g., cardiovascular disease, diabetes, skeletal muscle wasting, obstructive sleep apnea, osteoporosis, depression, and lung cancer) [Citation3]. Some of these diseases share the same risk factors as COPD (e.g., cigarette smoking), while COPD itself may pose an increased risk of developing these diseases (e.g., deterioration in pulmonary function due to COPD can reduce physical activity and increase risk of fractures) [Citation1].
Osteoporosis is a chronic skeletal disorder, characterized by low bone mineral density (BMD) and microarchitectural deterioration of bone tissue, which increases the risk of fracture [Citation4]. Osteoporosis has a significant burden, with health, social and economic impact [Citation5]. Based on data of the ScoreCard for OsteoPorosis in Europe (SCOPE) collaboration, in 2034, 5.34 million people will suffer with osteoporotic fractures in the European Union plus United Kingdom and Switzerland [Citation6].
An imbalance between bone resorption and development can be the point of start of osteoporosis. Several risk factors can contribute for the development of this condition, namely modifiable (e.g., nutritional deficiency, reduced physical activity, cigarette smoking, weight loss, air pollution) and non-modifiable risk factors (e.g., older age, female, Caucasian, family history, previous history of fractures). There are also comorbidities or situations that can be involved in osteoporosis, such as the chronic use of corticosteroids, vitamin D deficiency, COPD, etc. [Citation7,Citation8].
Some features often seen in COPD patients may put them at a greater risk for osteoporosis. For example, chronic inflammation in the airways and lungs can promote bone resorption and impair bone formation, physical inactivity due to breathing difficulties can contribute to decrease BMD, and prolonged use of corticosteroids by COPD patients can promote bone loss, increasing the risk of osteoporosis [Citation9–12].
The link between COPD and osteoporosis is still under research. A systematic literature review (SLR) found a high prevalence of osteoporosis and high risk of fracture in patients with COPD and identified various common risk factors, such as cigarette smoking, low physical activity, reduced weight, and disease-specific risk factors [Citation13]. In 2019, a SLR on prevalence, severity and therapeutic outcomes observed that osteoporosis was prevalent in patients with COPD. The authors of this study suggested that a proper screening in patients with COPD with some risk factors, such as older age, advanced stages of the disease, low body mass index (BMI) or low fat-free mass index, should be performed [Citation14]. In another SLR, a high prevalence of osteoporosis was also identified among patients with COPD, with low BMI and sarcopenia being identified as major risk factors for osteoporosis [Citation15]. Despite the research on this area, existing studies present some methodological limitations, such as the absence of control for confounding factors, while there is lack of consensus on given aspects, such as the association between COPD severity and osteoporosis activity, or the therapeutic management of patients with COPD and osteoporosis [Citation13–17]. Thus, it is still important to investigate epidemiological associations, risk factors, clinical manifestations, and quality of life in patients with COPD and osteoporosis.
This scoping review aims to identify the published evidence on the epidemiological relationship between COPD and osteoporosis, as well as to characterize it to identify knowledge gaps, challenges, and further research needs.
2. Methods
The scoping review was conducted according to the Joanna Briggs Institute (JBI) Manual for Evidence Synthesis: Scoping Reviews Chapter and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Extension for Scoping Reviews (PRISMA-ScR) [Citation18,Citation19].
2.1. Information sources and search
A comprehensive search was performed in two main bibliographic databases: PubMed (www.ncbi.nlm.nih.gov/pubmed/), and Embase (www.embase.com/) to identify relevant evidence. Search terms comprised the combination of index and free terms of “osteoporosis” and “chronic obstructive pulmonary disease.” No time horizon limit was applied. The full search strategy is described in the “Appendix I: Search strategy.”
Following the search, all search results were uploaded into EndNoteTM 21 (Clarivate Analytics, PA, USA) and duplicates removed. Then, the search results were screened independently by two investigators against eligibility criteria. Disagreements were solved by a third investigator. The reference list of SLRs, meta-analyses and reviews were reviewed to identify additional relevant studies.
2.2. Eligibility criteria
Evidence studying the link between COPD and osteoporosis were eligible for this scoping review, if it fitted the following criteria:
Evaluated epidemiology, such as prevalence, potential physiologic mechanisms, among others.
Evaluated clinical manifestations, such as COPD severity, BMD, fractures, among others.
Evaluated risk factors, such as older age, low BMI, lifestyle, smoking, systemic inflammation, pulmonary dysfunction, glucocorticoid use, lack of vitamin D, among others.
Evaluated therapeutic management, such as previous evaluation of treatment risks, subsequent treatment, among others.
Evaluated quality of life, through validated instruments.
Described diagnosed COPD and osteoporosis, this is, articles describing only symptoms and signs that could be COPD or osteoporosis, but without a diagnosis, were excluded.
Were clinical trials or observational studies (e.g., cohort studies, case-control studies, cross-sectional studies, etc.).
Were written in English.
2.3. Data charting process and data items
Each relevant study was categorized into five main domains, namely epidemiology, clinical manifestations, risk factors, therapeutic management, and quality of life, representing the objective of each included study. Additionally, a form was used to extract the main characteristics of each study, namely the following:
Reference and date.
Study design as reported by the article’s authors, such as clinical trial, cohort study, case-control study, cross-sectional study, and other observational studies.
Type of included population, namely COPD, osteoporosis, or both.
Method of diagnosis of COPD, such as exams, exams plus GOLD (Global Initiative for Chronic Obstructive Lung Disease), exams plus other guidelines, ICD (International Classification of Diseases) codes, or unknown.
Method of diagnosis of osteoporosis, such as exams, exams plus WHO criteria, exams plus other guidelines, ICD codes, or unknown.
Setting, namely ambulatory or hospital (outpatient, inpatient, hospital not specified), or both, or unknown.
Object of the study (e.g., prevalence, number of fractures, older age, etc).
Number of patients included in the study.
Region.
The data extraction was performed by two investigators. Both extracted the data using Microsoft Excel™ 365 (Microsoft Corporation) based on the eligibility criteria, as described in the “Appendix II: Data extraction instrument.”
2.4. Data analysis and presentation
The results were analyzed through descriptive statistics and interpreted and discussed by all. The data were presented graphically, or in diagrammatic or tabulated form.
3. Results
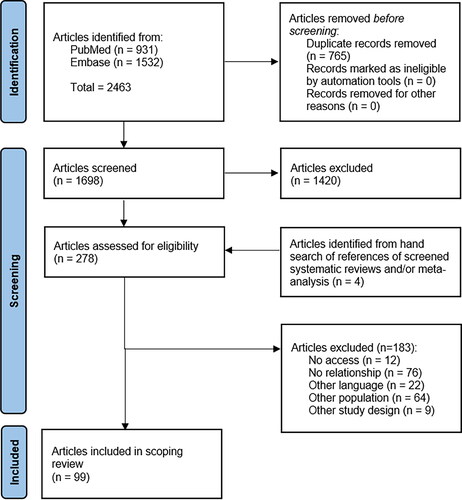
A total of 2,463 articles were identified from literature search. After duplicates removal, 1,698 articles were screened for eligibility criteria. In addition, 4 articles were identified form hand search of references of SLRs and/or meta-analyses. In total, 278 references were selected for full-text assessment. A final sample of 99 articles met the eligibility criteria. The selection of articles is presented in . The references of the included and excluded articles are listed in “Appendix III: List of included studies” and “Appendix IV: List of excluded studies, with reasons,” respectively.
Figure 1. Flow diagram of search literature. Adapted from “PRISMA 2020 flow diagram for new systematic reviews which included searches of databases, registers and other sources.”
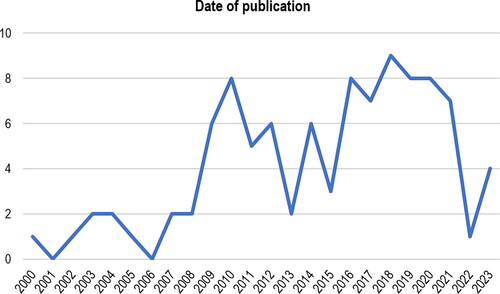
Sixty-two percent (n = 61/99) of included articles were published in the last 10 years (between 2014 and 2023) ().
3.1. Objective of the study
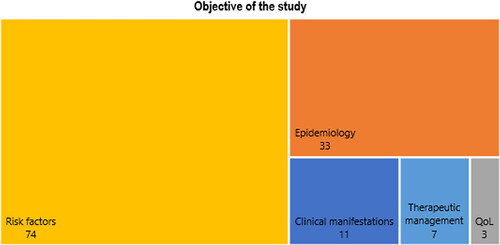
The studies were classified into one of five domains, according to their main objective: epidemiology (n = 33), clinical manifestations (n = 11), risk factors (n = 74), therapeutic management (n = 7), and quality of life (n = 3) (). Twenty-seven (27.6%) studies evaluated two or more different domains.
Figure 3. Categorization of studies into five domains: epidemiology, clinical manifestations, risk factors, therapeutic management, and quality of life. Abbreviations: QoL = quality of life.
The epidemiologic measure most frequently evaluated in the included studies was “prevalence” (n = 31; 93.9%). Eleven studies assessed clinical manifestations, being the most evaluated “fractures” (n = 5; 45.4%).
Most of the studies evaluating risk factors (n = 66/74; 89.2%) for disease development included a population with COPD diagnosis at baseline. Among these, 43 different risk factors for development of osteoporosis in patients with COPD were assessed. The most frequents were “BMI” (n = 22/66; 33.3%), followed by the intake of “corticosteroids” (n = 19/66; 28.8%), “COPD severity” (n = 13/66; 19.7%), “age” (n = 12/66; 18.2%), and “pulmonary parameters” (n = 9/66; 13.6%).
Only three studies assessed COPD as an independent risk factor for osteoporosis, and only study assessed osteoporosis as an independent risk factor for COPD.
The study of several “diagnostic methods” was the most common subject in studies from the domain “therapeutic management” (n = 3; 37.5%). The three studies that assessed quality of life, evaluated seven different topics. The full list of epidemiologic measures, clinical manifestations, risk factors (), therapeutic management and quality of life instruments is described in .
Figure 4. Type of risk factors assessed in the included studies. Abbreviations: BMD = bone mineral disease; BMI = body mass index; COPD = chronic obstructive pulmonary disease.
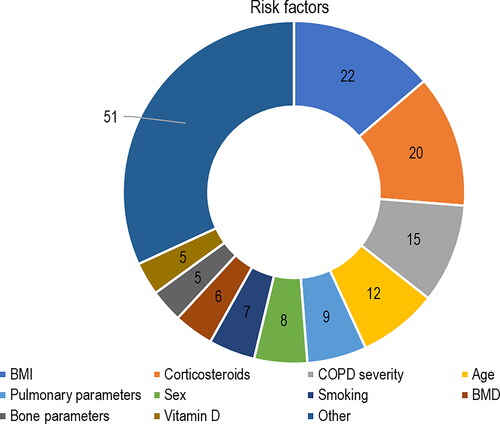
Table 1. Summary of domains and Sub-domains found within scoping review.
3.2. Study design
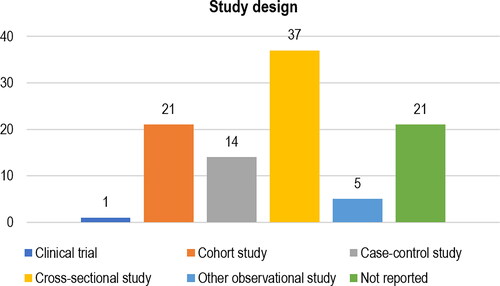
The most frequently reported type of study design was the cross-sectional design (n = 37; 37.4%), followed by the cohort design (n = 21; 21.2%). Twenty-one (21.2%) studies did not report their type of study design ().
3.3. Population
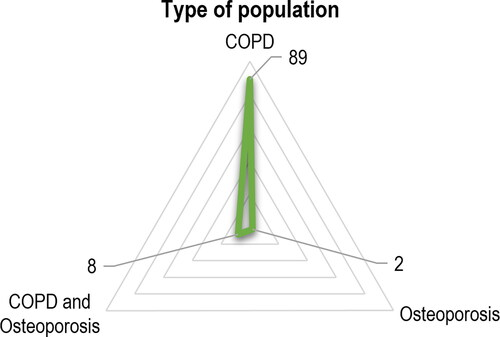
Almost 90% (n = 89) of the studies assessed a population with a COPD diagnosis and studied their relationship with osteoporosis. Only eight (8.1%) studies assessed a population with both COPD and osteoporosis diagnosis (). The studies included a median of 104 patients (min = 23; max = 1,271,072).
Figure 6. Type of population included in the study. Abbreviations: COPD = chronic obstructive pulmonary disease.
3.3.1. Method of diagnosis of COPD or osteoporosis
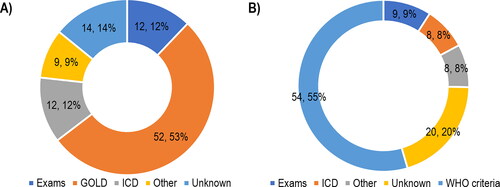
More than half of the studies (n = 52; 52.5%) diagnosed COPD through exams and classification according to GOLD. Exams and the WHO criteria were the method of diagnosis most reported for osteoporosis (n = 54; 54.5%) ().
Figure 7. Methods of diagnosis of (A) COPD or (B) osteoporosis. Abbreviations: GOLD = global initiative for chronic obstructive lung disease; ICD = international classification of diseases; WHO = world health organization. Note: For the methods of diagnosis of “GOLD” and “WHO criteria,” it was assumed that exams were performed before the patient severity classification/ diagnosis.
3.4. Setting
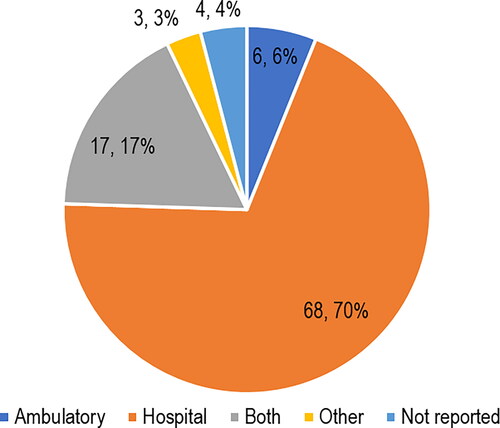
Seventy percent (n = 68) of the included studies were performed in hospital, and of that, three (4.4%) included inpatient patients, 28 (41.2%) included outpatient patients, and 37 (54.4%) studies did not specify the type of patients. Three (3.1%) studies included patients from other settings, such as rehabilitation centers. The distribution of studies by setting is described in .
3.5. Region
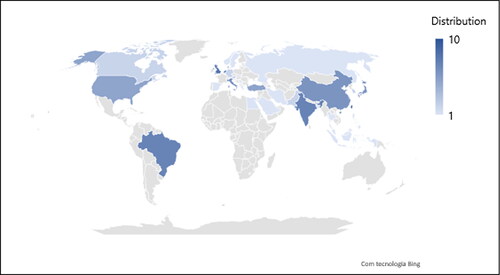
shows a distribution of the included studies by regions. Taiwan (n = 10; 10.1%); United Kingdom (n = 8; 8.1%); Brazil, India, and The Netherlands (n = 7; 7.1%; each) where the regions were most studies were performed. In Africa, it was recorded only one (1.0%) study, in Egypt.
4. Discussion
4.1. Summary of evidence found in the scoping review
Ninety-nine epidemiological studies evaluated the relationship between COPD and osteoporosis, on topics related to epidemiology, clinical manifestations, risk factors, therapeutic management, and quality of life. Most studies were published in the last ten years, suggesting an increased interest in researching the relationship between both diseases. The studies have a diverse geographic distribution, with dominance in Asia and Europe. Nearly 90% of studies included patients with a diagnosis of COPD at the baseline and further explored associations between that condition and osteoporosis. Diagnoses were mainly made using GOLD for COPD and WHO criteria for osteoporosis, which is in line with international recommendations for clinicians [Citation1,Citation4]. Most patients in the studies were recruited and followed in hospitals, which is expected since diagnoses, medical examinations and monitoring tests are often made at hospitals [Citation20].
4.1.1. Epidemiology
Prevalence was the most reported epidemiologic measure in the studies (only two evaluated incidence). The design of such studies is in line with methodological recommendations, since cross-sectional and cohort are the preferred study designs to assess prevalence and incidence, respectively [Citation21]. The prevalence of osteoporosis in COPD patients ranged between approximately 6% in the UK and 69% in Bangladesh [Citation22,Citation23]. For context, the prevalence of osteoporosis in the world’s general population was reported to be 18.3% (95% CI 16.2–20.7) (23.1% in women, and 11.7% in men), with the lowest prevalence in America (12.4% [95% CI 7.4–19.5]) and the highest in Africa (39.5% [95% CI 22.3–59.7]) [Citation24]. According to the results of a SLR with meta-analysis, the prevalence of osteoporosis can vary between 14% to 67% among patients with COPD [Citation14]. In another study, the overall pooled prevalence of osteoporosis was 38% in COPD patients and 15% in non-COPD patients, meaning that the presence of COPD more than double the risk of developing osteoporosis (odds ratio [OR] = 2.83; 95% CI 2.00–4.03) [Citation15].
4.1.2. Clinical manifestations
Fractures are one of the most important clinical manifestations of osteoporosis [Citation20]. It was also the most studied clinical manifestation in the group of retrieved studies. Evidence suggests that there is an increase in the number of osteoporotic fractures in patients with COPD, and proportionally a reduction in patients’ quality of life [Citation5]. In the studies identified in this scoping review, the risk of fracture was mainly assessed through FRAX (fracture risk assessment tool) and was suggested to be higher in osteoporotic patients with COPD than in non-osteoporotic patients with COPD. One study reported conflicting results, suggesting no correlation between FRAX score and prevalence of vertebral fractures in COPD patients [Citation25]. Osteoporotic fractures were used as an outcome measure in only one study, a population-based retrospective cohort analysis, revealing marginal increased risks of any osteoporotic fracture (hazard ratio [HR] = 1.24; 95% CI 1.02–1.51) and vertebral osteoporotic fractures (HR = 1.297; 95% CI 1.020–1.649) in COPD patients versus individuals without COPD [Citation26].
Further evidence suggests that having osteoporosis diminishes the likelihood of improvement of functional exercise capacity (i.e., 6-min walking distance) in COPD patients undergoing pulmonary rehabilitation programs [Citation27]. This finding is supported by the results of systematic reviews of literature, with one meta-analysis reporting an OR of 0.28 (95% CI 0.11–0.70) for reduced physical performance in association with the presence of osteoporosis [Citation28,Citation29]. A meta-analysis by Kakoullis and colleagues found that osteoporosis was associated with a significant reduction in survival and pulmonary function among patients with COPD [Citation30].
4.1.3. Risk factors
Risk factors constituted the most evaluated domain among the studies included in this review (74 out of 98 studies). Most studies (66 out of 74 studies) were designed to assess the impact of specific risk factors in the development of osteoporosis in COPD patients. In total, 43 different risk factors for development of osteoporosis in patients with COPD were assessed. The most frequents were “BMI” (n = 22/66; 33.3%), followed by the intake of “corticosteroids” (n = 19/66; 28.8%), “COPD severity” (n = 13/66; 19.7%), “age” (n = 12/66; 18.2%), and “pulmonary parameters” (n = 9/66; 13.6%).
Although the mechanism of osteoporosis in COPD is not clearly described, some risk factors have been identified. Those risk factors can be classified into general risk factors (e.g., age, BMI, smoking, sex), while others as disease-related risk factors (e.g., corticosteroids intake, COPD severity, bone and pulmonary parameters) [Citation13]. For example, the use of systemic corticosteroids in COPD patients is a known risk factor for developing osteoporosis, while low BMD in osteoporotic patients is a risk factor for COPD [Citation8,Citation13,Citation31,Citation32]. The smoking history, as well as the low physical activity which often characterizes COPD patients also increases the likelihood of osteoporosis. Nevertheless, it would be important to carry out prospective studies that could clarify the contribution of each individual factor to the risk of developing osteoporosis in these patients [Citation33]. For example, Chen and colleagues concluded that sarcopenia (OR = 3.65; 95% CI 1.45–9.16) and a BMI < 18.5 kg/m2 (OR = 4.26; 95% CI 1.07–16.99) were risk factors for osteoporosis among patients with COPD [Citation15]. In the scoping review, only three studies assessed COPD as an independent risk factor for osteoporosis. The results of these case-control studies (in Bangladesh, Brazil and China) suggest that persons with COPD have a greater chance of developing osteoporosis compared to persons without COPD [Citation23,Citation34,Citation35].
4.1.4. Therapeutic management
The results of this scoping review indicate that there is a lack of information on clinical approaches to prevent or decrease the likelihood of developing osteoporosis in patients with COPD, as well as on monitoring the disease and therapeutic management (e.g., prescribing medication) in patients with COPD and established osteoporosis. Studies have addressed the use of diagnostic procedures (e.g., DXA, chest CT) to monitor osteoporosis activity in patients with COPD, surgical techniques to improve lung function in patients with OVCFs, the potential importance of performing DXA to guide the prescription of osteoporosis medications in patients on corticosteroids, as well as patterns of pharmacological treatments (i.e., corticosteroids and osteoporosis medications) in patients with COPD with or without concomitant osteoporosis. For example, routine chest CT and DXA may be useful to monitor bone health in patients with COPD [Citation36–38]. Regarding pharmacotherapy, it appears that osteoporosis may have been overlooked in COPD patients entering pulmonary rehabilitation, as 82% of osteoporosis patients were not treated with bone medication in the Netherlands [Citation39]. In a cohort study carried out in Taiwan, 16,204 (55%) of 29,611 patients with COPD and osteoporosis did not receive anti-osteoporotic treatment [Citation40]. In Denmark, patients using corticosteroids are more frequently scanned with DXA and more frequently prescribed with osteoporosis medications than non-users of corticosteroids [Citation36].
4.1.5. Quality of life
Six different instruments were used to assess quality of life of patients diagnosed with COPD and osteoporosis, and one study assessed these diseases’ burden (direct and indirect costs). One study used a generic instrument to measure health-related quality of life, namely the EQ-5D [Citation41]. The other five studies used instruments that are disease-specific, and which are mainly used to capture patients’ values and preferences, called patient reported outcomes (PRO). The identification of these studies is relevant, since to our knowledge previous systematic reviews did not assess this domain.
4.2. Strengths and limitations of the scoping review
To our knowledge, this is the first scoping review identifying clinical studies on the epidemiological relationship between COPD and osteoporosis, in five major domains. This review was designed to explore existing epidemiological evidence rather than explore pathological, mechanistic, or biological explanations for the relationship between the two conditions. Other recently published reviews have addressed these issues [Citation42,Citation43].
As it is a scoping review, a methodological quality of the included studies was not performed. However, it is possible to perform a critical appraisal of them, considering relevant aspects of both internal validity (methodology) and external validity (representativeness). Methodologically, all included studies are observational studies, except one study that was a clinical trial [Citation44]. That was in accordance with the appropriateness of these designs in studying aspects related with risk factors, epidemiology, among others, in experimental designs [Citation21]. Among observational evidence, different study designs were found, varying from cohort design, case-control design to cross-sectional design or other not reported by studies’ authors. Each study design is subject to potential bias and confounding [Citation21]. It was also perceived that some studies chose a study design that, according to their objective, was not the most appropriate. For instance, a clinical trial evaluating a risk factor [Citation21]. In addition, some studies did not present complete information, namely the method of diagnosis, setting, among others. This makes difficult to assess the representativeness of the results and, consequently, to extrapolate them into clinical practice [Citation45].
In the present scoping review, international recommendations were followed. Despite that, some limitations can be pointed out. The literature search was performed in only two bibliographic databases (PubMed, and EMBASE). However, these two databases were the largest databases in the field, and each included more than 36 million articles. Additionally, the scoping review only included articles published in English, due to authors limitations in translating other languages. Nonetheless, this language restriction was not applied in literature search strategy, which could lead to potential language bias, but it was part of the eligibility criteria [Citation46].
4.3. Which are the topics already well covered by existing reviews?
Several studies evaluating prevalence of osteoporosis in individuals with COPD were identified in this scoping review. One systematic review and meta-analysis has addressed this topic, concluding that the COPD increase the risk of osteoporosis compared to the absence of the disease [Citation15].
Regarding clinical manifestations, osteoporosis is associated with poorer functional capacity, pulmonary function, and survival in patients with COPD [Citation28,Citation30].
Advanced age, smoking, alcohol consumption, and physical inactivity are examples of characteristics often seen in patients with COPD that are well-established risk factors for osteoporosis [Citation8,Citation13,Citation31]. The use of long-term oral corticosteroids is also a known risk factor for increased mortality and vertebral fractures in patients with COPD [Citation32]. In fact, oral glucocorticoids should only be used in the acute management of exacerbations of COPD [Citation1]. The results of systematic reviews preclude conclusions about the relationship between the use of inhaled corticosteroids and the risk of fracture and osteoporosis [Citation16,Citation17]. Furthermore, although COPD severity (e.g., GOLD stage III/IV) has been correlated with osteoporosis in a meta-analysis, other has reported opposite findings [Citation15,Citation16].
4.4. Which areas within broad topics are suitable for systematic review?
Evidence synthesis and further primary research are needed to better support clinical decision making in treating patients with COPD and osteoporosis. Although there are adequate guidelines for treating primary osteoporosis, guidance on the treatment and management of osteoporosis secondary to COPD is lacking [Citation13]. Considering the results of the present scoping review, topics that may be worth studying in future systematic reviews and potentially meta-analyses include the following: the utility of FRAX to predict and prevent osteoporosis and fractures in patients with COPD; the relationship between routes of administration, doses and duration of corticosteroid therapy and the incidence of osteoporotic outcomes; the impact of COPD severity stage, duration, and number of exacerbations on osteoporosis risk, adjusted for confounders (as current meta-analyses report conflicting results); and the effectiveness of performing routine diagnostic tests, such as DXA or chest CT, to monitor osteoporosis activity and its related outcomes in patients with COPD.
4.5. Which are the topics needing further primary research?
From an epidemiological point of view, the relationship between COPD and osteoporosis deserves further investigation into some aspects, such as the following: the correlation between FRAX scores and the occurrence of vertebral fractures in COPD patients (as most studies use FRAX to estimate risk, rather than measuring actual fractures); the impact of osteoporosis on COPD treatment goals, such as improving physical performance; the association between the use of long-term inhaled corticosteroids and the incidence of fractures (since results from meta-analyses are conflicting [Citation16,Citation17], and individual randomized controlled trials [RCTs] found no differences between using inhaled corticosteroids and inhaled non-steroid anti-inflammatory drugs regarding BMD changes and the incidence of fractures [Citation47–49]; the role of COPD as an independent risk factor for fractures (for example, a study in Italy revealed that COPD increased the risk of vertebral and non-vertebral fractures, independently from corticosteroids use and other risk factors) [Citation50]; prescription patterns of anti-resorptive medications or others (e.g., vitamin D) in patients with COPD and their relationship with osteoporotic outcomes; the impact of osteoporosis on the quality of life of patients with COPD. Such epidemiological studies should be carried out with controlling for confounding factors, such as age, smoking, BMI, BMD, and others.
5. Conclusion
Several studies investigating the relationship between COPD and osteoporosis have been carried out in recent years. Although there are aspects of the association between COPD and osteoporosis that are well-established (e.g., shared risk factors such as smoking, elderly, physical inactivity or low BMI), there are still questions to answer, namely the impact of the severity of COPD, and the long-term use of inhaled corticosteroids on the incidence of osteoporosis and fractures, as well as the value of performing routine DXA or chest CT, and of prescribing anti-resorptive medications to patients with COPD to prevent osteoporotic-related outcomes. Studies on the quality of life of patients with COPD and osteoporosis are also lacking. Investigating such issues would be important to propose clinical guidelines for screening, prevention, and treatment of osteoporosis in patients with COPD.
Supplemental Material
Download PDF (261.7 KB)Disclosure statement
The authors of this scoping review declare that they do not have any conflict of interest.
Additional information
Funding
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for prevention, diagnosis and management of COPD: 2024 report; 2024. [cited 2024 Apr 30]. https://goldcopd.org/2024-gold-report/.
- WHO. Chronic obstructive pulmonary disease (COPD). 2024. [cited 2024 May 3]. https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd).
- Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351. doi: 10.1016/S0140-6736(11)60968-9.
- Kanis JA, Cooper C, Rizzoli R, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3–44. doi: 10.1007/S00198-018-4704-5.
- Borgström F, Karlsson L, Ortsäter G, et al. Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos. 2020;15(1):59. doi: 10.1007/S11657-020-0706-Y.
- Kanis JA, Norton N, Harvey NC, et al. SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos. 2021;16(1):82. doi: 10.1007/S11657-020-00871-9.
- Sozen T, Ozisik L, Calik Basaran N. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4(1):46–56. doi: 10.5152/eurjrheum.2016.048.
- Pouresmaeili F, Kamalidehghan B, Kamarehei M, et al. A comprehensive overview on osteoporosis and its risk factors. Ther Clin Risk Manag. 2018;14:2029–2049. doi: 10.2147/TCRM.S138000.
- Stanojkovic I, Kotur-Stevuljevic J, Spasic S, et al. Relationship between bone resorption, oxidative stress and inflammation in severe COPD exacerbation. Clin Biochem. 2013;46(16–17):1678–1682. doi: 10.1016/J.CLINBIOCHEM.2013.08.003.
- Bossenbroek L, De Greef MHG, Wempe JB, et al. Daily physical activity in patients with chronic obstructive pulmonary disease: a systematic review. COPD. 2011;8(4):306–319. doi: 10.3109/15412555.2011.578601.
- Vorrink SNW, Kort HSM, Troosters T, et al. Level of daily physical activity in individuals with COPD compared with healthy controls. Respir Res. 2011;12(1):33. 10.1186/1465-9921-12-33.
- Rice JB, White AG, Scarpati LM, et al. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017;39(11):2216–2229. doi: 10.1016/J.CLINTHERA.2017.09.011.
- Li Y, Gao H, Zhao L, et al. Osteoporosis in COPD patients: risk factors and pulmonary rehabilitation. Clin Respir J. 2022;16(7):487–496. doi: 10.1111/CRJ.13514.
- Bitar A, Syed Sulaiman S, Ali I, et al. Osteoporosis among patients with chronic obstructive pulmonary disease: systematic review and meta-analysis of prevalence, severity, and therapeutic outcomes. J Pharm Bioallied Sci. 2019;11(4):310–320. doi: 10.4103/JPBS.JPBS_126_19.
- Chen YW, Ramsook AH, Coxson HO, et al. Prevalence and risk factors for osteoporosis in individuals with COPD: a systematic review and meta-analysis. Chest. 2019;156(6):1092–1110. doi: 10.1016/J.CHEST.2019.06.036.
- Peng S, Tan C, Du L, et al. Effect of fracture risk in inhaled corticosteroids in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. BMC Pulm Med. 2023;23(1):304. doi: 10.1186/s12890-023-02602-5.
- Zhang N, Fan X, Zhang Y, et al. Risk of fracture and osteoporosis in patients with COPD and inhaled corticosteroids treatment. Respir Care. 2023;68(12):1719–1727. doi: 10.4187/RESPCARE.10933.
- Peters MDJ, Godfrey C, McInerney P, et al. Chapter 11: scoping reviews (2020 version). In: Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. JBI; 2020. doi: 10.46658/JBIMES-20-12.
- Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850.
- Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393(10169):364–376. doi: 10.1016/S0140-6736(18)32112-3.
- Munn Z, Moola S, Lisy K, et al. Chapter 5: systematic reviews of prevalence and incidence. In: Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. JBI; 2020. doi: 10.46658/JBIMES-20-06.
- Akyea RK, McKeever TM, Gibson J, et al. Predicting fracture risk in patients with chronic obstructive pulmonary disease: a UK-based population-based cohort study. BMJ Open. 2019;9(4):e024951. doi: 10.1136/BMJOPEN-2018-024951.
- Bari MZJ, Patwary I, Hussain D, et al. Association of COPD with osteoporosis in male smokers: a case control study in a tertiary medical college hospital in Bangladesh. J Back Musculoskelet Rehabil. 2020;33(1):119–125. doi: 10.3233/BMR-181303.
- Salari N, Ghasemi H, Mohammadi L, et al. The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg Res. 2021;16(1):609. doi: 10.1186/S13018-021-02772-0.
- Ogura-Tomomatsu H, Asano K, Tomomatsu K, et al. Predictors of osteoporosis and vertebral fractures in patients presenting with moderate-to-severe chronic obstructive lung disease. COPD. 2012;9(4):332–337. doi: 10.3109/15412555.2012.667850.
- Lee PH, Kok VC, Chou PL, et al. Risk and clinical predictors of osteoporotic fracture in east Asian patients with chronic obstructive pulmonary disease: a population-based cohort study. PeerJ. 2016;4:e2634. doi: 10.7717/peerj.2634.
- Crisafulli E, Gorgone P, Vagaggini B, et al. Efficacy of standard rehabilitation in COPD outpatients with comorbidities. Eur Respir J. 2010;36(5):1042–1048. doi: 10.1183/09031936.00203809.
- Hornikx M, Van Remoortel H, Demeyer H, et al. The influence of comorbidities on outcomes of pulmonary rehabilitation programs in patients with COPD: a systematic review. Biomed Res Int. 2013;2013:146148–146148. doi: 10.1155/2013/146148.
- Li LSK, Caughey GE, Johnston KN. The association between co-morbidities and physical performance in people with chronic obstructive pulmonary disease: a systematic review. Chron Respir Dis. 2014;11(1):3–13. doi: 10.1177/1479972313516879.
- Kakoullis L, Sampsonas F, Karamouzos V, et al. The impact of osteoporosis and vertebral compression fractures on mortality and association with pulmonary function in COPD: a meta-analysis. Joint Bone Spine. 2022;89(1):105249. doi: 10.1016/J.JBSPIN.2021.
- Long G, Liu C, Liang T, et al. Predictors of osteoporotic fracture in postmenopausal women: a meta-analysis. J Orthop Surg Res. 2023;18(1):574. doi: 10.1186/S13018-023-04051-6.
- Chang YP, Lai CH, Lin CY, et al. Mortality and vertebral fracture risk associated with long-term oral steroid use in patients with chronic obstructive pulmonary disease: a systemic review and meta-analysis. Chron Respir Dis. 2019;16 doi: 10.1177/1479973119838280.
- Singh JM, Palda VA, Stanbrook MB, et al. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease: a systematic review. Arch Intern Med. 2002;162(22):2527–2536. doi: 10.1001/ARCHINTE.162.22.2527.
- Adas-Okuma MG, Maeda SS, Gazzotti MR, et al. COPD as an independent risk factor for osteoporosis and fractures. Osteoporos Int. 2020;31(4):687–697. doi: 10.1007/S00198-019-05235-9.
- Xu R, Zhang Y, Chen XC, et al. Association of bone mineral density with chronic obstructive pulmonary disease in postmenopausal women. Rev Invest Clin. 2019;71(3):204–210. doi: 10.24875/RIC.19002935.
- Madsen H, Brixen K, Hallas J. Screening, prevention and treatment of osteoporosis in patients with chronic obstructive pulmonary disease - a population-based database study. Clin Respir J. 2010;4(1):22–29. doi: 10.1111/j.1752-699X.2009.00138.x.
- Oschatz E, Prosch H, Kohansal R, et al. COPD and osteoporosis: detection and grading of vertebral fractures on lateral chest radiography. J Thorac Imaging. 2009;24(3):212–215. doi: 10.1097/RTI.0b013e3181aa8de9.
- Romme EA, Murchison JT, Phang KF, et al. Bone attenuation on routine chest CT correlates with bone mineral density on DXA in patients with COPD. J Bone Miner Res. 2012;27(11):2338–2343. doi: 10.1002/jbmr.1678.
- Graat-Verboom L, Spruit MA, van den Borne BE, et al. Correlates of osteoporosis in chronic obstructive pulmonary disease: an underestimated systemic component. Respir Med. 2009;103(8):1143–1151. doi: 10.1016/j.rmed.2009.02.014.
- Liao KM, Chiu KL, Chen CY. Prescription patterns in patients with chronic obstructive pulmonary disease and osteoporosis. Int J Chron Obstruct Pulmon Dis. 2021;16:761–769. doi: 10.2147/copd.S289799.
- Lee SH, Kwon HY. Prevalence of osteoporosis in Korean patients with chronic obstructive pulmonary disease and their health-related quality of life according to the Korea national health and nutrition examination survey 2008-2011. J Bone Metab. 2017;24(4):241–248. doi: 10.11005/JBM.2017.24.4.241.
- Tsukamoto M, Nabeshima T, Wang KY, et al. The impact of chronic obstructive pulmonary disease on bone strength. J Bone Miner Metab. 2024. doi: 10.1007/S00774-024-01496-5.
- Mou K, Chan SMH, Vlahos R. Musculoskeletal crosstalk in chronic obstructive pulmonary disease and comorbidities: emerging roles and therapeutic potentials. Pharmacol Ther. 2024;257:108635. doi: 10.1016/J.PHARMTHERA.2024.108635.
- Scanlon PD, Connett JE, Wise RA, et al. Loss of bone density with inhaled triamcinolone in lung health study II. Am J Respir Crit Care Med. 2004;170(12):1302–1309. doi: 10.1164/rccm.200310-1349OC.
- Whiting P, Wolff R, Mallett S, et al. A proposed framework for developing quality assessment tools. Syst Rev. 2017;6(1):204. doi: 10.1186/S13643-017-0604-6.
- Scharfe J, Pfisterer-Heise S, Kugler CM, et al. Language restrictions in systematic reviews should not be imposed in the search strategy but in the eligibility criteria if necessary. Syst Rev. 2021;12(1):11–147. doi: 10.1016/J.JCLINEPI.2020.12.027.
- Maltais F, Schenkenberger I, Wielders PLML, et al. Effect of once-daily fluticasone furoate/vilanterol versus vilanterol alone on bone mineral density in patients with COPD: a randomized, controlled trial. Ther Adv Respir Dis. 2020;14:1753466620965145. doi: 10.1177/1753466620965145.
- Kerwin EM, Ferguson GT, Mo M, et al. Bone and ocular safety of budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler in COPD: a 52-week randomized study. Respir Res. 2019;20(1):167. doi: 10.1186/S12931-019-1126-7.
- Ferguson GT, Calverley PMA, Anderson JA, et al. Prevalence and progression of osteoporosis in patients with COPD: results from the towards a revolution in COPD health study. Chest. 2009;136(6):1456–1465. doi: 10.1378/CHEST.08-3016.
- Adami G, Gatti D, Rossini M, et al. Risk of fracture in women with glucocorticoid requiring diseases is independent from glucocorticoid use: an analysis on a nation-wide database. Bone. 2024;179:116958. doi: 10.1016/J.BONE.2023.