Abstract
It is often difficult to determine molecular mechanisms leading to early embryonic lethality of genetically modified mice due to lack of cells for further analyses. The authors describe here establishment of mouse embryonic fibroblast (MEF) cell lines from gastrulation stage embryos. In this example, using a combination of in vivo and in vitro techniques, the authors successfully generated MEF cell lines that lack both fibronectin (FN) and focal adhesion kinase (FAK).
INTRODUCTION
Mouse embryonic fibroblast (MEF) cell lines established from genetically manipulated mouse embryos can be used as powerful tools to address molecular mechanisms of protein function and cellular signaling. Although primary MEF growth in culture can be facilitated by spontaneous mutations that induce cell proliferation and inhibit cell apoptosis, genetic changes may interfere with data interpretation when comparing wild-type and mutant MEFs created under these conditions. Multiple methods are currently in use to transform primary MEFs into immortalized cell lines. The most common is viral transformation with Epstein-Barr virus (EBV), Simian virus 40 (SV40) T antigen, adenovirus E1A and E1B, and human papillomavirus (HPV) E6 and E7. However, when genetic manipulation in mice leads to early embryonic death, even the most efficient gene delivery systems often fail to live upto our expectations due to low numbers of starting cellular material. Here, we provide an example of MEF generation from individual and combined fibronectin (FN)- and focal adhesion kinase (FAK)-null mutant embryos that exhibit early embryonic lethality and cellular proliferation defects in culture. This method uses the inactivation of the p53 tumor suppressor as well as Cre-lox inactivation of FAK for the establishment of permanent MEF cell lines with a minimum of internal variations due to the immortalization process.
RESULTS
Signaling from extracellular matrix (ECM) is important for regulation of cell proliferation, survival, and motility (Akamatsu et al. Citation1996; Giancottii and Ruoslahti Citation1999; Hynes Citation1999; Ruoslahti Citation1999; Schlaepfer et al. Citation2004; Mclean et al. Citation2005). FN, a large ECM glycoprotein, forms fibrils on or near the surface of cells that are usually aligned with adjacent intracellular actin stress fibers. The interaction of extracellular FN and intracellular actin is mediated by integrin receptors and signaling proteins such as FAK cocluster with the cytoplasmic domains of integrins to deliver signals that control cell survival and motility (Giancottii and Ruoslahti Citation1999; Hynes Citation1999). The phenotype of FN knockout mouse embryos is very similar to that of FAK, suggesting a tight functional link between these two molecules (George et al. Citation1993; Furuta et al. Citation1995). Because FN matrix composition as well as FAK activity are altered in tumors and invasive cells (Akamatsu et al. Citation1996; Giancottii and Ruoslahti Citation1999; Hynes Citation1999; Ruoslahti Citation1999; Schlaepfer et al. Citation2004; Mclean et al. Citation2005), and FAK activity can lead to alterations in FN matrix organization (Ilic et al. Citation2004), it is important to develop cells lacking both FN and FAK so that FAK-associated signaling connections regulating FN matrix organization can be studied through cell reconstitution approaches. In addition, understanding how signals from FN are transduced into cells through FAK may provide novel insights for cancer or tissue therapies.
Potential source of cells that lack both FN and FAK are mouse embryos with null-mutations in both genes. Because deletion of either FN or FAK is embryonic lethal at embryonic day 8.5 (E8.5), we could generate mice that are only heterozygous for both genes (FAK+/ − FN+/ − ). In crossing of these mice, only 1 in 16 embryos would lack both genes (FAK−/ − FN−/ − ). A phenotype of such double mutants might be quite severe with embryos dying before E8.5 and would seriously complicate the extraction of sufficient embryonic cellular material for MEF generation. Therefore, we decided to take an advantage of Cre/loxP technology and FAKloxP/loxP mice (Beggs et al. Citation2003). By generating and then crossing FAKloxP/loxPFN+/ − mice, we could get litters in which one out of four embryos has the FAKloxP/loxPFN−/ − genotype. Transiently expressing Cre recombinase in these cells would delete the floxed FAK exon and generate FAK−/ − FN−/ − double-knockout cells in vitro.
However, we were facing one other important problem. Primary MEFs can divide only for a limited number of passages. Because FN−/ − embryos are dying at E8.5, the number of FAKloxP/loxPFN−/ − cells that could be obtained from early embryos would be insufficient for extensive cell culture experiments. It is known that mutation of the p53 tumor suppressor can extend the proliferative capacity of the primary cells in vitro (Tsukada et al. Citation1993). We have previously shown that primary FAK-null MEFs do not proliferate in culture due to a p53-dependent block in cell growth (Lim et al. Citation2008). However, introduction of a p53−/ − mutation on the background of FAK+/ − mice resulted in a successful generation of FAK−/ − MEFs and keratinocytes that could be propagated indefinitely in vitro (Ilic et al. Citation1995, Citation2007). The same strategy of using a p53-null mutation was used here to generate FAK−/ − FN−/ − MEFs.
In the first step, FAKloxP/loxP mice were crossed with p53−/ − mice. In the second generation (F2), 1 of 16 mice had a FAKloxP/loxPp53−/ − genotype. Although the number of mice with a suitable genotype was again low, they were alive and fertile, and we could cross them with FN+/ − p53−/ − mice obtained in parallel by breeding FN+/ − with p53−/ − mice. All pups from the crossing of FAKloxP/loxPp53−/ − and FN+/ − p53−/ − mice were FAKloxP/ + p53−/ − , but only 50% of them were FN+/ − . Breeding of FAKloxP/ + FN+/ − p53−/ − mice gave litters in which one of eight embryos had the FAKloxP/loxPFN+/ − p53−/ − genotype. Finally, from the crossing of these mice, one of four embryos possessed the desired FAKloxP/loxPFN−/ − p53−/ − genotype.
At E8.0–8.5, two pregnant FAKloxP/loxPFN+/ − p53−/ − females were euthanized. Seven embryos from each were dissected (numbered 1 to 7 and 8 to 14), and placed individually in 50 µl of Matrigel at the center of a 6-cm tissue culture dish. Matrigel polymerized to produce biologically active matrix material resembling the mammalian cellular basement membrane and in such a way forms a supportive environment for primary embryo cell growth. The Matrigel was supplemented with 20% fetal calf serum (FCS) and an antibiotic/antimicotic mixture. After the Matrigel polymerized around the extracted embryo, it was overlaid with 5 ml Dulbecco's modified Eagle medium (DMEM) (containing 4.5 g/L glucose and l-glutamine) and supplemented with 20% FCS, antibiotics, nonessential amino acids, sodium pyruvate, and 0.1 µM β-mercaptoethanol. Extraembryonic membranes were removed by dissection prior to Matrigel implantation and were used for genomic DNA isolation and genotype determination. Two (embroys number #5 and #8) out of 14 embryos were FN−/ − (a).
Figure 1. Generating FAKloxP/loxPFN−/ − p53−/ − cell lines. (a) Genotyping of embryos from FAKloxP/loxPFN+/ − p53−/ − crosses, by PCR for FN. Embryos #1, #4, and #10 were wild-type, #5 and #8 were FN−/ − mutants, whereas others were FN+/ − . Genomic DNA isolated from primary wild-type MEF and FN−/ − cell line was used as a positive and negative control, respectively. (b) Outgrowth of fibroblast-like cells from E8.0–8.5 mouse embryo cultured in a drop of Matrigel for 3, 5, or 7 days. (c) Organization of FN matrix. Cells were cultured in the presence of 20 µg/ml exogenously added FN (Sigma) for 2 days. FN matrix organization is assessed by immunostaining of fixed and permeabilized cells. Rabbit polyclonal anti-FN antibody was from Sigma. FITC-conjugated donkey anti-rabbit antibody was from Jackson Immunoresearch.
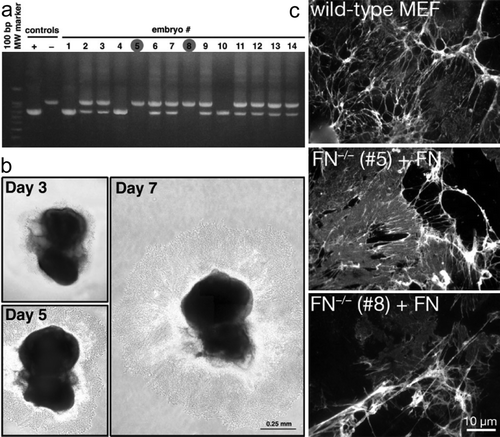
The embryos were cultured in a tissue culture incubator under standard conditions (37°C, 5% CO2) for 7 to 10 days. During that period, mesodermal cells proliferated and expanded from the embryo proper in an extended sheet (b). FAKloxP/loxPFN−/ − p53−/ − embryos #5 and #8 were then removed with surrounding sheet of cells from the Matrigel, and mechanically disaggregated in 50 to 100 µl 0.05% trypsin for several minutes. Tissue clumps and cells were transferred into FN-coated 4-well dish and cultured until they were confluent. Cells were subsequently expanded in medium containing 10% FCS.
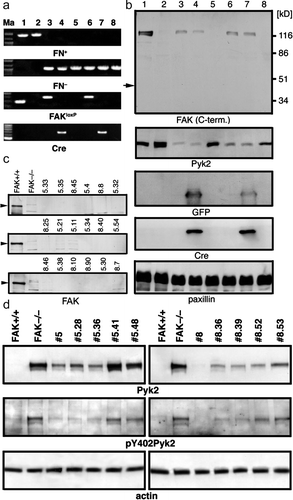
After the second passage, we reconfirmed the FAKloxP/loxPFN−/ − p53−/ − genotype by PCR and examined the ability of these cells to assemble exogenously added FN into three-dimensional (3D) matrix. As expected in FAK-expressing cells there were no differences in exogenous FN matrix assembly between primary wild-type and FN−/ − MEFs (c). Part of cultures were used to propagate cells and make frozen vials stocks for future studies. The remaining FAKloxP/loxPFN−/ − p53−/ − MEFs were exposed to adenoviral (Ad) Cre plus Ad-green fluorescent protein (GFP) as a marker to track the transduced cells (viral transduction is performed at ∼25 Ad particles per cell). After 48 h, cells were trypsinized; one half was serially diluted and plated into 96-well flat bottom tissue culture dishes, whereas the other half was used to isolate genomic DNA for PCR (a) or Western blotting (b) analyses. Single GFP-expressing colonies were marked and expanded for MEFs derived from FN−/ − embryos #5 and #8. Polymerase chain reaction (PCR) and Western blotting confirmed the transient expression of Cre and GFP as well as the deletion of FAK during the steps in this process (a, b). Of 18 FN−/ − p53−/ − clones analyzed, all were FAK negative by Western blotting analyses (c). The expression of the FAK-related Pyk2 kinase is elevated in cells upon FAK deletion (d) as has been documented previously upon FAK inactivation (Sieg et al. Citation1998).
Figure 2. Generating FAK−/ − FN−/ − p53−/ − cell lines. (a) PCR verification of knocking out FAK in vitro. Marker lane (Ma): the 100-bp marker; lanes 1 and 2: FAK+/ + and FAK−/ − control cell lines, respectively; lane 3: parental FAKloxP/loxPFN−/ − p53−/ − line #5; lane 4: line #5 transduced with Cre-expressing adenovirus for 48 h; lane 5: single clone #5.41; lane 6: parental FAKloxP/loxPFN−/ − p53−/ − line #8; lane 7: line #8 transduced with Cre-expressing adenovirus for 48 h; lane #8: single clone #8.36. PCR product size: FN+, 900 bp; FN− 1060 bp; FAK+ 290 bp; FAKloxP, 400 bp; Cre, 419 bp. The same pair of primers is used to detect both FAK+ (290 bp) and FAK loxP (400 bp) alleles. (b) Western blot analyses of FAK knockout in vitro. Lane 1, FAK wild-type control cell line; lane 2, FAK-null control cell line; lane 3, cell line 5 (FAKloxP/loxPFN−/ − p53−/ − ); lane 4, cell line 5 (FAKloxP/loxPFN−/ − p53−/ − ) plus Cre; lane 5, cell line 5.41 (FAK−/ − FN−/ − p53−/ − ) 48 h after adding Cre; lane 6, cell line 8 (FAKloxP/loxPFN−/ − p53−/ − ); lane 7, cell line 8 (FAKloxP/loxPFN−/ − p53−/ − ) plus Cre; lane 8, cell line 8.36 (FAK−/ − FN−/ − p53−/ − ) 48 h after adding Cre. Arrow indicates size of FRNK, independently expressed C-terminal region of FAK. GFP and Cre expression are detected 48 h upon infection with adenovirus and they are gone in single cell clones. Paxillin used as a loading control. (c) Western blot analysis of FAK expression in clones derived from cell lines #5 and #8 after in vitro deletion of floxed region of FAK with adnovirus-delivered Cre. FAK+/ + , control cell line that express FAK; FAK−/ − , negative control line obtained directly from FAK−/ − embryos (Furuta et al. Citation1995). (d) Western blot analysis of Pyk2 expression and (auto)phosphorylation on Y402. FAK+/ + , control cell line that express FAK; FAK−/ − , negative control line obtained directly from FAK−/ − embryos (Ilic et al. Citation1995). Actin was used as a loading control. Anti-FAK antibodies were purchased from BD Transduction Laboratories and from Santa Cruz Biotechnology. Anti-Pyk2 antibody was from from BD Transduction Laboratories, anti-paxillin and ant-GFP from Zymed, anti-phosphoY402 Pyk2 from BioSource, anti-Cre from Covance. All secondary Abs were from Jackson Immunoresearch.
DISCUSSION
Here, we described a method for generation of cell lines from E8.0–8.5 mouse embryos using a combination of in vivo and in vitro gene knockout techniques. At this stage of development, mouse embryos are composed mainly of ectoderm and mesoderm tissue. Culturing embryos ex vivo within Matrigel favors growth of a uniform population of mesodermal cells that will start to show signs of senescence after 10 to 12 passages. Early embryonic lethality of gene-targeted mice raises obstacles for addressing molecular mechanisms. With ∼25% of embryos in litter being mutant homozygotes, it is difficult, if not impossible, to generate sufficient cellular material for further analyses, especially if a given mutation affects cell proliferation. To bypass this obstacle, we crossed mice onto a p53-null background, which is known to enhance proliferative potential of cells in culture (Tsukada et al. Citation1993). Although immortalization of cells by deleting p53 would interfere with analyses of p53-dependent events, this system has a number of benefits: the starting number of cells can be low, there are no exogenously introduced factors that could cause heterogeneity of cell populations, the molecular mechanism leading to immortalization is defined, and wild-type MEFs from embryos of the same age can be derived as controls.
For in vitro knockout, the delivery of Cre recombinase with GFP-expressing Ad vectors to delete floxed genes has also several advantages: transduction efficiency is high, adenoviral vectors transfect many cell types, GFP coexpression enables easy detection or selection of transduced cells, and because Ad vectors do not integrate in genome, GFP and Cre expression is transient. The techniques described herein could be used also to obtain sufficient material for multiple analyses from tissue of any floxed mouse.
Acknowledgements
The authors thank Dr. R. Hynes (MIT) for FN + /− mice. D.I. was supported by grant CA87652. D.D.S. is an American Heart Association Established Investigator (0540115N) and is supported by CA102310 from the National Institutes of Health. None of the authors have a financial interest related to this work.
References
- Akamatsu H, Ichihara-Tanaka K, Ozono K, Kamiike W, Matsuda H, Sekiguchi K. Suppression of transformed phenotypes of human fibrosarcoma cells by overexpression of recombinant fibronectin. Cancer Res. 1996; 56: 4541–4546
- Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003; 40: 501–514
- Furuta Y, Ilic D, Kanazawa S, Takeda N, Yamamoto T, Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995; 11: 1989–1995
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993; 119: 1079–1091
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999; 285: 1028–1032
- Hynes RO. Cell adhesion: Old and new questions. Trends Cell Biol. 1999; 9: M33–M37
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995; 377: 539–544
- Ilic D, Kovacic B, Johkura K, Schlaepfer DD, Tomasevic N, Han Q, Kim JB, Howerton K, Baumbusch C, Ogiwara N, et al. FAK promotes organization of fibronectin matrix and fibrillar adhesions. J Cell Sci. 2004; 117: 177–187
- Ilic D, Mao-Qiang M, Crumrine D, Dolganov G, Larocque N, Xu P, Demerjian M, Brown BE, Lim ST, Ossovskaya V, et al. Focal adhesion kinase controls pH-dependent epidermal barrier homeostasis by regulating actin-directed Na+/H+ exchanger 1 plasma membrane localization. Am J Pathol. 2007; 170: 2055–2067
- Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008; 29: 9–22
- McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer-a new therapeutic opportunity. Nat Rev Cancer. 2005; 5: 505–515
- Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999; 76: 1–20
- Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta. 2004; 1692: 77–102
- Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK- cell migration. EMBO J. 1998; 17: 5933–5947
- Tsukada T, Tomooka Y, Takai S, Ueda Y, Nishikawa S, Yagi T, Tokunaga T, Takeda N, Suda Y, Abe S, et al. Enhanced proliferative potential in culture of cells from p53-deficient mice. Oncogene. 1993; 8: 3313–3322