Abstract
Inositol 1,4,5-trisphosphate (IP3) is an important second messenger that can trigger a Ca2+ wave prolongated between cells. This intercellular signaling was found defective in some gap junction connexin deafness mutants. In this study, the mechanism underlying IP3 intercellular signaling in the cochlea was investigated. A gap junction channel is composed of two hemichannels. By using a fluorescence polarization technique to measure IP3 concentration, the authors found that IP3 could be released by gap junction hemichannels in the cochlea. The IP3 release was increased about three- to fivefold by the reduction of extracellular Ca2+ concentration or by mechanical stress. This incremental release could be blocked by gap junction blockers but not eliminated by a purinergic P2x receptor antagonist and verapamil, which is a selective P-glycoprotein inhibitor inhibiting the ATP-binding cassette transporters. The authors also found that IP3 receptors were extensively expressed in the cochlear sensory epithelium, including on the cell surface. Extracellular application of IP3 could trigger cellular Ca2+ elevation. This Ca2+ elevation was eliminated by the gap junction hemichannel blocker. These data reveal that IP3 can pass through hemichannels acting as an extracellular mediator to participate in intercellular signaling. This hemichannel-mediated extracellular pathway may play an important role in long-distance intercellular communication in the cochlea, given that IP3 only has a short lifetime in the cytoplasm.
INTRODUCTION
The gap junction is an intercellular channel that forms an intracellular conduit allowing direct exchange of small molecules and ions between cells. A gap junction channel is formed by two hemichannels. Each hemichannel is composed of six subunits, which are hexagonally arranged, forming a relatively large pore (∼10 Å) through which ions and small molecules up to 1 kDa can pass (Harris Citation2001). Recent studies have shown that the role of hemichannels extends beyond the formation of gap junction channels between cells. They can function solely on the cell surface to construct an alternative communication pathway for extracellular space rather than intracellular cytoplasm between adjacent cells (Bennett et al. Citation2003; Goodenough and Paul Citation2003). This hemichannel communicative potency has been directly demonstrated by dye-uptake assays and the measurement of the release of cell signaling molecules, such as ATP, glutamate, and NAD+ (Bruzzone et al. Citation2001; Contreras et al. Citation2002; Stout et al. Citation2002; Ye et al. Citation2003; Zhao et al. Citation2005). The opening of hemichannels can be mediated by Ca2 + and member tension (Harris Citation2001; Bennett et al. Citation2003; Goodenough and Paul Citation2003). Depending on conditions, the opening can be physiological or pathological (Kamermans et al. Citation2001; Zhao et al. Citation2005; Thompson et al. Citation2006).
Inositol 1,4,5-triphosphate (IP3) is an important second messenger, whose primary function is to elevate intracellular Ca2 + concentration by activating IP3 receptors to release Ca2 + from the intracellular store and to allow external Ca2 + influxing (Berridge Citation1993; Dellis et al. Citation2006). IP3 can also evoke a Ca2 + wave propagated between cells via gap-junctional coupling (Niessen et al. Citation2000). Recently, it has been found that this IP3 intercellular signaling may play an important role in hearing: some Cx26 deafness mutants impaired the propagation of IP3-evoked Ca2 + waves between cells (Beltramello et al. Citation2005). However, the IP3 permeability and the mechanisms underlying the IP3 intercellular signaling in the cochlea remain undetermined. In this study, by using the fluorescence polarization technique to directly measure the IP3 concentration, we found that hemichannels in the cochlear sensory epithelium could release IP3 under physiological conditions. We also found that the extracellular application of IP3 could elevate the intracellular Ca2 + concentration. These data suggest that IP3 can pass through hemichannels to participate in intercellular communication via an extracellular pathway in the cochlea.
MATERIALS AND METHODS
Cochlear Isolation and Incubation for Hemichannel-Releasing Measurement
The cochlea was freshly isolated from adult guinea pigs (250 to 450 g). Cochlear isolation and the procedure of measurement for hemichannel release have been fully described elsewhere (Zhao Citation2005a; Zhao et al. Citation2005). Briefly, the cochlea was microdissected in a sterile normal extracellular solution (ECS) (in mM: 142 NaCl, 5.37 KCl, 1.47 MgCl2, 2 CaCl2, 10 HEPES, 25 dextrose; osmolarity 300 mOsm and pH 7.2). After removal of the bone, the organ of Corti was isolated. Then, the isolated cochlea was incubated in 200 µl ECS in an incubation chamber. Every 5 min, the incubation solution (200 µl) was collected and replaced with fresh solution. After two collections, the cochlea was switched to be incubated with a sterile, nominally Ca2 + - and Mg2 + -free extracellular solution (in mM: 142 NaCl, 5.37 KCl, 10 HEPES, 25 dextrose; 300 mOsm and pH 7.2) for 15 min (three collections) to test hemichannel release. Then, the cochlea was returned back to incubation in the normal ECS and sampled three more times (see the trace at the bottom of A). All experiments were performed at room temperature (23°C).
IP3 Measurement by Fluorescence Polarization Technique
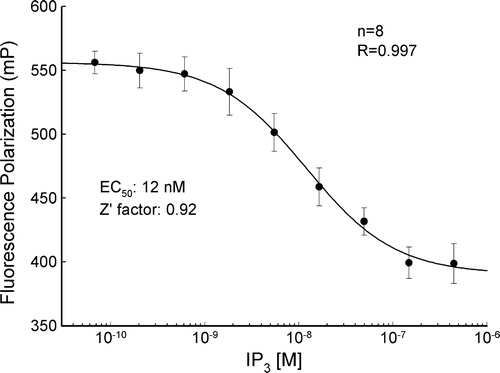
The amount of IP3 was measured using a fluorescence polarization assay, which is based on competitive binding between an IP3 fluorescence tracer and unlabeled IP3 from the sample solution. Free IP3 competes for binding to the IP3-binding protein and allows the IP3 tracer to rotate freely upon excitation with plain polarized light. The polarized signal (mP) is inversely proportional to the amount of the free unlabeled IP3; high levels of free IP3 result in fluorescence emission being depolarized (low mP) (Owicki Citation2000). The measurement was performed by a HitHunter fluorescence polarization assay kit (catalog number 90-0037; DiscoveRx Tech, Fremont, CA). The incubating solution sample (20 µl) was mixed with the reagents following the manufacturer's instructions. A black 96-well plate was used for measurement to avoid optical cross-talk between wells. The fluorescence emission was read by a Wallac 1420 Victor Multilabel Plate Reader (PerkinElmer, Wellesley, MA). The IP3 concentration was calculated from the IP3 standard curve, which was measured from threefold serial dilutions of a IP3 standard solution (20 µM) and fitted with a four-parameter Hill equation: y=y0+a/(1+(x/c)b). The measured standard curve showed a high regression (R=0.997) (). The data were compiled and presented as mean±SD.
Mechanical Stimulation and Membrane Stress Application
Mechanical stimulation was performed by gently pipetting (once every 3 to 4 s) the incubated cochlea with 10 mg/ml micro glass beads (φ = 30–50 µm; Polysciences, Warrington, PA) in the normal ECS solution (Zhao et al. Citation2005). To apply membrane stress, the normal ECS (300 mOsm) was replaced with a hypotonic ECS (275 mOsm) and the same procedure described above was implemented to as replace 0 mM Ca2 + ECS. The osmolarity of all solutions was measured by a microcomputer-controlled osmometer (Model 3300; Advanced Instruments, Norwood, MA) and adjusted by dextrose.
Fluorescence Dye-Uptake Assay
A membrane-impermeable fluorescence dye Lucifer Yellow (LY; MW = 444; charge −2), which has similar size and charge to IP3, was used for dye-uptake assay. As previously reported (Zhao Citation2005a), cells or cochlear sensory epithelia were incubated with 1 mM LY in the Ca2 + -free ECS for 10 min and then washed out by normal ECS. The cellular dye uptake was observed under a microscope (Nikon, TE300) with corresponding fluorescence filters. The image was captured by a CCD camera.
Ca2 + Fluorescence Imaging and Measurement
The cochlear sensory epithelium was incubated in normal extracellular solution with 5 µM Fluo-4 AM (F-14217; Molecular Probes) for 30 min at room temperature. After washout and de-esterification for 20 to 30 min, the intensity of the Fluo-4 fluorescence emission was continuously recorded online by a photometer (Photon Tech. Int., Birmingham, NJ) under a Nikon microscope. The fluorescence image was also captured by a CCD camera.
Applications of Gap Junction Blockers and BAPTA-AM
The gap junction channel blockers 18α-glycyrrhetinic acid (18-AGA; 50 µM), carbenoxolone (CBX; 0.1 mM), and octanol (10 mM) were used in the experiments. These gap junction blockers are known to block hemichannel dye uptake in cochlear supporting cells (Zhao Citation2005a). The blocker was added into both Ca2 + -free and normal extracellular solutions (Zhao et al. Citation2005). For intracellularly loading of the calcium-chelating reagent BAPTA, the cochlea was preincubated in theECS with 20 µM BAPTA-AM (B6769; Molecular Probes) for 45 min, followed by 20 min incubation in normal ESC for de-esterification.
Immunofluorescence Staining
The detailed method for immunofluorescence staining has been described in our previous report (Zhao and Yu Citation2006). The cochlear sensory epithelium or the dissociated cochlear cells were fixed with 4% paraformaldehyde for 30 min. After washout, the epithelium was incubated in a blocking solution (10% goat serum and 1% bovine serum albumin [BSA] in phosphate-buffered saline [PBS]) with 0.1% Triton X-100 for 20 min. The epithelium was then incubated with rabbit polyclonal anti-IP3R (IP3 receptor) antibody (1:100; sc-28163; Santa Cruz Biotechnology, Santa Cruz, CA) in the blocking solution at 4°C overnight. After washing out, the epithelium was allowed to react with the secondary antibody Alexa Fluor 488 (1:500; Molecular Probes) in the blocking solution, at room temperature for 1 h, to visualize staining. The stained epithelia or cells were observed under a Leica confocal microscope (Leica TCS SP2).
RESULTS
Hemichannel-Mediated IP3 Release in the Cochlear Sensory Epithelium
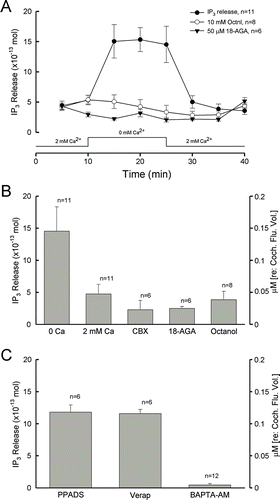
The hemichannel-mediated IP3 release was measured by use of a fluorescence polarization assay (). As the cochlear sensory epithelium was incubated in nominally Ca2 + -free extracellular solution (ECS) (presented by the bottom trace in A) to interfere with hemichannel openings, the IP3 release was increased. The amount of IP3 increased from 4.8±1.5×10−13 to 14.6±3.8×10−13 moles, almost a threefold increase (A and B). The IP3 concentration normalized to the guinea pig's cochlear space volume, which is about 10 µl (Thorne et al. Citation1999), was increased from 48±15 nM to 0.15±0.04 µM (B). The level of IP3 release returned to the baseline when the cochlea was reincubated in the 2 mM Ca2 + ECS.This incremental increase in IP3 release could be blocked by application of the gap junction blockers carbenoxolone (CBX; 0.1 mM), 18a-glycyrrhentinic acid (18-AGA; 50 µM), and octanol (10 mM) (A and B). The IP3 levels in the presence of the gap junction blockers CBX, 18-AGA, and octanol were 2.29±1.44, 2.5±0.3, and 3.85±1.33×10−13 moles, respectively. It has been reported that elevation of intracellular Ca2 + can also interfere with hemichannel opening (De Vuyst et al. Citation2006). BAPTA is a fast calcium-chelating reagent. Preincubation with BAPTA-AM to buffer intracellular Ca2 + significantly reduced the IP3 release (C). However, incubation with the P2x receptor blocker pyridoxalphosphate-6-azophenyl-2',4'-disulfonic acid (PPADS; 50 µM) did not eliminate the IP3 release (C). The IP3 release was still increased to 11.82±1.13×10−13 moles by the reduction of extracellular Ca2 + in the presence of 50 µM PPADS; there was no significant decrease (p=0.52, t test) in comparison with the IP3 release at 0 mM Ca2 + (B). Verapamil is a selective P-glycoprotein inhibitor and can inhibit the adenosine triphosphate (ATP)-binding cassette (ABC) transporters. Application of 10 µM verapamil also did not eliminate IP3 release (C). The IP3 release at 0 mM Ca2 + was 11.57±0.66×10−13 moles in the presence of 10 µM verapamil. This further supports that IP3 is released by hemichannels.
Figure 2. Hemichannel-mediated IP3 release in the cochlear sensory epithelium. (A, B) IP3 release by the reduction of extracellular Ca2 + and blockage by gap junction channel blockers. The trace along the x-axis of A shows the procedure of cochlea incubating in different Ca2 + concentrations. The right y-axis indicates the concentration of IP3 released normalized to the cochlear fluid volume (10 µl). (C) The IP3 release under treatment with the P2x receptor blocker PPADS (50 µM), the ABC transporter inhibitor verapamil (Verap; 10 µM), and preincubation with the intracellular calcium chelating reagent BAPTA.
Membrane Tension Induced IP3 Release
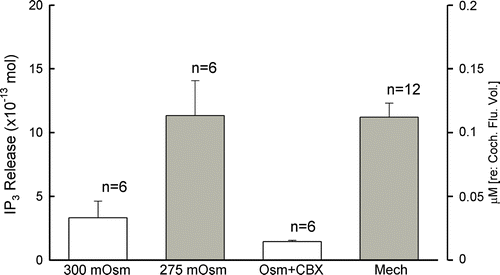
Mechanical stimulation can induce membrane tension to interfere with the hemichannel opening (Leybaert et al. Citation2003; Bao et al. Citation2004). Under mechanical stimulation, the IP3 release was increased to 11.2±1.1×10−13 moles (). In this experiment, we also applied a hypotonic extracellular solution to induce cell membrane stress. The IP3 release was increased from 3.3±1.3 to 11.3±2.7×10−13 moles as the osmolarity of the incubating solution was reduced from 300 to 275 mOsm (). This incremental release was also eliminated by CBX. After application of 0.1 mM CBX, the IP3 release was reduced to 1.44±0.11×10−13 moles ().
Figure 3. IP3 release in the cochlear sensory epithelium by osmolarity stress and mechanical stimulation. The cochlea was preincubated in normal extracellular solution (300 mOsm) and then switched to be incubated in a hypotonic extracellular solution (275 mOsm). The IP3 release was increased by the hypotonic challenge and blocked by cotreatment with 0.1 mM CBX.
Hemichannel Dye Uptake in the Cochlear Sensory Epithelium
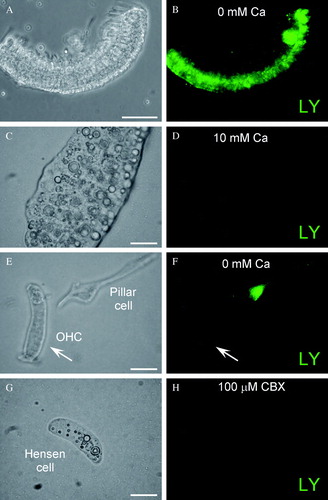
Hemichannel activity can be demonstrated by the dye-uptake assay. shows hemichannel dye uptake in the cochlear sensory epithelium. As previously reported (Zhao Citation2005a), the cochlear sensory epithelium had LY dye uptake under the low-bivalent condition (A and B). Also, elevation of extracellular Ca2 + could diminish this dye uptake; the epithelium had no dye uptake in the incubation in the 10 mM Ca2 + ECS (C and D). Further examination in the dissociated cell preparation showed that the cochlear supporting cell had dye uptake, whereas the outer hair cell, which has no connexin expression, had no dye uptake in the same incubation (E and F). Coincubation with CBX (0.1 mM) also eliminated the dye uptake in the cochlear supporting cells (G and H). These experiments further demonstrated that hemichannels in the cochlear sensory epithelium can also influx small molecules.
Figure 4. Hemichannel dye uptake assay in the cochlear sensory epithelium. (A, B) LY dye uptake in the cochlear sensory epithelium under low-bivalent condition. A is a phase-contrast image of the epithelium. B is its fluorescence image, which had dye uptake after incubation with LY. (C, D) The epithelial dye uptake was eliminated by elevation of extracellular Ca2 + . The incubation was performed at 10 mM extracellular Ca2 + concentration. (E, F) Dye uptake in dissociated cochlear cells. The supporting pillar cell had dye uptake, but the outer hair cell, which has no connexin expression, did not have dye uptake in the same incubation (indicated by arrows). (G, H) Gap junction blocker blocks dye uptake in the cochlear supporting cell. An isolated cochlear supporting Hensen cell had no dye uptake in coincubation with 100 µM CBX. Scale bars = 100 µm (A to D); 10 µm (E to H).
IP3 Receptor Expression and Extracellular IP3 Evoked Elevation of Ca2 + in the Cochlear Sensory Epithelium
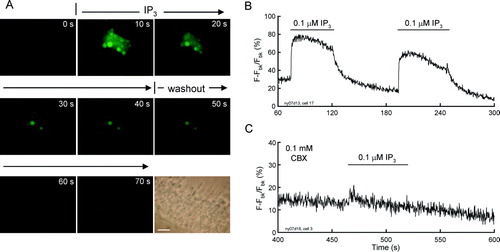
IP3 activates IP3 receptors (IP3Rs) to stimulate Ca2 + release from the intracellular Ca2 + store and external Ca2 + influxing to induce intracellular Ca2 + elevation (Berridge Citation1993; Dellis et al. Citation2006). Immunofluorescence staining showed that IP3Rs are extensively expressed in the cochlear sensory epithelium (). The intense labeling for IP3Rs is visible in the cytoplasm (A) and on the cochlear cell surface (C to F).We further found that the extracellular application of IP3 could elevate the intracellular Ca2 + concentration in the cochlear sensory epithelium (). Ca2 + fluorescence images show that the cellular Ca2 + in the epithelium was elevated by perfusion of extracellularIP3 (A). After washout of IP3, the Ca2 + fluorescence was diminished. This IP3-evoked Ca2 + elevation was reversible and repeatable (B), and could also be eliminated by gap junction hemichannel blockers (C). Treatment with 0.1 mM CBX abolished the Ca2 + elevation (C).
Figure 5. Expression of IP3 receptors (IP3Rs) in the cochlear sensory epithelium. (A, B) Immunofluorescence labeling for IP3R in the cochlear sensory epithelium. (C to F) Expression of IP3Rs in the cochlear-supporting cells. Labeling is visible on the cell surface, in the cytosol, and on the nuclear membrane. Asterisks in E indicate lipid bubbles in Hensen cells that have no IP3R labeling. Scale bars = 50 µm (A, B); 20 µm (C, D); 10 µm (E, F).
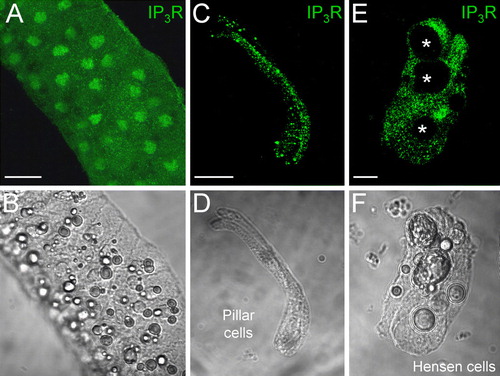
Figure 6. Extracellular IP3 evoked intracellular Ca2 + elevation in the cochlear sensory epithelium. (A) Time-lapse recording of Ca2 + fluorescence images in the cochlear sensory epithelium evoked by extracellular perfusion of 0.1 µM IP3. Scale bar = 20 µm. (B) Ca2 + fluorescence intensity in the cochlear sensory epithelium was continuously recorded. Horizontal bars represent extracellular perfusion of 0.1 µM IP3. (C) Blockage of Ca2 + elevation by coapplication of CBX. CBX eliminated the extracellular IP3-triggerred elevation in intracellular Ca2 + concentration. The sensory epithelium was coincubated with 0.1 mM CBX. Note a small increase at the beginning of the IP3 perfusion induced by perfusion-induced mechanical disturbance.
DISCUSSION
In this study, we used a fluorescence polarization technique to directly measure IP3 concentration for assaying hemichannel IP3 release in the cochlear sensory epithelium ( and ). We found that hemichannels in the cochlear sensory epithelium could release IP3. The IP3 release was increased from the nanomolar to micromolar levels by the reduction of extracellular Ca2 + and mechanical stress. We also found that the application of extracellular IP3 elevated cellular Ca2 + in the cochlear sensory epithelium (), indicating that IP3 can act as an extracellular mediator to perform intercellular communication.
It has been well documented that hemichannels can function on the cell surface to release small cell-signaling molecules such as ATP and glutamate (Bennett et al. Citation2003; Goodenough and Paul Citation2003). This hemichannel-mediated ATP release can play an important role in intercellular signaling in the brain and retina (Kamermans et al. Citation2001; Thompson et al. Citation2006). In the cochlea, we have also reported that hemichannels can function in the cochlear sensory epithelium to release ATP to modify outer hair cell electromotility and regulate hearing function (Zhao Citation2005a; Zhao et al. Citation2005). In this experiment, we have found that hemichannels in the cochlear sensory epithelium can also release IP3 ( and ). This result not only provides direct evidence that gap junction channels in the cochlea are permeable to IP3, but also provides the first evidence that hemichannels can release IP3 as well.
In this study, we have further demonstrated that extracellular application of IP3 can elevate intracellular Ca2 + concentration (), indicating that IP3 can act as an extracellular mediator to participate in intercellular communication (). IP3 can mediate the adrenergic response and elevate intracellular Ca2 + concentration by activation of IP3 receptors (Berridge Citation1993). Recently, it has been found that IP3 can activate only a few (2∼5) IP3 receptors on the cell surface to dominant intracellular Ca2 + concentration (Dellis et al. Citation2006). IP3 receptors are expressed extensively in the cochlear sensory epithelium (). As shown by hemichannel dye uptake (; also see Zhao Citation2005a), the extracellular IP3 may reenter into the neighboring cells via hemichannel uptake and subsequently binds to IP3 receptors to elevate intracellular Ca2 + concentration ( and ).
Figure 7. Schematic drawing of hemichannel-mediated intercellular signaling pathway in the cochlea. IP3 can pass through not only intact gap-junctional channels but also hemichannels to participate in intercellular signaling.
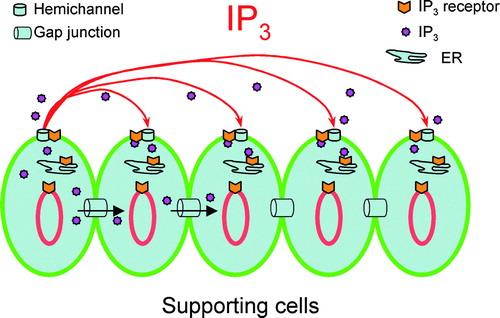
This hemichannel-mediated intercellular signaling may be able to achieve broad, long-distance intercellular communication and plays an important role in the IP3-mediated intercellular signaling, given that IP3 is rapidly degraded by enzymes in the cytoplasm. The lifetime of IP3 in most cells is less than a few seconds (e.g., t1/2=4 s in liver) (Berg et al. Citation2001). So, it is difficult for IP3 to travel a long distance in the cytoplasm via intracellular gap junction channels between cells. However, IP3 in the extracellular space can survive long time because there are no specific enzymes to degrade it quickly. Indeed, we found that the IP3 could be stable in the incubation solution up to1 h and had no significant degradation (data not shown). Thus, IP3 may be able to travel a long distance in the extracellular space to achieve broad, long-distance intercellular signaling ().
Connexin mutations account for 70% to 80% of nonsyndromic deafness in children (Kelsell et al. Citation1997; Zhao et al. Citation2006). Although gap junctions play important roles in hearing, their roles in cochlear function are poorly understood. In the cochlea, the supporting cells employ gap junctions extensively. In contrast, the auditory sensory hair cells do not have gap junctions or connexin expressions (Kikuchi et al. Citation1995; Lautermann et al. Citation1998; Forge et al. Citation2003; Zhao and Yu Citation2006). Previously we proposed that the hair cells, which have no direct blood supply, receive nutrient and energy supplies from the supporting cells by means of mediators released through hemichannels (Zhao Citation2005b; Zhao et al. Citation2005). Our present study further supports this concept. Hemichannels in the cochlea can release ATP and IP3, which can elevate intracellular Ca2 + to trigger and regulate a wide range of cellular processes, including cell fertilization (Berridge Citation1993). By extension, these data may also have implications for hearing loss related to connexin mutations and the possible mechanism by which these mutations cause hair cell dysfunction (Zhao et al. Citation2006). Mutations in these proteins may be likely to interrupt this crucial cochlear signaling function as well as the possible nutrient supply to the hair cells.
Recently, it has been found that pannexin as a new gap junction gene gamily can also encode gap junction proteins in vertebrates (Baranova et al. Citation2004; Shestopalov and Panchin Citation2008). Pannexin proteins can also form functional hemichannels on the cell surface to release ATP. Like connexin hemichannels, pannexin hemichannels can be gated by elevation of intracellular Ca2 + and mechanical stress, and are sensitive to CBX inhibition but insensitive to extracellular Ca2 + (Shestopalov and Panchin Citation2008). Our preliminary data show that pannexin has expression in the cochlear sensory epithelium (unpublished observation in our laboratory). IP3 may also be able to be released via pannexin hemichannels. However, the measured IP3 release was significantly reduced in 2 mM extracellular Ca2 + ( and ), indicating that pannexin hemichannels may not be a major contributor to this IP3 release in the cochlear sensory epithelium. However, it cannot be completely ruled out that pannexin hemichannels can act as a partner to coparticipate in IP3 release, just as they can release ATP, so as to play an important role in intercellular signaling.
Acknowledgements
This work was supported by a grant (R01 DC 05989) from the National Institute of Deafness and Other Communication Disorders.
References
- Bao L, Sachs F, Dahl G. Connexins are mechanosensitive. Am J Physiol Cell Physiol. 2004; 287: C1389–C1395
- Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 2004; 83: 706–716
- Beltramello M, Piazza V, Bukauskas FF, Pozzan T, Mammano F. Impaired permeability to Ins(1,4,5)P3 in a mutant connexin underlies recessive hereditary deafness. Nat Cell Biol. 2005; 7: 63–69
- Bennett MVL, Contreras JE, Bukauskas FF, Saez JC. New roles for astrocytes: Gap junction hemichannels have something to communicate. Trends Neurosci. 2003; 26: 610–617
- Berg JM, Tymoczko JL, Stryer L. Biochemistry5th ed. W.H. Freeman and Company, New York 2001; 405–406
- Berridge MJ. Inositol trisphosphate and calcium signaling. Nature 1993; 361: 315–325
- Bruzzone S, Guida L., Zocchi E, Franco L, De Flora A. Connexin 43 hemi channels mediate Ca2 + -regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001; 15: 10–12
- Contreras JE, Sanchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Saez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA. 2002; 99: 495–500
- Dellis O, Dedos SG, Tovey SC, Taufiq-Ur-Rahman, Dubel SJ, Taylor CW. (2006) Ca2 + entry through plasma membrane IP3 receptors. Science 313: 229–233.
- De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006; 25: 34–44
- Forge A, Becker D, Casalotti S, Edwards J, Marziano N, Nevill G. Gap junctions in the inner ear: Comparison of distribution patterns in different vertebrates and assessement of connexin composition in mammals. J Comp Neurol. 2003; 467: 207–231
- Goodenough DA, Paul DL. Beyond the gap: Functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003; 4: 284–294
- Harris AL. Emerging issues of connexin channels: Biophysics fills the gap. Q Rev Biophys. 2001; 34: 325–472
- Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science 2001; 292: 1178–1180
- Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Liang JN, Parry G, Mueller RF, Leigh IM. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 1997; 387: 80–83
- Kikuchi T, Kimura RS, Paul DL, Adams JC. Gap junctions in the rat cochlea: Immunohistochemical and ultrastructural analysis. Anat Embryol. 1995; 191: 101–118
- Lautermann J, ten Cate WJ, Altenhoff P, Grummer R, Traub O, Frank H, Jahnke K, Winterhager E. Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res. 1998; 294: 415–420
- Leybaert L, Braet K, Vandamme W, Cabooter L, Martin PE, Evans WH. Connexin channels, connexin mimetic peptides and ATP release. Cell Commun Adhes. 2003; 10: 251–257
- Niessen H, Harz H, Bedner P, Kramer K, Willecke K. Selective permeability of different connexin channels to the second messenger inositol 1,4,5-trisphosphate. J Cell Sci. 2000; 113: 1365–1372
- Owicki JC. Fluorescence polarization and anisotropy in high throughput screening: Perspectives and primer. J Biomol Screen. 2000; 5: 297–306
- Shestopalov VI, Panchin Y. Pannexins and gap junction protein diversity. Cell Mol Life Sci. 2008; 65: 376–394
- Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002; 277: 10482–10488
- Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science 2006; 312: 924–927
- Thorne M, Salt AN, DeMott JE, Henson MM, Henson OW, Jr, Gewalt SL. Cochlear fluid space dimensions for six species derived from reconstructions of three-dimensional magnetic resonance images. Laryngoscope 1999; 109: 1661–1668
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: A novel mechanism of glutamate release. J Neurosci. 2003; 23: 3588–3596
- Zhao HB. Connexin26 is responsible for anionic molecule permeability in the cochlea for intercellular signaling and metabolic communications. Eur J Neurosci. 2005a; 21: 1859–1868
- Zhao HB. (April, 2005) (2005b) What is the function of connexin 26 in the cochlea? Potassium recycling or intercellular signaling and nutrient/energy supplies? In: Meniere's Disease and Inner Ear Homeostasis Disorders. Proceedings of the 5th International Symposium, Lim DJ (ed.). , Los Angeles, CA pp. 254–255.
- Zhao HB, Kikuchi T, Ngezahayo A, White TW. Gap junctions and cochlear homeostasis. J Membr Biol. 2006; 209: 177–186
- Zhao HB, Yu N. Distinct and gradient distributions of connexin26 and connexin30 in the cochlear sensory epithelium of guinea pigs. J Comp Neurol. 2006; 499: 506–518
- Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci. USA. 2005; 102: 18724–18729