Abstract
Gap junction channels composed of connexin43 (Cx43) are essential for normal myogenic differentiation and skeletal muscle regeneration. Here, the aim was to study whether lithium chloride (LiCl) could regulate Cx43 expression and gap junction channel function by mimicking the Wnt/β-catenin pathway in primary myoblasts. Cx43 mRNA expression in myoblasts was up-regulated in response to 5 mM LiCl. The enhanced Cx43 protein expression resulting from treatment with 5 and 10 mM LiCl for 24 h increased gap-junctional coupling in myoblasts. However, no obvious changes were observed with 20 mM LiCl. Furthermore, chronic treatment with 10 mM LiCl decreased Cx43 protein expression compared with untreated cells. The authors showed that LiCl mimicked the active canonical Wnt/β-catenin signaling by glycogen synthase kinase-3β (GSK-3β) inactivation and accumulation of the effector protein β-catenin into the nucleus. These results suggest that LiCl regulates Cx43 expression in skeletal myoblasts in vitro partly by a Wnt/β-catenin–dependent pathway.
INTRODUCTION
Gap junctions are clusters of intercellular hemichannels (connexons) that enable direct cell-cell signaling and propagation of electrical activity, as well as allowing for the exchange of ions and small molecules such as secondary messengers (Loewenstein Citation1981; Bennett et al. Citation1991; Kumar and Gilula Citation1996). Each connexon consists of six tetraspan connexin (Cx) protein molecules and several different connexins have been detected in early developing myoblasts, but only connexin43 (Cx43) may be needed for normal regenerative myogenesis (Araya et al. Citation2005). However, there are no gap junctions in adult skeletal muscles (Araya et al. Citation2005; Dermietzel et al. Citation1990; Gorbe et al. Citation2005). A previous study shows that myogenin mRNA expression and myotube formation during differentiation in the L6 myogenic cell line are reversibly inhibited by pharmacological gap junction blockers (Proulx et al. Citation1997). Recently, Gorbe and his colleagues argue that Cx43 expression in regenerating muscle is correlated with myoblast proliferation and fusion into myotubes. Therefore, these reports highlight that gap junction coupling plays a critical role in myotube formation (Gorbe et al. Citation2005, Citation2006, Citation2007).
Lithium chloride (LiCl), an agent used to treat manic depressive illness, has been shown to inhibit glycogen synthase kinase-3β (GSK-3β) and mimic the active effects of the canonical Wnt signaling on gene expression and cell proliferation (Klein and Melton Citation1996; Williams et al. Citation2002). The Wnt family consists of secreted glycosylated lipid–modified proteins, which are powerful regulators in embryonic development, cell differentiation, proliferation, and migration (Logan and Nusse Citation2004). In cells lacking Wnt signaling, GSK-3β phosphorylates β-catenin, inducing rapid degradation of β-catenin via the ubiquitin/proteasome pathway. However, the canonical Wnt signaling causes the stabilization of β-catenin and the translocation of which into nuclei where it forms a complex with T-cell transcription factors/lymphoid–enhancer-binding factor (TCF/LEF). Recently, the Wnt family of genes was confirmed to influence cell-cell communication during both embryonic development and adult life (Cadigan and Nusse Citation1997). Wnt-1 gene has been shown to promote gap-junctional communication between ventral cells of the developing Xenopus embryo (Olson et al. Citation1991, Citation1992). In addition, the ectopic expression of Wnt-1 in the limb mesenchyme of transgenic mice increases the local accumulation of Cx43 transcripts (Meyer et al. Citation1997). In rat pheochromocytoma (PC12) cells, a study suggests that Cx43 gene may be a downstream target of Wnt-1 signaling (van der Heyden et al. Citation1998). Ai and his colleagues also report that Wnt signaling is an important modulator of Cx43-dependent intercellular coupling in the heart (Ai et al. Citation2000). Furthermore, some evidence demonstrate that the Wnt pathway is critical for skeletal muscle development (Cossu and Borello Citation1999; Snider and Tapscott Citation2003; Tajbakhsh et al. Citation1998). For example, Wnt-1 and Wnt-3 are secreted by the dorsal neural tube and required for induction of myogenesis during mouse development through the canonical Wnt/β-catenin pathway. GSK-3β, as a component in Wnt signaling, plays an important role in muscle differentiation during embryogenesis and also has an important negative regulatory role in myogenesis (Rochat et al. Citation2004).
However, the relationship between Wnt signaling and Cx43 expression in skeletal myoblasts has not, to our knowledge, been previously investigated. Accordingly, we embarked upon this study to determine whether LiCl regulated Cx43 expression and gap-junctional communication in myoblasts though a Wnt/β-catenin–dependent pathway.
MATERIALS AND METHODS
Cell Culture
Primary muscle cell cultures from forelimbs of neonatal Wistar rats were isolated followed by an enzymatic dissociation method using collagenase I (1.0 mg/ml; Sigma) and then trypsine (0.25%; Sigma). Cells were resuspended in growth medium (GM; F-10 medium containing 20% fetal bovine serum [FBS]) until ∼80% confluence and then changed to differentiation medium (DM; F-10 medium containing 5% FBS) supplied with 5, 10, or 20 mM LiCl for various lengths of time (24, 48, and 72 h). The primary muscle cells contained >90% desmin-positive myoblasts. The specificity of LiCl action was investigated with 20 mM NaCl. All experimental protocols were approved by the Harbin Medical University Medical Animal Care and Use Committee.
RT-PCR Analysis
Total RNA was isolated with TRIzol RNA extraction reagents (Invitrogen) and reverse transcription proceeded with 1 µg of total RNA using ThermoScript reverse transcriptase–polymerase cahin reaction (RT-PCR) System (Invitrogen). The primers sequences for PCR were Cx43 (297 bp): forward 5′-ATGGCTGCTCCTCACCAACG-3′, reverse 5′-GGTCGTTGGTCCACGATGGC-3′; β-actin (490 bp): forward 5'-GGCTACAGCTTCACCACCAC-3′, reverse 5′-GCTTGCTGATCCACATCTGC-3′. Thirty cycles of amplification were carried out as follows: at 94°C for 30 s, at 60°C for 30 s, and at 72°C for 1 min using an BIOER XP cycler. PCR products were analyzed by running on a 2.0% agarose gel and using the Tanon GIS2010 Analysis software.
Western Blot Analysis
Total cell homogenates were harvested by lysing washed primary myoblasts in RIPA: 1× phosphate-buffered saline (PBS), 1% NP40, 0.1% sodium dodecyl sulfate (SDS), 5 mM EDTA, 0.5% sodium deoxycholate, 1 mM sodium orthovanadate, and 1/100 phenylmethylsulfonyl fluoride (PMSF). Fifty micrograms of the total protein, which was assayed by Bradford Protein Assay Kit (Beyotime), was separated on 10% SDS–polyacrylamide gel electrophoresis (PAGE) and then transferred to PVDF membrane. After blocking, the membranes were probed with a rabbit monoclonal antibody for total GSK-3β (1:1000; Cell Signaling), a rabbit polyclonal antibody against phospho-GSK-3β (Ser9) (1:1000; Cell Signaling), and a mouse monoclonal antibody for Cx43 (1:500; Zymed). Detection of alkaline phosphatase–conjugated secondary antibodies (1:1000; Santa Cruz) was done by reaction with freshly prepared Nitro blue tetrazolium chloride(NBT)/5-Bromo-4-chloro-3-indolyl phosphate,toluidine salt (BCIP) substrate solution. Blots were scanned and analyzed using the Tanon GIS2010 Analysis software.
Confocal Immunofluorescence Microscopy
Coverslips covered with myoblasts were rinsed in PBS and fixed with cold 4% paraformaldehyde for 30 min, and washed a further three times. After blocking with goat serum, slides were incubated with the primary antibodies directed against Cx43 (1:100; Zymed) and β-catenin (1:50; Santa Cruz) for overnight at 4°C and after several washes, with flourescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody for 60 min at 37°C. All nuclei were stained by adding 4′,6-diamidino-2-phenylindole (DAPI) (0.1 µg/ml; Sigma) for 5 min. Images were recorded with a confocal laser-scanning microscope (Olympus Fluoview V5.0 FV300).
Dye Transfer Assay
Gap-junctional dye transfer was measured between skeletal myoblasts with a flow-cytometric assay as described previously (Tomasetto et al. Citation1993). Donor myoblasts were labeled with calcein AM (5 µmol/L; Molecular Probe) for 45 min at 37°C. Recipient myoblasts were labeled with PKH26 (2×106 M; Sigma) and then added to a monolayer of myoblasts labeled with calcein AM. Cells were cocultured until the recipient myoblasts attached and then were changed to the medium with 5, 10, and 20 mM LiCl or 20 mM NaCl for 24 h. Dye transfer measured by flow cytometry was carried out using a Becton-Dickenson FACSORT flow cytometer. In addition, fluorescence images were captured by using a confocal laser scanning microscope.
Statistical Analysis
Statistical differences between means were determined by analysis of variance (ANOVA) followed by least significant difference (LSD) analysis, the student's t test for the analysis of the immunostaining intensity for Cx43, and paired-sample t test for different time-course comparision. Values are expressed as means±SEM, with p<0.05 considered to be significant.
RESULTS
LiCl Regulates Cx43 Expression and Gap-Junctional Communication in Skeletal Myoblasts
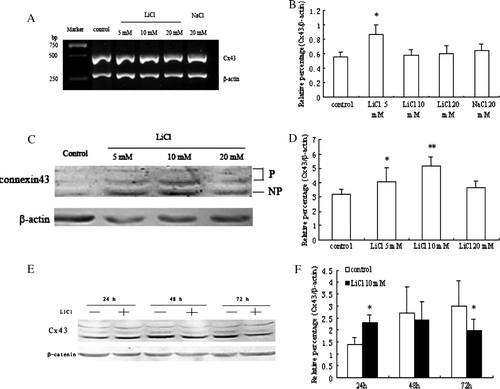
We first determined whether Cx43 expression can be regulated by LiCl in skeletal myoblasts. As shown in A and B, Cx43 expression was increased at the mRNA level in cells treated with 5 mM LiCl. Western blot analysis showed that the enhanced expression of Cx43 protein (phosphorylated and nonphosphorylated forms) was observed in the presence of 5 and 10 mM LiCl for 24 h compared with control cells and there was no obvious change with treatment with 20 mM LiCl (C, D). Treatment with 20 mM NaCl was without any effect (data not shown). The expression of Cx43 protein in primary myoblasts with DM was up-regulated from 24 up to 72 h. Compared with untreated cells, treatment with 10 mM LiCl for 24 h enhanced Cx43 expression in myoblasts, but led to the decrease of Cx43 expression from 48 to 72 h (E, F).
Figure 1. The effects of LiCl on Cx43 expression in myoblasts. (A) RT-PCR analysis of Cx43 mRNA expression in different treatment groups. (C) Western blot analysis of Cx43 expression in skeletal myoblasts with various concentration of LiCl treatment. (E) Treatment with 10 mM LiCl from 24 to 72 h. Cx43 was detected as three bands corresponding to the phosphorylation states and unphosphorylation states of this connexin. (B, D, F) Quantitative analyses of Cx43 mRNA and protein expression are shown in the graphs as relative percentage (means±SEM) from n =3–4 independent experiments. *p < 0.05 and **p < 0.01 versus untreated cells.
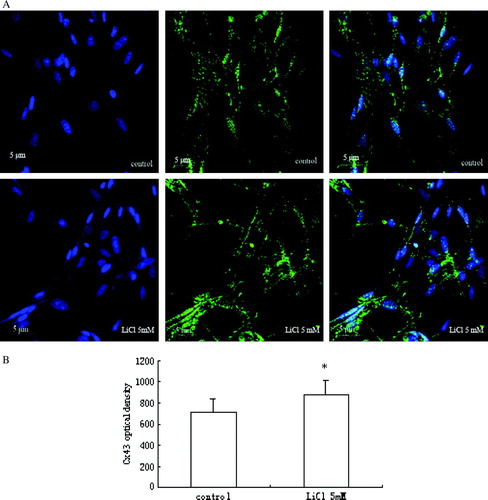
As shown in A and B, the results of confocal immunofluorescence microscopy revealed that Cx43 expression in myoblasts was increased after treatment with 5 mM LiCl for 24 h, as compared to the low level detected in untreated cells. Small green fluorescent dots, which represented Cx43 expression, were distributed both in the cytoplasm, particularly in the perinuclear regions, and at the cell surface.
Figure 2. (A) Fluorescent images of Cx43 expression in skeletal myoblasts. Cx43 is expressed less in control cells with DM, and after LiCl stimulation, its expression increases. (B) A quantitative analysis of the immunostaining intensity for Cx43 expression is shown in the graph as relative percentage (mean±SEM) from three independent experiments. *p < 0.05 versus control.
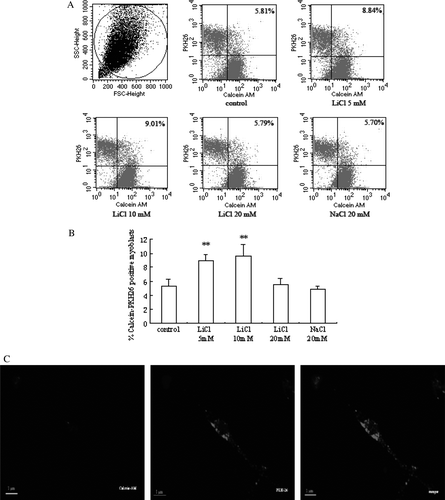
To see if there was functional improvement of gap junction permeability in myoblasts due to LiCl stimulation, intercelluar communication via gap junctions was detected by dye transfer assay (A). The efficacy of dye spreading in control myoblasts was very low. After myoblasts were incubated in medium containing LiCl or NaCl for 24 h, the extent of dye transfer increased in the presence of 5 and 10 mM LiCl, and there were no obvious changes observed in cells treated with 20 mM LiCl and NaCl (B). In addition, we also observed the coupling between myoblasts stained with the gap junction–permeable fluorescent dye calcein (green fluorescence) and PKH26-labeled myoblasts (red fluorescence) with a confocal laser scanning microscope. The cells had red and green fluorescence simultaneously, suggesting that they are linked to other myoblasts by gap junctions (C).
Figure 3. Gap-junctional communication between myoblasts. (A) Representative flow-cytometric analyses after recipient myoblasts labeled with PKH26 and donor myoblasts stained with calcein AM were cocultured for 24 h in culture medium containing 5, 10, and 20 mM LiCl and 20 mM NaCl, respectively. PKH26-calcein–positive myoblasts are in right upper quadrant. (B) A quantitative analysis of PKH26-calcein–positive myoblasts is shown in the graph as relative percentage (means±SEM) (n=3). **p < 0.01 versus control cells. (C) Fluorescent images were made after coculture for 24 h.
LiCl Phosphorylate and Inactivate GSK-3β
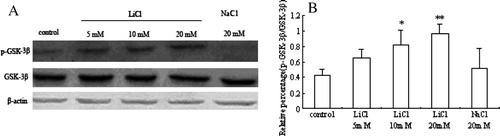
To gain insight into the potential mechanism of LiCl regulating Cx43 expression in skeletal myoblasts, the effect of GSK-3β on the Wnt pathway was investigated. The amount of total GSK-3β and phospho-GSK-3β (Ser9) was observed in the study. The level of phospho-GSK-3β expression was relatively low in control cells and was significantly increased at the presence of LiCl in a dose-dependent manner (A). However, the exposure to LiCl didn't alter total GSK-3β content in myoblasts ( A). Therefore, the ratio of phospho-GSK-3β to total GSK-3β was up-regulated concentration dependantly (B).
Figure 4. The effects of LiCl on phospho-GSK-3β and total GSK-3β in skeletal myoblasts. (A) Representative immunoblots of phospho-GSK-3β and total GSK-3β from different treatment groups. (B) A quantitative analysis of the ratio of phospho-GSK-3β (Ser9) to total GSK-3β from five independent experiments is shown in the graph. Values are mean±SEM. *p < 0.05 and **p < 0.01 versus control.
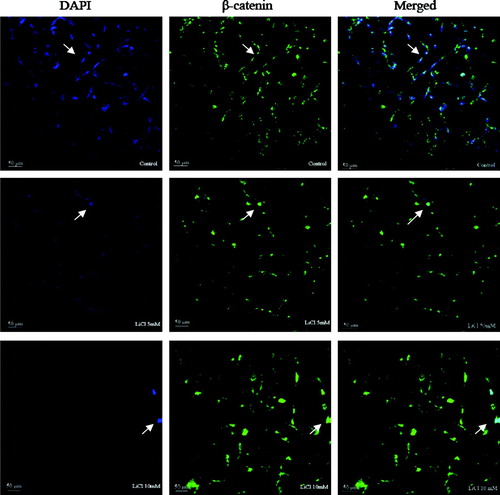
LiCl Induces β-Catenin Accumulation and Translation into the Nucleus
To provide additional evidence that LiCl activated Wnt signaling in these cells, we next investigated the distribution of β-catenin in myoblasts. β-Catenin is stabilized in the cytoplasm by the activation of the canonical Wnt pathway, enabling it then to translocate into the nucleus, where it is shown to be required for Wnt-dependent effects (Cong et al. Citation2003; Kawano and Kypta Citation2003). In this study, as shown in , β-catenin was mostly localized to cytoplasmic membrane and cytoplasm in untreated cells. In contrast with control, the treatment with LiCl significantly induced clear accumulation of β-catenin in the nucleus. The blue imunofluorescence staining (DAPI) became unclear but the green imunofluorescence staining (β-catenin) in the nucleus was enhanced greatly in the images of LiCl-treated cells.
Figure 5. The effect of LiCl on the nuclear localization of β-catenin. β-Catenin localizes at cytomembrane and cytoplasm in control cells, whereas it translocates into nucleus after treatment with 5 and 10 mM LiCl for 24 h. The green channel reflects β-catenin expression and the blue channel reflects DAPI. The green and blue channels are merged for viewing.
DISCUSSION
In the present study, we demonstrate that the concentration and time course of LiCl treatment may play an important role in regulating Cx43 expression and gap-junctional communication in skeletal myoblasts, and LiCl regulates Cx43 expression not mainly at transcriptional level but also via phosphorylated modification. Furthermore, the effects of LiCl are by mimicking the activation of the canonical Wnt pathway through increasing phospho-GSK-3β expression and inducing nuclear accumulation of β-catenin in skeletal myoblasts.
Recently, it has been described that Wnt-1 signaling can be mimicked by LiCl treatment. The action of LiCl, like that of Wnt-1, which is thought to act throught the canonical pathway, ultimately leads to the accumulation of β-catenin in the nucleus. Previous studies suggest that the increased Cx43 mRNA in response to Wnt-1 signaling is, at least in part, through nuclear translocation of β-catenin, binding to Tcf-family members, and subsequent transcriptional transactivation. In this paper, Cx43 mRNA expression was increased by the treatment with 5 mM LiCl but not with 10 and 20 mM LiCl. It may be that Wnt signaling plays different roles in various cell types. Recently, Anderson and colleagues report that two microRNAs, miR-206 and miR-1, are assocaited with the inhibition of Cx43 protein expression during myoblast differentiation (Anderson et al. Citation2006). Therefore, perhaps other interacting pathways in coordination with Wnt signaling might influence Cx43 expression during myoblast differentiation.
In addition, Cx43 protein expression was increased with 5 and 10 mM LiCl treatment in a dose-dependent manner. In contrast with a previous study by Ai and his colleagues who enhanced Cx43 expression of cardiac myocytes with 20 mM LiCl, our data showed that 20 mM LiCl did not increase Cx43 expression of myoblasts. Furthermore, the expression of Cx43 was up-regulated with 10 mM LiCl for 24 h and down-regulated for 48 and 72 h compared with untreated cells. MTT: 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide assay has been used to test the cell proliferation at the presence of LiCl. At 5, 10, and 20 mM, LiCl promoted cell proliferation compared with control (data not shown). Therefore, the difference may be explained by some evidence suggesting that the inhibition of GSK-3β activity is sufficient to stimulate myogenic differentiation (van der Velden et al. Citation2006, Citation2007). We think that higher dose and chronic time of LiCl stimulation could induce the overexpression of phospho-GSK-3β, promote myoblast fusion and differentiation, and then lead to the reduced Cx43 expression. Thus, in this study, we demonstrated that LiCl regulated Cx43 expression in myoblasts, which was in agreement with previous reports showing a clear correlation between Wnt signaling and Cx43 protein expression. Furthermore, we found that the concentration and time course of LiCl treatment could be critical to the regulation of Cx43 expression in skeletal myoblasts. From the comparison of Cx43 expression as measured at the mRNA and protein levels, it appears that the increased Cx43 protein expression cannot be mainly caused by an enhanced transcriptional activity; it also may be regulated by phosphorylated modification. Moreover, the results of flow-cytometric analysis showed that lower concentration of LiCl increased gap-junctional communication between skeletal myoblasts in vitro, accompanied by the up-regulated Cx43 expression. Previous studies demonstrated that Cx43 gap junctions synchronize the maturation of prefusion myoblasts to assist in their differentiation. Therefore, LiCl regulates Cx43 protein expression and gap-junctional communication in skeletal myoblasts, which may play an important role in LiCl-induced myoblast differentiation and myotube formation.
Some studies show that the phopsphorylated regulation of GSK-3 (phospho-GSK-3α Ser21 and phospho-GSK-3β Ser9) is specifically associated with skeletal muscle differentiation. In this study, the expression of phospho-GSK-3β (Ser9) and the ratio of phospho-GSK-3β (Ser9) to total GSK-3β were increased during the exposure to LiCl in a concentration-dependant manner. Furthermore, there were no significant changes in total GSK-3β, which was in line with the effects of GSK-3β inhibition by Wnt-1. In addition, LiCl induced the nuclear translocation of β-catenin. Therefore, we confirm that LiCl mimics the activated Wnt signaling in skeletal myoblasts.
In conclusion, the results of the present study provide evidence that Cx43 expression and gap-junctional functionality are regulated by LiCl in skeletal myoblasts through mimicking the canonical Wnt signaling. Notably, the limitation of the current study is that all studies on Wnt signaling were obtained by pharmacological inhibition using LiCl. In addtion, the accumulation of β-catenin by GSK-3β inhibition via phosphorylation at the Ser9 site may be involved with pathways, such as the phosphoinositide 3-kinase–protein kinase B (PI3K-PKB)/Akt–dependent pathway, other than Wnt signaling (Haq et al. Citation2003). But, this study cannot rule out a role of PI3K/Akt pathway.
Acknowledgements
The authors thank all of the members of their laboratory for technical assistance and helpful discussions.
References
- Ai Z, Fischer A, Spray DC, Brown AM, Fishman. Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest. 2000; 105: 161–171
- Anderson C., Catoe H., Werner R. MIR-206 regulates connexin43 expression during skeletal muscle development. Nucleic Acids Res. 2006; 34: 5863–5871
- Araya R., Eckardt D., Maxeiner S., Krüger O., Theis M., Willecke K., Sáez J.C. Expression of connexins during differentiation and regeneration of skeletal muscle: Functional relevance of connexin43. J Cell Sci. 2005; 118(Pt 1)27–37
- Bennett M.V., Barrio L.C., Bargiello T.A., Spray D.C., Hertzberg E., Sáez J.C. Gap junctions: New tools, new answers, new questions. Neuron. 1991; 6: 305–320
- Cadigan K.M., Nusse R. Wnt signaling: A common theme in animal development. Genes Dev. 1997; 11: 3286–3305
- Cong F., Schweizer L., Chamorro M., Varmus H. Requirement for a nuclear function of beta-catenin in Wnt signaling. Mol. Cell. Biol. 2003; 23: 8462–8470
- Cossu G., Borello U. Wnt signaling and the activation of myogenesis in mammals. EMBO J. 1999; 18: 6867–6872
- Dermietzel R., Hwang T.K., Spray D.S. The gap junction family: Structure function and chemistry. Anat Embryol (Berl) 1990; 182: 517–528
- Gorbe A., Becker D.L., Dux L., Krenacs L., Krenacs T. In differentiating prefusion myoblasts connexin43 gap junction coupling is upregulated before myoblast alignment then reduced in post-mitotic cells. Histochem Cell Biol. 2006; 125: 705–716
- Gorbe A., Becker D.L., Dux L., Stelkovics E., Krenacs L., Bagdi E., Krenacs T. Transient upregulation of connexin43 gap junctions and synchronized cell cycle control precede myoblast fusion in regenerating skeletal muscle in vivo. Histochem Cell Biol. 2005; 123: 573–583
- Gorbe A., Krenacs T., Cook J.E., Becker D.L. Myoblast proliferation and syncytial fusion both depend on connexin43 function in transfected skeletal muscle primary cultures. Exp Cell Res. 2007; 313: 1135–1148
- Klein P. S., Melton D. A. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996; 93: 8455–8459
- Kumar N.M., Gilula N.B. The gap junction communication channel. Cell. 1996; 84: 381–388
- Haq S., Michael A., Andreucci M., Bhattacharya K., Dotto P., Walters B., Woodgett J., Kilter H., Force T. Stabilization of β-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc Natl Acad Sci U S A. 2003; 100: 4610–4615
- Kawano Y., Kypta R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003; 116: 2627–2634
- Loewenstein W.R. Junctional intercellular communication: The cell-to-cell membrane channel. Physiol Rev. 1981; 61: 829–913
- Logan C.Y., Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004; 20: 781–810
- Meyer R.A., Cohen M.F., Recalde S., Zakany J, Bell S.M., Scott W.J., Lo C.W. Developmental regulation and asymmetric expression of the gene encoding Cx43 gap junctions in the mouse limb bud. Dev Genet. 1997; 21: 290–300
- Olson D.J., Christian J.L., Moon R.T. Effect of wnt-1 and related proteins on gap junctional communication in Xenopus embryos. Science. 1991; 252: 1173–1176
- Olson D.J., Moon R.T. Distinct effects of ectopic expression of Wnt-1, activin B, and bFGF on gap junctional permeability in 32-cell Xenopus embryos. Dev Biol. 1992; 151: 204–212
- Proulx A., Merrifield P.A., Naus C.C. Blocking gap junctional intercellular communication in myoblasts inhibits myogenin and MRF4 expression. Dev Genet. 1997; 20: 133–144
- Rochat A., Fernandez A., Vandromme M., Molès J.P., Bouschet T., Carnac G., Lamb N.J. Insulin and Wnt1 pathways cooperate to induce reserve cell activation in differentiation and myotube hypertrophy. Mol Biol Cell. 2004; 15: 4544–4555
- Snider L., Tapscott S. Emerging parallels in the generation and regeneration of skeletal muscle. Cell. 2003; 113: 811–812
- Tajbakhsh S., Borello U., Vivarelli E., Kelly R., Papkoff J., Duprez D., Buckingham M., Cossu G. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development. 1998; 125: 4155–4162
- Tomasetto C., Neveu M.J., Daley J., Horan P.K., Sager R. Specificity of gap junction communication among human mammary cells and connexin transfectants in culture. J Cell Biol. 1993; 122: 157–167
- van der Heyden M.A., Rook M.B., Hermans M.M., Rijksen G., Boonstra J., Defize L.H., Destrée O.H. Identification of connexin43 as a functional target for Wnt signalling. J Cell Sci. 1998; 111: 1741–1749
- van der Velden J. L., Langen R. C., Kelders M. C., Willems J., Wouters E. F., Janssen-Heininger Y. M., Schols A. M. Myogenic differentiation during regrowth of atrophied skeletal muscle is associated with inactivation of GSK-3beta. Am J Physiol Cell Physiol. 2007; 292: C1636–C1644
- van der Velden J. L., Langen R. C., Kelders M. C., Wouters E. F., Janssen-Heininger Y. M., Schols A.M. Inhibition of glycogen synthase kinase-3beta activity is sufficient to stimulate myogenic differentiation. Am J Physiol Cell Physiol. 2006; 290: C453–C462
- Williams R.S., Cheng L., Mudge A.W., Harwood A.J. A common mechanism of action for three mood-stabilizing drugs. Nature 2002; 417: 295–295