Abstract
Chronic obstructive pulmonary disease (COPD) is preventable and requires early screening. The study aimed to examine the clinical values of long non-coding RNA (lncRNA) SNHG5 in COPD diagnosis and prognosis. Out of 160 COPD patients, 80 were in the stable stage and 80 were in the acute exacerbation of COPD stage (AECOPD). SNHG5 expression was detected via qRT-PCR. The survival analysis was conducted using Cox regression analysis and K-M curve. SNHG5 levels significantly reduced in both stable COPD and AECOPD groups compared with the control group, with AECOPD group recording the lowest values. SNHG5 levels were negatively correlated with GOLD stage. Serum SNHG5 can differentiate stable COPD patients from healthy individuals (AUC = 0.805), and can screen AECOPD from stable ones (AUC = 0.910). SNHG5 negatively influenced the release of inflammatory cytokines. For AECOPD patients, those with severe cough and wheezing dyspnea symptoms exhibited the lowest values of SNUG5. Among the 80 AECOPD patients, 16 cases died in the one-year follow-up, all of whom had low levels of SNHG5. SNHG5 levels independently influenced survival outcomes, patients with low SNHG5 levels had a poor prognosis. Thus, lncRNA SNHG5, which is downregulated in patients with COPD (especially AECOPD), can potentially protect against AECOPD and serve as a novel prognostic biomarker for AECOPD.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable, and treatable disease. It is characterized by persistent respiratory symptoms and limited airflow [Citation1]. The World Health Organization reports that about 210 million people worldwide are affected by COPD globally, with 3 million people dying from the disease annually [Citation2]. In China, nearly 100 million people have COPD, and its prevalence in people over 40 years old is 13.7% [Citation3]. Various factors, including environmental factors and genetic factors, contribute to COPD’s development, with smoking being a major cause [Citation4]. COPD’s clinical course is primarily marked by exacerbation, defined as a sudden worsening of respiratory symptoms requiring additional therapy. An acute exacerbation of COPD (AECOPD) can lead to severe respiratory failure and, in some cases, death, placing a significant burden on the healthcare system [Citation5]. AECOPD patients often have a poor short-term or long-term prognosis, making it crucial to evaluate their prognosis accurately.
Biological indicators have long been a research focus in predicting COPD, yet no definitive supporting evidence exists [Citation6]. Long non-coding RNAs (lncRNAs) are functional non-coding RNAs longer than 200 nucleotides, lacking an open read frame. Recent evidence suggests that lncRNA plays an important role in cellular inflammation, malignant tumors, autoimmunity, and vascular diseases [Citation7,Citation8]. They are considered potential biomarkers in disease screening and diagnosis due to their sensitivity and specificity [Citation9]. Small nucleolar RNA host gene 5 (SNHG5), a well-defined cytoplasmic lncRNA, is identified to be a downregulated lncRNA in COPD tissues based on microarray analysis [Citation10]. Recent studies also found decreased expression of SNHG5 in cigarette smoke extract (CSE)-induced COPD cell models. Overexpression of SNHG5 is suggested to alleviate CSE-caused 16HBE cell apoptosis and inflammation [Citation11]. However, the diagnostic accuracy of lncRNA SNHG5 in COPD remains unexamined.
Hence, this study aims to explore the expression pattern of lncRNA SNHG5 in COPD and AECOPD cases, highlighting the protective role of SNHG5 in COPD and its impact on disease prognosis.
Materials and methods
Study cohort
A total of 160 patients with COPD were recruited from Zhangjiakou First Hospital. Of these, 80 were in the stable stage and 80 were in the AECOPD stage. Stable COPD patients met the following criteria: (1) fulfilled the Global Initiative for Obstructive Lung Disease (GOLD) criteria for COPD based on the ratio of post-bronchodilator FEV1 to FVC < 0.70 [Citation12]; (2) without exacerbations for at least 3 months; (3) without other lung diseases; (4) without severe infection; (5) with no history of tumors, malignant hematologic diseases, and autoimmune diseases. The inclusion criteria of AECOPD patients were the following: (1) met the criteria for COPD based on the diagnostic criteria according to the 2020 GOLD report; (2) were in acute exacerbation phase according to the definition of an acute worsening of respiratory symptoms (including an increased degree of dyspnea and cough, purulent sputum, or increases in sputum) that resulted in the need for additional treatment; (3) did not receive oral or intravenous corticosteroids or any other anti-inflammatory drugs in the preceding four weeks, given the possibility that anti-inflammatory drugs may be able to change the expression of lncRNA. Patients with a history of severe infection, tumors, autoimmune diseases and malignant hematologic diseases were excluded from the study, and they all did not complicate with relevant lung diseases, such as asthma and lung cancer. All AECOPD cases were followed up for one year to record the survival outcome. In addition, 80 healthy individuals who visited the same hospital for physical examination were included in the healthy control group. These individuals had no history of respiratory diseases, including COPD, asthma, interstitial lung disease, bronchiectasis, and more.
Each participant signed an informed consent before enrollment. The Ethics Committee of Zhangjiakou First Hospital provided the ethical approval.
Sampling and laboratory investigations
Each subject’s demographic characteristics, including age, sex, body mass index (BMI) and smoking status were recorded. Their comorbidities, such as cardiovascular disease, diabetes mellitus and hypertension were also recorded. The cough frequency was recorded and the wheezing dyspnea was assessed using the scores of modified British Medical Research Council (mMRC) questionnaire. Participants were categorized as having no (mMRC score = 0), mild (mMRC score = 1), moderate (mMRC score = 2 or 3), or severe (mMRC score = 4) dyspnea. The predicted forced expiratory volume in one second (FEV1%) was measured to evaluate the lung function.
Of 5 mL serum samples were taken from each participant in the morning for laboratory tests. A Mindray BC-5000 automatic blood cell analyzer (Mindray, Beijing, China) was used for the measurement of white blood cell count (WBC), platelet and neutrophil count, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) in all subjects. Human enzyme-linked immunosorbent assay (ELISA) assay was performed for the test of the inflammatory cytokines including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in the serum of all subjects.
Real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted and isolated from serum samples using TRIzol reagent. The RNA purity and concentration were determined by NanoDrop ND2000 spectrophotometer. Then 1 μg of total RNA was reverse transcribed into cDNA by PrimeScriptTM RT reagent kit (Takara, San Jose, CA), followed by RT-qPCR by adding TB Green Fast qPCR mix kit and primers. The relative expression of lncRNA was calculated using the comparative Ct method and normalized to the levels of GAPDH.
Statistical analysis
SPSS version 23.0 software (Chicago, IL) and GraphPad Prism version 9.0 (La Jolla, CA) were applied for data analysis and visualization. The continuous variables were presented as mean ± standard deviation (SD), and compared between groups via student’s t test or one-way ANOVA followed by Tukey’s post-test for multiple comparisons. Categorical variables were shown as numbers and percentages (%), while chi-square test was used for the difference comparison. The correlation of lncRNA SNHG5 expression with inflammation-related parameters was evaluated using the Pearson correlation coefficient, while the association of SNHG5 with GOLD stage was assessed via Spearman correlation analysis. The survival analysis was conducted using Cox-regression survival analysis and Kaplan–Meier (K–M) survival analysis. In the multiple Cox regression model, SNHG5 level (0: ≤ mean value; 1: > mean value), CRP (0: ≤ mean value; 1: > mean value), Platelet (0: ≤ mean value; 1: > mean value), GOLD stage (0: 1–2; 1: 3–4), disease course (0: ≤ mean value; 1: > mean value), cardiovascular disease (0: No; 1: Yes), smoke (0: No; 1: Yes) were considered as variables. A p value of less than 0.05 was considered statistically significant.
Results
Demographic and clinical data of the study groups
presents the demographic and clinical data of the three study groups. The groups were age and sex-matched with no significant distribution differences in distribution (p > 0.05). There were also no significant differences in BMI, percentage of current smokers, and comorbidities (p > 0.05). Compared to the control group, both the stable COPD and AECOPD groups had lower values of FEV1, FVC, FEV1/FVC, and FEV1% predicted (p < 0.001). However, there was no significant difference in the disease course between stable COPD and AECOPD groups. A larger proportion of cases in the AECOPD group were in GOLD Stages 2 and 3 compared with the stable COPD group (p < 0.001). Regarding laboratory examination indexes, the values of WBC, ESR, CRP, TNF-α, and IL-6 were higher in both stable COPD and AECOPD groups than the control group with the significant difference among three groups (p < 0.001), and the AECOPD group had the highest values. But there was no significant difference in the values of neutrophil and platelet (p > 0.05). The clinical symptoms of all AECOPD patients were also recorded. It can be seen that most cases were coughed morning only or episodes during day, less cases had no cough or nearly cough continuous. Based on mMRC score, the wheezing dyspnea was also recorded. Most cases had a score of 2–3, fewer cases had score 1 and 4, while only one case had score 0.
Table 1. Clinical characteristics of the study population.
The levels of lncRNA SNHG5 in COPD patients
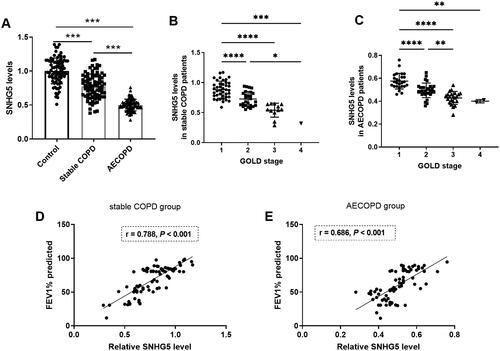
shows a significant difference in the expression of lncRNA SNHG5 in the serum among the three groups (p < 0.05). Both the stable COPD and AECOPD groups had significantly lower SNHG5 levels than the control group. Notably, AECOPD group had the lowest SNHG5 levels, significantly lower than the stable COPD group (p < 0.05). Based on the GOLD stage, cases in stable COPD and AECOPD groups were divided into four groups, it was found that cases with high GOLD stage had low SNHG5 levels in both stable COPD and AECOPD groups (). As depicted in , levels of SNHG5 were positively correlated with FEV1% predicted in both stable COPD (r = 0.788, p < 0.001) and AECOPD groups (r = 0.686, p < 0.001).
Figure 1. Levels of lncRNA SNHG5 in COPD patients. A) SNHG5 levels significantly decreased in both stable COPD and AECOPD groups compared with the control group. B) Levels of SNHG5 in stable COPD patients with different GOLD stage. C) Levels of SNHG5 in AECOPD patients with different GOLD stage. D) Levels of SNHG5 were positively correlated with FEV1% predicted in stable COPD group. E) Levels of SNHG5 were positively correlated with FEV1% predicted in AECOPD group. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
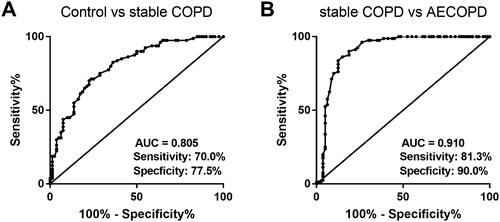
The diagnostic value of lncRNA SNHG5 in COPD
ROC curve was plotted to assess the diagnostic value of SNHG5 in COPD. As seen in , serum SNHG5 can differentiate stable COPD patients from healthy individuals. The AUC was 0.805 with the sensitivity of 70.0% and the specificity of 77.5%. illustrates the predictive effectiveness of SNHG5 in differentiating AECOPD patients from stable ones, with an AUC of 0.910, a sensitivity of 81.3% and a specificity of 90.0%.
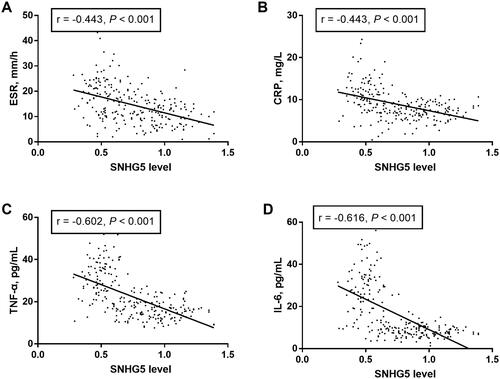
The relationship of lncRNA SNHG5 with inflammatory response in COPD patients
Pearson’s correlation analysis was used to determine the linear correlation between lncRNA SNHG5 and concentrations of inflammation-related factors (). We found significantly negative correlations of SNHG5 levels with ESR (r = −0.443, p < 0.001; ), CRP (r = −0.443, p < 0.001; ), TNF-α (r = −0.602, p < 0.001; ) and IL-6 (r = −0.616, p < 0.001; ), indicating that SNHG5 negatively regulates inflammation in patients.
Prognostic value analysis of SNHG5 in COPD
The association of SNHG5 values with the clinical symptoms in AECOPD cases was evaluated. According to the cough status, all AECOPD patients were divided into three groups. As shown in , patients who coughed nearly continuously had the lowest values of SNHG5. Based on mMRC score, all AECOPD patients were divided into three groups, namely no and mild dyspnea (n = 12), moderate dyspnea (n = 58), and severe dyspnea (n = 10). It can be seen that patients with moderate to severe dyspnea had lower serum SNHG5 levels compared to those with no or mild dyspnea, with the lowest levels observed in the severe group (). The results indicated that SNHG5 was closely related to worse clinical symptoms of AECOPD patients, reflecting its possible link to patients’ survival.
Figure 4. Prognostic value analysis of SNHG5 in COPD. A) Serum SNHG5 levels in AECOPD patients with various cough frequencies. *p < 0.05; ***p < 0.001 B) Serum SNHG5 levels in AECOPD patients with different degree of wheezing dyspnea. ***p < 0.001. C) Serum SNHG5 levels were downregulated in deceased patients compared to survivors. ***p < 0.001. D) Forest map of factors related to increased risks of death based on cox regression analysis. E) Kaplan–Meier plot of SNHG5 in predicting patients’ outcomes.
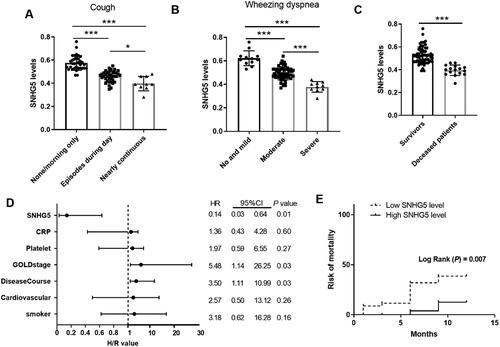
According to the one-year follow-up, 16 cases out of 80 AECOPD patients died from COPD. The causes of death included chronic pulmonary heart disease (n = 8), chronic respiratory failure (n = 7), and pneumothorax (n = 1). The demographic and clinical data of survivors and deceased patients were initially compared. As shown in , among deceased patients, there was a higher proportion of current smokers and those with cardiovascular disease compared with the survivors (p < 0.05). In addition, the GOLD stage, platelet, and CRP concentration differed significantly between the two groups (p < 0.05). SNHG5 levels were also compared between survivors and the deceased group. In , a significant downtrend of serum SNHG5 levels was detected in deceased patients compared to survivors (p < 0.001). To explore the independent relationship between SNHG5 and patients’ survival, cox regression analysis was done. As seen in , SNHG5 (HR = 0.14, 95% CI = 0.03–0.64, p = 0.01), GOLD stage (HR = 5.48, 95% CI = 1.14–26.25, p = 0.03) and disease course (HR = 3.50, 95% CI = 1.11–10.99, p = 0.03) were independently influence factors for the survival outcome of AECOPD patients. Then K–M plot showed that there was a significant difference in the survival rate of AECOPD patients between low SNHG5 expression and high SNHG5 expression groups (p = 0.007), and patients with low SNHG5 levels had high risk of mortality ().
Table 2. Comparison of clinical characteristics between survival and deceased group.
Discussion
COPD has a high incidence and poses a heavy economic burden, making its screening and management challenging [Citation13]. Therefore, its scientific and reasonable prevention and control of COPD are of great importance [Citation14]. In our study, we detected the abnormal expression of lncRNA SNHG5 in the serum of COPD patients, which correlated with disease severity. Moreover, serum SNHG5 levels were correlated with the inflammatory response of COPD, it may serve as a biomarker for disease severity and the clinical prognosis of COPD patients.
In recent decades, accumulating evidence has suggested the involvement of lncRNAs in the molecular mechanisms of COPD [Citation15]. For example, highly expressed CCAT1 is detected in lung tissues of COPD patients, and CCAT1 knockdown can ameliorate cigarette smoke-mediated airway inflammation [Citation16]. In addition, differentially expressed lncRNAs in COPD lung tissues have been identified by microarray analysis, indicating their key role in the diagnosis of patients with COPD [Citation10]. Our recent findings indicate lower levels of SNHG5 in the serum of stable COPD patients. Previous evidence has also reported the low expression of lncRNA SNHG5 in COPD patients, and its protective role against human bronchial epithelial cell injury [Citation11]. In addition, in the progress of acute respiratory distress syndrome, acute lung injury occurs along with the downregulation, and upregulation of SNHG5 can inhibit cell apoptosis and inflammation of LPS-induced A549 cells [Citation17]. These findings underscore the significant role of SNHG5 in COPD. Moreover, serum SNHG5 levels can differentiate stable COPD patients from healthy people based on the ROC curve. All COPD patients were staged based on the GOLD stage, and SNHG5 levels were compared across groups. We observed a negative correlation between serum SNHG5 levels and COPD severity. Additionally, the ROC curve demonstrated that SNHG5 effectively differentiates AECOPD patients from stable ones, indicating its predictive value in AECOPD. Despite these findings, only two independent stable COPD and AECOPD groups were enrolled in this study. Further research should examine the dynamic changes of serum SNHG5 levels during both stable state and exacerbated stage for COPD cases to reflect its predictive value in the development of AECOPD.
COPD, a chronic inflammatory disease, can intensify during acute exacerbations [Citation18]. The inflammatory response plays a key role in the development of COPD [Citation19]. As previous evidence reported, SNHG5 is regarded as a master regulator of inflammatory responses [Citation20]. This study explored the correlation of SNHG5 with inflammatory response in COPD patients. The results showed a negative correlation between serum SNHG5 levels and ESR, CRP, TNF-α, and IL-6 concentrations. IL-6, a common inflammatory factor in lung diseases, is mainly produced by mononuclear macrophages, T cells, B cells, and other cells. It is closely related to immune response and inflammation, and plays an important role in the body’s anti-infection immune response. IL-6’s significant role in the pathogenesis of COPD, indicating the existence of an inflammatory response, is notably increased in COPD patients [Citation21]. ESR can be used as one of the inflammatory response markers of inflammation and tissue injury, while CRP is one of the sensitive indicators of bacterial infection and can be used as one of the indicators to reflect inflammation, tissue injury degree, and evaluate treatment effect [Citation22]. When there is inflammation and tissue damage in the body, ESR is accelerated, and CRP, IL-6, and other things are released rapidly [Citation23]. These findings suggest that SNHG5 negatively regulates inflammation in patients.
AECOPD can severely damage lung function, with a mortality rate 20–25% within one year [Citation24]. As previous clinical studies reported, COPD can lead to serious complications and a poor prognosis for the patients [Citation25]. Based on the follow-up information, 16 out of 80 AECOPD patients died from COPD within a year, which aligns with previous reports [Citation24]. Moreover, a significant downtrend of serum SNHG5 levels was detected in patients who eventually died compared to survivors. Therefore, the prognostic value of serum SNHG5 in AECOPD was further explored. As expected, SNHG5 was identified s an independent factor influencing the survival outcome of AECOPD patients. Patients with low SNHG5 levels had a worse prognosis. In addition, disease course and GOLD stage were also identified to be independently related to patients’ prognosis.
In conclusion, lncRNA SNHG5 was determined to be downregulated in patients with COPD, especially those with AECOPD. Serum SNHG5 could serve as a novel diagnostic and prognostic biomarker for COPD. Our data may provide a novel insight into the clinical value of lncRNA SNHG5 in managing COPD.
Ethical approval and consent to participate
An informed consent was signed by each participant before enrollment. The ethical approval was gained from the Ethics Committee of Zhangjiakou First Hospital.
Author contributions
Study concept and design: X. T. Y., X. P. L.; analysis and interpretation of data: W. P. F., Z. P. G.; drafting of the manuscript: X. T. Y., W. P. F.; critical revision of the manuscript for important intellectual content: X. P. L.; statistical analysis: Z. P. G.
Declaration of interest
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Yu C, Zhang L. Methylprednisolone up-regulates annexin A1 (ANXA1) to inhibit the inflammation, apoptosis and oxidative stress of cigarette smoke extract (CSE)-induced bronchial epithelial cells, a chronic obstructive pulmonary disease in vitro model, through the formyl peptide receptor 2 (FPR2) receptors and the adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK) pathway. Bioengineered. 2022;13(2):1–9. doi: 10.1080/21655979.2022.2031769.
- Alupo P, Wosu AC, Mahofa A, et al. Incidence and predictors of COPD mortality in Uganda: a 2-year prospective cohort study. PLoS One. 2021;16(2):e0246850. doi: 10.1371/journal.pone.0246850.
- Xu Z, Zhu L, Zhan J, et al. The efficacy and safety of high-flow nasal cannula therapy in patients with COPD and type II respiratory failure: a meta-analysis and systematic review. Eur J Med Res. 2021;26(1):122. doi: 10.1186/s40001-021-00587-7.
- Skurikhin E, Pershina O, Zhukova M, et al. Spiperone stimulates regeneration in pulmonary endothelium damaged by cigarette smoke and lipopolysaccharide. Int J Chron Obstruct Pulmon Dis. 2021;16:3575–3591. doi: 10.2147/COPD.S336410.
- Hoult G, Gillespie D, Wilkinson TMA, et al. Biomarkers to guide the use of antibiotics for acute exacerbations of COPD (AECOPD): a systematic review and meta-analysis. BMC Pulm Med. 2022;22(1):194. doi: 10.1186/s12890-022-01958-4.
- Furci F, Murdaca G, Pelaia C, et al. TSLP and HMGB1: inflammatory targets and potential biomarkers for precision medicine in asthma and COPD. Biomedicines. 2023;11(2):437. doi: 10.3390/biomedicines11020437.
- Zheng PF, Chen LZ, Liu P, et al. A novel lncRNA-miRNA-mRNA triple network identifies lncRNA XIST as a biomarker for acute myocardial infarction. Aging (Albany NY). 2022;14(9):4085–4106. doi: 10.18632/aging.204075.
- Han T, Liao A. CASC7: a LncRNA with potential clinical application. Int J Radiat Biol. 2022;98(10):1510–1518.
- Xu X, Liang Y, Gareev I, et al. LncRNA as potential biomarker and therapeutic target in glioma. Mol Biol Rep. 2023;50(1):841–851. doi: 10.1007/s11033-022-08056-y.
- Tang W, Shen Z, Guo J, et al. Screening of long non-coding RNA and TUG1 inhibits proliferation with TGF-beta induction in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2951–2964. doi: 10.2147/COPD.S109570.
- Shen Q, Zheng J, Wang X, et al. LncRNA SNHG5 regulates cell apoptosis and inflammation by miR-132/PTEN axis in COPD. Biomed Pharmacother. 2020;126:110016. doi: 10.1016/j.biopha.2020.110016.
- Neumeier A, Keith R. Clinical guideline highlights for the hospitalist: the GOLD and NICE guidelines for the management of COPD. J Hosp Med. 2020;15(4):240–241. doi: 10.12788/jhm.3368.
- Şerifoğlu İ, Ulubay G. The methods other than spirometry in the early diagnosis of COPD. Tuberk Toraks. 2019;67(1):63–70. doi: 10.5578/tt.68162.
- Fazleen A, Wilkinson T. Early COPD: current evidence for diagnosis and management. Ther Adv Respir Dis. 2020;14:1753466620942128. doi: 10.1177/1753466620942128.
- Xie J, Wu Y, Tao Q, et al. The role of lncRNA in the pathogenesis of chronic obstructive pulmonary disease. Heliyon. 2023;9(11):e22460. doi: 10.1016/j.heliyon.2023.e22460.
- Zong D, Liu X, Li J, et al. LncRNA-CCAT1/miR-152-3p is involved in CSE-induced inflammation in HBE cells via regulating ERK signaling pathway. Int Immunopharmacol. 2022;109:108818. doi: 10.1016/j.intimp.2022.108818.
- Wang J, Zhang Y, Zhang L. Long non-coding RNA SNHG5 suppresses the development of acute respiratory distress syndrome by targeting miR-205/COMMD1 axis. Mol Cell Biochem. 2021;476(2):1063–1074. doi: 10.1007/s11010-020-03972-8.
- Wang Y, Xu J, Meng Y, et al. Role of inflammatory cells in airway remodeling in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3341–3348. doi: 10.2147/COPD.S176122.
- Guo P, Li R, Piao TH, et al. Pathological mechanism and targeted drugs of COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1565–1575. doi: 10.2147/COPD.S366126.
- Li H, Xuan J, Zhang W, et al. Long non-coding RNA SNHG5 regulates ulcerative colitis via microRNA-375/Janus kinase-2 axis. Bioengineered. 2021;12(1):4150–4158. doi: 10.1080/21655979.2021.1953219.
- Grubek-Jaworska H, Paplinska M, Hermanowicz-Salamon J, et al. IL-6 and IL-13 in induced sputum of COPD and asthma patients: correlation with respiratory tests. Respiration. 2012;84(2):101–107. doi: 10.1159/000334900.
- Corsonello A, Pedone C, Battaglia S, et al. C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) as inflammation markers in elderly patients with stable chronic obstructive pulmonary disease (COPD). Arch Gerontol Geriatr. 2011;53(2):190–195. doi: 10.1016/j.archger.2010.10.015.
- Mosrane Y, Bougrida M, Alloui AS, et al. [Systemic inflammatory profile of smokers with and without COPD]. Rev Pneumol Clin. 2017;73(4):188–198. doi: 10.1016/j.pneumo.2017.07.003.
- Shu B, Li H, Zhou X, et al. Efficacy and safety of Re Du ning injection for acute exacerbations of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2022;2022:7479639–7479610. doi: 10.1155/2022/7479639.
- Peltola L, Pätsi H, Harju T. COPD comorbidities predict high mortality - asthma-COPD-overlap has better prognosis. COPD. 2020;17(4):366–372. doi: 10.1080/15412555.2020.1783647.