Abstract
Endothelial cells (ECs) express VE-cadherin and N-cadherin, and recent data suggest that VE-cadherin levels are dependent on N-cadherin expression. While investigating changes in N-cadherin levels during endothelial monolayer maturation, the authors found that VE-cadherin levels are maintained in ECs despite a decrease in N-cadherin, suggesting that VE-cadherin levels may not depend on N-cadherin. Knockdown of N-cadherin did not affect VE-cadherin levels in ECs with low endogenous N-cadherin expression. Surprisingly, however, knockdown of N-cadherin in ECs with high endogenous N-cadherin expression increased VE-cadherin levels, suggesting an inverse relationship between the two. This was further supported by a decrease in VE-cadherin following overexpression of N-cadherin. Experiments in which p120, a catenin that binds N- and VE-cadherin, was knocked down or overexpressed indicate that these two cadherins compete for p120. These data demonstrate that VE-cadherin levels are not directly related to N-cadherin levels but may be inversely related due to competition for p120.
INTORDUCTION
Endothelial cells (ECs) form a monolayer located at the inner lining of all blood vessels where they serve as a nonthrombogenic surface, contribute to the regulation of vascular tone, and act as a restrictive barrier between the vessel lumen and the underlying tissue. The integrity of the endothelial barrier is dependent on sufficient cell-cell adhesion, mediated in part by the cadherin-based adherens junction. Cadherins are a superfamily of cell adhesion molecules, two of which, VE-cadherin and N-cadherin, are expressed by endothelial cells. VE-cadherin is endothelial specific, whereas N-cadherin is expressed in several cell types and is the predominant cadherin found in neuronal cells. The overall amino acid sequences of VE- and N-cadherin share 38% homology. The regions of most similarity lie in the cytoplasmic domains, which share 47% homology and contain the binding domains for p120 and β-catenin/plakoglobin. p120 binds the juxtamembrane region of the cadherin. The interaction of p120 with this region of the cadherin is required for maintaining the levels of cadherins by preventing endocytosis (Iyer et al. Citation2004; Xiao et al. Citation2003a, Citation2007), and post-translational modifications of p120 have been implicated in mediating changes in adhesion strength (Yap et al. Citation1998). Either β-catenin or γ-catenin (plakoglobin) binds the catenin-binding region of the cadherin and through an interaction with α-catenin regulates the association of the actin cytoskeleton with the cadherin-catenin complex, although recently this model of adherens junction linkage to the actin cytoskeleton has been questioned (Drees et al. Citation2005; Yamada et al. Citation2005).
Although these two cadherins have similar structures and binding partners, i.e., p120, β-catenin, and plakoglobin, N- and VE-cadherin have been shown to have different functions in the endothelium. The current model suggests that VE-cadherin mediates homotypic endothelial cell-cell adhesion, whereas N-cadherin mediates heterotypic contacts between ECs and vascular smooth muscle cells (VSMCs) or pericytes on the abluminal surface of the ECs (Liebner et al. Citation2006). Both of these cadherins have been attributed functional roles in vasculogenesis consistent with their respective localizations. There have been two transgenic mouse models of VE-cadherin deficiency, both embryonic lethal due to vascular defects (Carmeliet et al. Citation1999; Gory-Faure et al. Citation1999). The N-cadherin gene has also been specifically deleted in the endothelium in a transgenic mouse model and, like the VE-cadherin knockouts, was embryonic lethal due to vascular defects (Luo and Radice Citation2005). Consistent with N-cadherin's presence at regions of heterotypic cell-cell contact, several studies have strongly asserted the importance of N-cadherin recruitment of mural cells to nascent vessels during angiogenesis, which is a key step in vessel maturation (Gerhardt and Betsholtz Citation2003; Paik et al. Citation2004; Tillet et al. Citation2005). However, Luo and Radice noted that embryonic death occurred in the N-cadherin conditional knockout (embryonic day 10.5 [E10.5]) chronologically before investment of mural cells, suggesting that N-cadherin serves additional functions in the endothelium prior to pericyte recruitment. Diminished VE-cadherin expression in these embryos led the authors to suggest that N-cadherin may be responsible for maintenance of VE-cadherin levels in the endothelium (Luo and Radice Citation2005). siRNA targeting N-cadherin in cultured HUVECs (human umbilical vein endothelial cells) produced similar results supporting their hypothesis.
Studies conducted in HUVECs have shown that the composition of the endothelial adherens junction changes as the junction matures. Plakoglobin expression and junctional localization were shown to increase as confluence progressed, whereas those of β-catenin remained fairly constant. VE-cadherin mRNA, as assessed by Northern blot, also remained fairly constant, although protein levels were not assessed (Lampugnani et al. Citation1995). In investigating junctional maturation, we noted that N-cadherin levels decreased as the endothelial junction matured. Interestingly, VE-cadherin levels remained constant or increased slightly as the N-cadherin levels decreased. This led us to further investigate the relationship between VE- and N-cadherin in endothelial cell monolayers. Herein, we report that VE-cadherin levels are not dependent on N-cadherin expression; furthermore, at high levels of N-cadherin expression, there is an inverse relationship between the levels of these two cadherins. In addition, we demonstrate that N-cadherin levels are not maintained by contact of endothelial cells with vascular smooth muscle cells supporting the concept that N-cadherin function is required before vessel formation and maturation, which is consistent with findings in the endothelial specific N-cadherin deficient mouse model.
METHODS
Cell Culture
Bovine pulmonary artery endothelial cells (BPAECs; VEC Technologies) were grown in minimum essential medium (Cellgro) supplemented with 10% fetal bovine serum (Atlanta Biologicals), 0.1 mg/ml heparin (Sigma), and Pen/Strep (Gibco), and were split 1:2 every third day. QBI-293A human embryonic kidney cells (Quantum Biotechnology) for production of adenovirus were grown in Dulbecco's modified Eagle's medium (Cellgro) supplemented with 10% fetal bovine serum, and were split 1:10 every third day. Human lung microvascular cells (HLMECs; Lonza) were plated on 20 µg/ml Vitrogen (Cohesion Technologies) and grown in EGM2-MV endothelial growth medium (Lonza) supplemented with 5% fetal bovine serum. Human pulmonary artery endothelial cells (HPAECs; Lonza) were plated on 0.2% gelatin and grown in EGM2 endothelial growth medium (Lonza) supplemented with 2% fetal bovine serum. Human umbilical vein endothelial cells (HUVECs; Cascade) were plated on 0.2% gelatin and grown in EGM2 endothelial growth medium (Lonza) supplemented with 10% fetal bovine serum. Bovine lung microvascular endothelial cells (BLMECs; VEC Technologies) were grown in minimum essential medium (Cellgro) supplemented with 20% fetal bovine serum and Pen/Strep. Rat vascular smooth muscle cells (VSMCs) were isolated and provided to us by the laboratory of Dr. H. Singer, and were then grown in the same medium as human dermal microvascular endothelial cells (HDMECs), with which they would be cocultured.
HDMECs were isolated from human foreskins. Human foreskins were collected in phosphate-buffered saline/penicillin/streptomycin (PBS-Pen/Strep), rinsed with ethanol, and then digested with 1.2 U/ml dispase II at 37°C for 1 h. Following passage of tissue through a 100-µm cell strainer, a mixed population of cells was plated on fibronectin. The following day, cells were trypsinized and endothelial cells were selected from the suspension by incubating with magnetic beads coated with an antibody to CD31 (Dynal; 1×104 beads/ml cell suspension) at 4°C for 20 min. Nonspecifically bound cells were removed by washing with 0.5% bovine serum albumin (BSA) in Hanks’ balanced salt solution (HBSS), and endothelial cells were plated and passaged once. Cells were then assessed for VE-cadherin and lectin binding to document that they were endothelial cells. Cells were then frozen and stored in liquid nitrogen. Upon resuspension, HDMECs were grown in EGM2-MV endothelial growth medium (Lonza) supplemented with 5% fetal bovine serum.
HDMEC/VSMC Coculture
We used a Transwell (Corning) coculture system as described by Fillinger et al. (Citation1997) and Isakson and Duling (Citation2005). VSMCs were seeded onto 100-mm plates (no contact) or onto the underside of a 75 mm diameter 0.4-µm pore cell culture insert (contact). After 4 h, the insert was flipped and placed in a 100-mm plate, and medium was added to both top and bottom chambers. The following day, HDMECs were seeded onto three cell culture inserts. One was placed into an empty 100-mm plate, one was placed in the 100-mm plate containing VSMCs, and the third was the insert with VSMCs growing on the opposite side. Fresh medium was added to the top and bottom chambers of each plate. RNA was isolated (from HDMECs only) 96 h after seeding HDMECs, and VE-cadherin, N-cadherin, and GAPDH mRNA levels were determined by quantitative real-time polymerase chain reaction (qRT-PCR).
RNA Interference
siRNA was purchased from either Dharmacon (Human N-cadherin SmartPools + OnTarget Plus chemically modified individual sequences, negative control siRNA) or Ambion (predesigned siRNA sequences for human N-cadherin: siRNA ID no. 10506 (N06; GGCCAAACAACUUUUAAUU) and siRNA ID no. 10325 (N25; GGCUUCUGGUGAAAUCGCA); custom siRNA design for the following target sequences: bovine N-cadherin GGAAUCACGAGAAAUAGAA, bovine p120 GCCAGAGAUCAGAUAAGAA). SiRNA was delivered into cells (N-cadherin: 0.7 µg siRNA per 375,000 cells; p120: 1.0 µg siRNA per 375,000 cells) via electroporation using Ambion siPort electroporation buffer and the BioRad GenePulser Xcell Electroporation System. Knockdown was evaluated by Western blot.
Quantitative Real-Time PCR
Copy number was calculated using the standard curve method described by Leong et al. (Citation2007). Briefly, two sets of primers were used. Primers positioned to generate slightly larger DNA fragments were used to produce standards. Spectrometer readings were used to determine concentrations of standards in µg/µl. These concentrations were converted to number of copies, and serial dilutions were used to produce standards of known copy number. These standards were run simultaneously with cDNA samples using the primer sets positioned to generate smaller DNA fragments (i.e., primers that would recognize the standards as well as the cDNA samples), and copy number of samples was extrapolated by plotting threshold cycle versus log copy number of the standards. Primers were as follows (5′→3′): human VE-cadherin, forward: GGCAAGATCAAGTCAAGCGTG; human VE-cadherin, reverse: ACGTCTCCTGTCTCTGCATCG (Kondapalli et al. Citation2004); N-cadherin, forward: GCCCCTCAAGTGTTACCTCAA; N-cadherin, reverse: AGCCGAGTGATGGTCCAATTT (Yoshinaga et al. Citation2004). Primers for production of standards were as follows (5′→3′): human VE-cadherin, forward: CCTCACTTCCCCATCATGTA; human VE-cadherin, reverse: CCAGCCTCTCAATGGCGAAC; N-cadherin, forward: TACTTGATATTAATGACAAT; N-cadherin, reverse: GCTGAGCAAAATCACCATTA. Reverse transcription was performed using the iScript cDNA Synthesis Kit (BioRad). Quantitative real-time PCR was carried out using the Bio-Rad iCycler system and SYBR green mastermix (BioRad).
Adenovirus
Generation of adenoviral recombinants was performed as described by He et al. (Citation1998). The pAdTrack-CMV, p-Shuttle-CMV shuttle vector, and pAdEasy-1 vector were kindly provided by Dr. B Vogelstein. Recombinant adenovirus was amplified in QBI-293A cells and purified using CsCl2 gradients. All infections were accompanied by a control GFP infection with multiplicity of infection (MOI) at or above greatest MOI used in the experimental groups. The p120-GFP (green fluorescence protein) cDNA construct was obtained from Dr. Keith Burridge. Myc-epitope tagged chimeric interleukin-2 receptor (IL-2R)/VE-cadherin constructs have been described previously (Xiao et al. Citation2003a; Calkins et al. Citation2003). IL-2R-VE-cadcyto contains the extracellular and transmembrane portions of the IL-2 receptor and the intact cytoplasmic domain of VE-cadherin. IL-2R–VE-cadAAA contains the extracellular and transmembrane portions of the IL-2 receptor and the VE-cadherin cytoplasmic domain with the AAA mutation at amino acids 562 to 564, and thus is incapable of binding p120.
Immunoprecipitation
Cells were lysed in cytoskeleton preserving buffer (10 mM PIPES, 100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 0.5% Igepal, 1 mM phenylmethylsulfonyl fluoride [PMSF], 5 µg/ml leupeptin, 2 µg/ml aprotinin, 1 mM Na3VO4, 1 mM EDTA), and lysates were precleared by centrifugation. Supernatants were incubated with primary antibody (p120; Santa Cruz Biotechnology) for 1 h at 4°C, then Protein A agarose beads (Invitrogen) were added and incubation was continued at 4°C for 1 more h. Beads were washed three times in buffer; immunoprecipitation procedure was repeated with the supernatant; Laemmli gel sample buffer was then added to the beads and boiled to remove bound proteins, and samples were analyzed by Western Blot.
Gel Electrophoresis and Western Blot
At designated time points following experimental procedures cells were lysed in Laemmli gel sample buffer, and lysates were run on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Primary antibodies used were as follows: VE-cadherin, p120 (Santa Cruz); β-actin (Sigma); N-cadherin (BD Transduction Laboratories; ECM biosciences); Myc-epitope tag (Cell Signaling Technologies). Secondary antibodies were horseradish peroxidase (HRP)-conjugated goat-anti-mouse, rabbit-anti-goat, or goat-anti-rabbit (Jackson ImmunoResearch). Super Signal West Pico and West Femto Chemiluminescent Substrates were purchased from Pierce Biotechnology. Bands were visualized using the Fuji LAS-3000 Image Station. Densitometric analysis was performed using Fujifilm Multigauge 3.0 software.
Immunofluorescence Microscopy
Cells seeded on 0.1% gelatin-coated coverglasses were fixed in 4% paraformaldehyde, permeabilized in Tris-buffered saline (TBS) with 0.1% Triton X-100, and blocked in 5.5% goat, donkey, or fetal bovine serum in TBS. Cells were then incubated with primary antibodies to VE-cadherin (Santa Cruz Biotechnology) or N-cadherin (Zymed) in TBS and fluorescent secondary antibodies (Molecular Probes, Jackson ImmunoResearch) in 3% BSA in TBS.
RESULTS
N-Cadherin Levels Decrease During Endothelial Monolayer Maturation
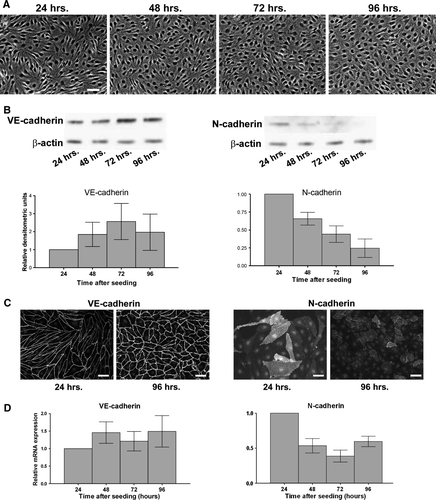
We were interested in studying the relationship between VE-cadherin and N-cadherin in the endothelium and first sought to determine whether VE- and N-cadherin levels, similar to plakoglobin levels, changed as cells become confluent. Human dermal microvascular endothelial cells (HDMECs) were plated at a density of 8×104 cells/cm2, and phase contrast images were taken to document the degree of confluence reached at 24, 48, 72, and 96 h after plating (A). Within 24 h, the monolayer appeared nearly confluent, and throughout the following 72 h we observed a further increase in cell density. RNA and protein were also isolated at these time points. Immunoblots revealed that VE-cadherin levels remained constant or slightly increased over this time course (B, left), whereas N-cadherin levels decreased sharply between 24 and 48 h after plating, continuing to decrease over the course of the experiment (B, right). N-cadherin is present in many endothelial cells at the 24-h time point and localized diffusely with some junctional localization, and a decrease in N-cadherin labeling intensity is seen at 96 h (C, right panels). In contrast, VE-cadherin labeling intensity increased at 96 h compared to 24 h (C, left panels). Determination of N-cadherin mRNA levels using quantitative real-time PCR (D, right) showed a decrease between 24 and 48 h, implying that the decrease in N-cadherin protein was due to a decrease in message. VE-cadherin mRNA levels, however, remained constant throughout the 96-h maturation period, in agreement with Lampugnani et al. (Citation1995).
Figure 1. N-cadherin expression decreases as the endothelial monolayer matures. (A) Human dermal microvascular endothelial cells (HDMECs) were plated at a density of 8.0×104 cells/cm2, and phase contrast images were taken every 24 h to document degree of confluence. Bar = 50 µm. (B) Top: Cells were lysed at each time point, and proteins were separated by SDS-PAGE. Following transfer to nitrocellulose membranes, lysates were immunoblotted for VE-cadherin, N-cadherin, and β-actin as a loading control. Bottom: Densitometric analysis of immunoblots. Values are normalized to β-actin and are expressed relative to the 24-h time point (mean±SEM; n=6). N-cadherin: p=.0011 by one-way ANOVA. (C) Localization of VE-cadherin and N-cadherin in HDMECs during growth to confluence Cells plated at 8.0×104 cells/cm2 were fixed at the 24- or 96-h time points, and immunofluorescence microscopy was performed by incubating with antibodies to either VE-cadherin (left panels) or N-cadherin (right panels) followed by fluorescent-conjugated secondary antibodies. Bar = 50 µm. (D) RNA was isolated at each time point, and quantitative real-time PCR was performed using primers to VE-cadherin, N-cadherin, and GAPDH, and copy number was determined using standard curves (Leong et al. Citation2007). Values for each time point are expressed relative to the 24-h time point, and are normalized to GAPDH (mean±SEM; n=6). N-cadherin mRNA: p = .0167 by one-way ANOVA.
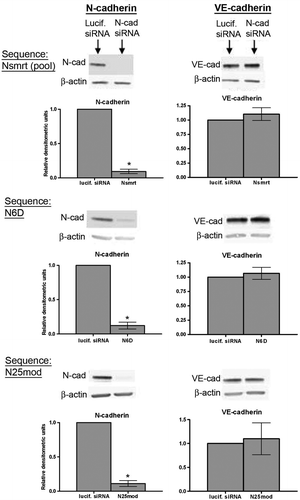
As shown in B, as HDMECs progressed to confluence, VE-cadherin levels were maintained despite a decrease in N-cadherin levels, suggesting that VE-cadherin is not dependent on N-cadherin levels. To further investigate the relationship between N- and VE-cadherin, we performed experiments using N-cadherin siRNA similar to those previously performed in HUVECs (Luo and Radice Citation2005). We first used the pool of sequences to produce successful knockdown of N-cadherin in HUVECs (siGENOME SmartPools from Dharmacon). This pool of siRNA (Nsmrt) was delivered into HUVECs by electroporation, and N- and VE-cadherin were assessed by Western blot 48 h later. As shown in , N-cadherin was successfully knocked down (, top left), and VE-cadherin levels were not altered (, top right). As RNA interference has become a more prevalent tool, methods to chemically modify siRNAs in order to minimize off-target effects have been developed, and these modified siRNAs have now become commercially available. Thus, to control for any possible off-target effects, we used additional siRNA sequences designed by two different companies. We first purchased the chemically modified pool of sequences targeting human N-cadherin from Dharmacon (OnTargetPlus SmartPools). We screened the four supplied sequences for efficiency of N-cadherin knockdown, and two of these sequences successfully depleted N-cadherin 48 hours after electroporation (N6D, , middle panels; N9D, not shown). Again we did not see a change in VE-cadherin levels. Finally, we used a chemically modified (by Dharmacon) version of a sequence we had originally obtained from Ambion (N25mod). Similar to the other sequences we used, N25mod also did not decrease VE-cadherin levels but did decrease N-cadherin levels (, bottom panels). Thus we have concluded that N-cadherin expression is not required for maintenance of VE-cadherin levels in HUVECs.
Figure 2. VE-cadherin levels in HUVECs are maintained in the absence of N-cadherin. Top panels: siRNA sequences targeting either luciferase (lucif. siRNA) or N-cadherin (Nsmrt, N6D, or N25mod) were delivered into HUVECs via electroporation, and cells were plated at confluence. Immunoblot analysis for N-cadherin (left), VE-cadherin (right), and β-actin was carried out 48 h after plating. Densitometric analysis of immunoblots is shown below. Values for N-cadherin and VE-cadherin are normalized to β-actin and are expressed relative to luciferase control (mean±SEM; minimum n=3, for N6D p<.0001, N25mod p=.0024, Nsmrt p<.0001). N6D: individual chemically modified sequence purchased from Dharmacon. Nsmrt: N-cadherin siGENOME Smartpools (unmodified) purchased from Dharmacon. N25mod: individual sequence purchased from Ambion, subsequently chemically modified by Dharmacon.
Heterogeneity of N-Cadherin Expression in Endothelial Cell Types
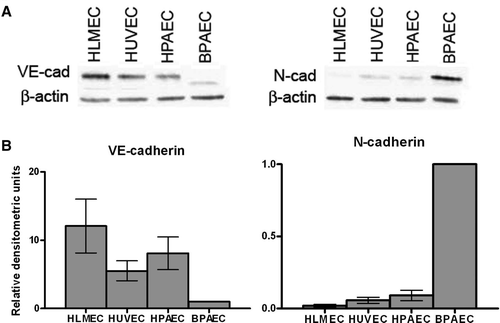
Our laboratory has worked with several different endothelial cell types, and we have observed that endothelial cells from different species and from different vascular beds express varying levels of N-cadherin protein. As shown in , confluent HLMECs, HUVECs, HPAECs, and BPAECs display different N-cadherin expression levels. The extremely low expression of N-cadherin in HLMECs relative to the other EC types was associated with high VE-cadherin expression, providing further evidence that VE-cadherin levels are not dependent on N-cadherin levels. In addition it appears that low N-cadherin expressors (HLMECs) had high VE-cadherin expression whereas high N-cadherin expressors (BPAECs) had low VE-cadherin expression; HUVECs and HPAECs fell between the two extremes. These data suggest that there may be an inverse relationship between N-cadherin and VE-cadherin at high expression levels of one of these cadherins. To determine whether the differences in cadherin expression were due to tissue of origin, we compared human and bovine microvascular endothelial cells from the same tissue of origin (HLMECs and BLMECs); BLMECs expressed N-cadherin abundantly, in contrast to HLMECs (data not shown) suggesting that bovine cells have a greater level of N-cadherin than human cells.
Figure 3. N-cadherin protein expression varies in endothelial cells from different sources. (A) Confluent monolayers of HLMECs, HUVECs, HPAECs, and BPAECs were lysed 72 to 96 h after plating, and proteins were separated by SDS-PAGE. Following transfer to nitrocellulose membranes, lysates were immunoblotted for VE-cadherin (left) and N-cadherin (right). β-Actin was used as a loading control. (B) Densitometric analysis of immunoblots shown in A. Densitometric values were normalized to β-actin and values are expressed relative to BPAECs (SEM; n = 3).
Changes in N-Cadherin Expression Levels Affect VE-Cadherin Levels, and Vice Versa
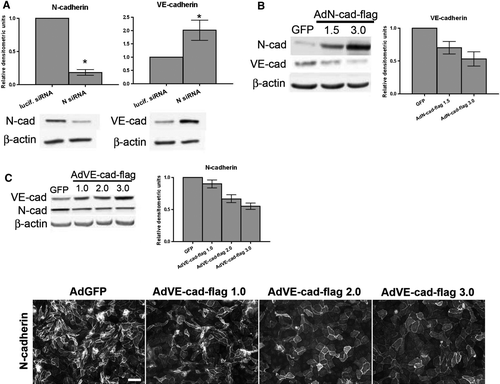
In order to determine whether the relatively low VE-cadherin expression in BPAECs was related to their high expression of N-cadherin, we depleted N-cadherin in these cells using siRNA. The decrease in N-cadherin resulted in an increase in VE-cadherin (A), supporting the idea that VE-cadherin levels are not dependent on N-cadherin, and also implying that the endogenously high N-cadherin expression in these cells limits VE-cadherin levels. This result further supports the idea that there is actually an inverse relationship between N-cadherin and VE-cadherin levels, rather than a direct relationship. Next, we expressed either N-cadherin or VE-cadherin using adenovirus containing Flag epitope-tagged-cadherin (AdN-cad-flag or AdVE-cad-flag) to determine if each would cause a decrease in the level of the other cadherin. Cells overexpressing N-cadherin displayed decreased levels of VE-cadherin in comparison to GFP-infected control BPAECs (B), whereas cells with increased expression of VE-cadherin showed a decrease in the level of N-cadherin (C, top). A corresponding decrease in N-cadherin labeling was also observed by immunofluorescence microscopy (C, bottom panels). These data demonstrate an inverse relationship between N- and VE-cadherin, such that when levels of one are high the levels of the other are decreased. Because p120, an intracellular binding partner of cadherins, is known to maintain VE-cadherin levels (Iyer et al. Citation2004) by preventing endocytosis of VE-cadherin in endothelial cells (Xiao et al. Citation2003a), we hypothesized that VE- and N-cadherin compete for p120 association in order to be stabilized at the membrane.
Figure 4. Relationship between VE-cadherin and N-cadherin protein levels in BPAECs. (A) siRNA sequences targeting either luciferase or N-cadherin were delivered into BPAECs via electroporation, and cells were plated at confluence. Immunoblot analysis for N-cadherin, VE-cadherin, and β-actin was carried out 48 h after plating. Densitometric analysis: Values for N-cadherin and VE-cadherin are normalized to β-actin and are expressed relative to luciferase control (mean±SEM, n=8; for N-cad p<.0001, VE-cad p=.0321 using single-sample t test. N siRNA: Individual sequence targeting bovine N-cadherin purchased from Ambion.) *Significance (p<.05) as determined using single-sample t test. (B) Confluent BPAEC monolayers were infected with either adenovirus containing GFP or one of two doses of flag epitope–tagged N-cadherin (AdN-cad-flag). After 48 h, cells were lysed, and N-cadherin and VE-cadherin were detected in lysates by immunoblot analysis. β-Actin was used as a loading control (bottom panels). (C) Top: Confluent BPAEC monolayers were infected with either GFP or one of three doses of VE-cadherin-flag adenovirus (AdVE-cad-flag) and immunoblotted for N-cadherin and VE-cadherin. Right, Densitometric analysis: Densitometric values for cadherins are normalized to β-actin expressed relative to control GFP infection (mean±SEM, n=3; for AdN-cad-flag, VE-cad p = .0197 by one-way ANOVA; for AdVE-cad-flag, N-cad p<.0001 by one-way ANOVA). Bottom: Following the 48-h infection with AdVE-cad-flag, immunofluorescence microscopy was performed on BPAECs using an antibody to N-cadherin, followed by a fluorescent-conjugated secondary antibody. Note the decrease in N-cadherin staining intensity with increasing dose of AdVE-cad-flag (left to right). Bar = 50 µm.
VE- and N-Cadherin Levels Are Dependent on p120
Previous studies have found that p120 maintains N-cadherin levels in neuronal cells (Davis et al. Citation2003), but this has not been shown in endothelial cells. Thus, we first depleted p120 in BPAECs using siRNA targeted to bovine p120 (A). As anticipated, VE-cadherin levels decreased (A, center), consistent with our previous findings that binding of p120 is required for maintaining VE-cadherin levels at the membrane (Iyer et al. Citation2004). N-cadherin levels decreased to a similar extent upon depletion of p120 (A, right), showing that p120 is required for maintenance of N-cadherin levels in endothelial cells. We next expressed exogenous p120 in order to increase p120 availability. Infecting confluent monolayers of BPAECs with adenovirus containing p120-GFP resulted in an increase in the levels of VE-cadherin. Interestingly, no significant change in the levels of N-cadherin (B) was observed with increased expression of p120, suggesting that, although N-cadherin is dependent on p120 association, basal levels of N-cadherin are not limited by p120 availability in the endothelium.
Figure 5. Regulation of cadherin levels by p120. (A) siRNA targeted to either luciferase (lucif. siRNA) or p120 was delivered via electroporation into BPAECs. After 48 h, cells were lysed and immunoblot analysis was carried out for p120 (left), VE-cadherin (center), and N-cadherin (right). β-Actin was used as a loading control (not shown). Densitometric values are normalized to β-actin and are expressed relative to luciferase siRNA (mean ± SEM, n=3; for p120 p=.0043, VE-cad p = .0199, N-cad p = .0166). (B) Confluent monolayers of BPAECs were infected with either GFP adenovirus or p120-GFP adenovirus. Forty-eight hours after infection, cells were lysed and immunoblot analysis was carried out for p120, VE-cadherin, N-cadherin, and β-actin as loading control. Densitometric values (right) for cadherins are normalized to β-actin and are expressed relative to GFP infection (mean ± SEM, n=5; for VE-cadherin p=.0132). *Significance (p<.05) as determined using single-sample t test. (C) Similar proportions of VE-cadherin and N-cadherin associate with p120 under control conditions. Lysates of BPAECs were subject to two consecutive cycles of immunoprecipitation for p120. Left: Immunoblot of each p120 immunoprecipitate for VE-cadherin (left lanes) and N-cadherin (right lanes). Center: Samples taken from lysate before the first immunoprecipitation (Pre-IP) and from supernatants following each immunoprecipitation (Post-IP (1) and (2)) were immunoblotted for VE-cadherin, N-cadherin, p120, and GAPDH. Right: graph represents percent of total VE-cadherin and N-cadherin precipitated from lysate in each p120 immunoprecipitation step, as analyzed by densitometry. Densitometric values for each cadherin were normalized to GAPDH to account for any dilution of the lysate and are displayed as percent of total (mean ± SEM, n=4). (D) BPAEC monolayers were infected with adenovirus containing either GFP (AdGFP), the extracellular and transmembrane portions of the IL-2 receptor fused to the cytoplasmic domain of VE-cadherin with a Myc-epitope tag (AdIL-2R-VE-cadcyto), or the extracellular and transmembrane portions of the IL-2 receptor fused to a p120-uncoupled cytoplasmic domain of VE-cadherin with a Myc-epitope tag (AdIL-2R-VE-cadAAA). 48 h after infection, cells were lysed and immunoblot analysis was carried out for N-cadherin, the Myc-epitope tag to detect the fusion proteins, and β-actin as a loading control. Blots representative of four separate experiments.
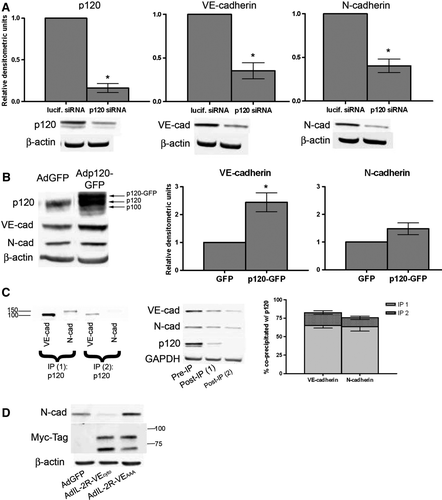
To determine whether the level of association between each cadherin and p120 was different, we performed two successive immunoprecipitations of p120 in BPAEC lysates to remove p120 and all its associated proteins from the lysate. Both cadherins were clearly present in the p120 immunoprecipitate (p120 IP, C, left). We also assessed the level of VE-cadherin or N-cadherin not associated with p120 by immunoblotting the remaining lysate following each IP (Post-IP, C, center) and comparing this to the whole lysate (Pre-IP). Densitometric analysis was performed on the VE- and N-cadherin immunoblots, and values were normalized to GAPDH to account for any dilution of the samples that occurred during the experiment. These values were used to approximate the percentage of total VE- and N-cadherin not bound to p120 (100×[VE- or N-cadherin in Post-IP lysate 1 + VE- or N-cadherin in Post-IP lysate 2]/VE-cadherin or N-cadherin in Pre-IP). These values were then subtracted from 100%, revealing that 82.61% ± 3.60% (65.25% in IP1 + 17.36% in IP2) of the total VE-cadherin and 75.54% ± 7.71% (63.25% in IP1 + 12.28% in IP2) of the total N-cadherin coprecipitated with p120 (C, right). Thus, the percentage of N-cadherin associated with p120 is similar to that of VE-cadherin in a confluent monolayer expressing both of these cadherins.
The experiments in A to C show that VE-cadherin and N-cadherin both associate with and are dependent on p120. We used a VE-cadherin mutant incapable of binding p120 in order to directly test whether competition for p120 was responsible for the decrease in N-cadherin upon VE-cadherin overexpression observed in C. The extracellular and transmembrane portions of the IL-2 receptor were fused to VE-cadherin cytoplasmic tail either without (AdIL-2R-VE-cadcyto) or with (AdIL-2R-VE-cadAAA) a mutation in the juxtamembrane domain which renders this mutant of VE-cadherin incapable of interacting with p120 (Xiao et al. Citation2003a). Expression of the intact cytoplasmic tail of VE-cadherin decreased N-cadherin levels, whereas the p120-uncoupled mutant did not (D, top panel). Thus, competition for p120 is responsible for the inverse relationship between VE- and N-cadherin protein levels.
Regulation of N-Cadherin mRNA Levels
In the absence of mural cells (pericytes and/or VSMCs), we showed that N-cadherin mRNA and protein expression decreased over time as HDMECs grew into a confluent monolayer (). Coculture of endothelial cells with mural cells has been shown in several studies to regulate endothelial cell behavior (Kurzen et al. Citation2002, Morel et al. Citation1997, Nakagawa et al. Citation2007, Korff et al. Citation2001, Orlidge and D'Amore Citation1987, Sato et al. 1989). In one of these studies, N-cadherin localization was also found to shift from EC-EC junctions to heterotypic junctions in the presence of pericytes (Kurzen et al. Citation2002). Therefore, we hypothesized that heterotypic contact of HDMECs with mural cells would prevent the drop in N-cadherin expression with confluence that we saw in . In order to discern effects of soluble factors produced by mural cells from those of direct cell-cell contact, we used two Transwell coculture systems (A): one in which endothelial cells and VSMCs were seeded on opposite sides of a 0.4-µm filter, which allowed direct contact (Fillinger et al. Citation1997 Isakson and Duling Citation2005), and another in which the ECs were seeded on the filter, and the VSMCs were seeded on the bottom of the cell culture plate not allowing direct heterotypic contact. VE- and N-cadherin mRNA expression in HDMECs was assessed 96 h after plating the HDMECs. Unexpectedly, N-cadherin mRNA levels not only failed to be maintained, but were reduced in both coculture systems as compared to HDMECs in monoculture (B). These data suggest that although N-cadherin is required for recruiting mural cells (Gerhardt et al. Citation1999; Paik et al. Citation2004; Tillet et al. Citation2005), heterotypic contact between these cells does not maintain N-cadherin mRNA expression as a nascent vessel matures. VE-cadherin mRNA expression was unchanged by coculture (B).
Figure 6. Modulation of N-cadherin mRNA expression by mural cells, serum treatment. (A) Coculture systems (Fillinger et al. Citation1997): Top: Direct contact. Rat vascular smooth muscle cells (VSMCs) were seeded on the bottom surface of a 0.4-µm pore Transwell cell culture insert. Twenty-four hours later, HDMECs were seeded on the top side of the wells, and were allowed to grow for 96 h. Bottom: No contact. VSMCs were seeded on 100-mm plate. Twenty-four hours later, HDMECs were seeded on a 0.4-µm pore Transwell cell culture insert that was placed in the 100-mm plate, and were allowed to grow for 96 h. HDMECs (not shown): cells were seeded on a 0.4-µm pore Transwell cell culture insert that was placed in an empty 100-mm plate and were allowed to grow for 96 h. (B) RNA was isolated from HDMECs. Graphs show VE-cadherin and N-cadherin mRNA expression. Values are normalized to GAPDH, and are expressed relative to HDMECs (mean±SEM, n=4; *significance [p<.05] as determined using single-sample t test; for N-cad no contact p = .0068, N-cad contact p = .0010). (C) HDMECs were seeded at confluence in growth medium. After 72 h medium was changed to either basal medium (EBM) or basal medium supplemented with 5% FBS (5%). After 24 h RNA was isolated. Graphs show VE-cadherin and N-cadherin mRNA expression. Values are normalized to GAPDH and are expressed relative to EBM-treated cells (mean±SEM, n=3; *significance [p<.05] as determined using single-sample t test; for N-cad p = .0182).
![Figure 6. Modulation of N-cadherin mRNA expression by mural cells, serum treatment. (A) Coculture systems (Fillinger et al. Citation1997): Top: Direct contact. Rat vascular smooth muscle cells (VSMCs) were seeded on the bottom surface of a 0.4-µm pore Transwell cell culture insert. Twenty-four hours later, HDMECs were seeded on the top side of the wells, and were allowed to grow for 96 h. Bottom: No contact. VSMCs were seeded on 100-mm plate. Twenty-four hours later, HDMECs were seeded on a 0.4-µm pore Transwell cell culture insert that was placed in the 100-mm plate, and were allowed to grow for 96 h. HDMECs (not shown): cells were seeded on a 0.4-µm pore Transwell cell culture insert that was placed in an empty 100-mm plate and were allowed to grow for 96 h. (B) RNA was isolated from HDMECs. Graphs show VE-cadherin and N-cadherin mRNA expression. Values are normalized to GAPDH, and are expressed relative to HDMECs (mean±SEM, n=4; *significance [p<.05] as determined using single-sample t test; for N-cad no contact p = .0068, N-cad contact p = .0010). (C) HDMECs were seeded at confluence in growth medium. After 72 h medium was changed to either basal medium (EBM) or basal medium supplemented with 5% FBS (5%). After 24 h RNA was isolated. Graphs show VE-cadherin and N-cadherin mRNA expression. Values are normalized to GAPDH and are expressed relative to EBM-treated cells (mean±SEM, n=3; *significance [p<.05] as determined using single-sample t test; for N-cad p = .0182).](/cms/asset/ab2077f7-3bca-44fe-85ef-a3d21b2bd2fc/icac_a_344205_f0006_b.gif)
Because heterotypic contact with VSMCs simulates vessel maturation, we hypothesized that activated ECs would show increased N-cadherin expression as compared to quiescent ECs. To test this hypothesis, we incubated confluent HDMECs (72 h post seeding) in either basal medium (EBM) or 5% serum-supplemented basal medium (5%) for 24 h, and assessed N- and VE-cadherin mRNA levels. As shown in C, serum stimulation increased N-cadherin mRNA expression and had no effect on VE-cadherin mRNA expression. Thus, N-cadherin expression is negatively regulated at the level of message by EC monolayer maturation (C, B), and positively regulated by activating stimuli such as growth factors present in serum (C).
DISCUSSION
Changes in the expression of adherens junction components during endothelial monolayer maturation have been previously reported: plakoglobin (γ-catenin) expression and localization were shown to increase as confluence progressed while those of β-catenin remained fairly constant (Lampugnani et al. Citation1995). We investigated the relationship between N-cadherin and VE-cadherin levels in developing endothelial monolayers and made an interesting observation: as an endothelial monolayer matures, N-cadherin levels decreased whereas VE-cadherin levels remained the same or increased slightly. The finding that VE-cadherin remained elevated in the absence of N-cadherin was inconsistent with the conclusion of Luo and Radice (Citation2005)—that VE-cadherin levels are dependent on the level of N-cadherin—thus, we performed further experiments to investigate the relationship between N- and VE-cadherin in endothelial monolayers.
Through the use of pooled siRNA sequences and several different individual siRNA sequences from two different commercial sources, we demonstrated that VE-cadherin levels are not decreased by a decrease in N-cadherin levels following treatment of HUVECs with N-cadherin siRNA. However, using the same pool of sequences purchased from Dharmacon, Luo and Radice (Citation2005) did observe a decrease in VE-cadherin levels. Several factors could have contributed to this discrepancy. For example, we used electroporation rather than lipid-based transfection to deliver siRNA. Our laboratory has used both methods, and we have achieved greater efficiency with the electroporation method. Notably, this difference in methods involves delivery of siRNA to the cells at different degrees of confluence: Electroporation is performed on cells while in suspension, therefore our cells were plated after siRNA had been delivered, whereas lipid-based transfection was carried out by Luo and Radice in 70% confluent cells. Different doses of siRNA could also be a factor. In our electroporation method, we used a specified mass of dsRNA per cell number (0.7 µg per 3.75e5 cells) rather than a molar concentration because the electroporation is carried out in a very small volume (75 µl). In reference to culture volume in which the cells are then plated, this concentration would translate to approximately 50 nM. Luo and Radice used a concentration of 100 nM to knock down N-cadherin in their system. We cannot be sure how these two doses of siRNA compare because of the difference in delivery; however, higher doses of siRNA are believed to be associated with a greater likelihood of off-target effects.
The conclusion that VE-cadherin levels are not dependent on N-cadherin expression is further supported by the findings in —heterogeneous expression of these two cadherins in endothelial cells from different species and different vascular beds. The heterogeneity in N-cadherin expression that we have observed is consistent with the global heterogeneity in endothelial cells that has been previously documented (reviewed in Aird Citation2003, Citation2006). Of note is that endothelial cells with low N-cadherin expression (HLMECs) display similar or elevated VE-cadherin levels as compared to cells that have a greater level of N-cadherin. Also, BPAECs, which had the greatest amount of N-cadherin had the lowest amount of VE-cadherin. This would suggest that N- and VE-cadherin levels may be inversely related rather than directly related. This idea is supported by studies in , showing that in addition to not being required for maintaining VE-cadherin levels, N-cadherin expression, at high levels, in fact negatively regulates VE-cadherin expression. The result shown in A is especially notable. If VE-cadherin levels were indeed dependent on N-cadherin, one would expect a decrease in VE-cadherin upon treatment of high N-cadherin expressing cells with N-cadherin siRNA. However, we saw the opposite in these cells. The increase in VE-cadherin observed following depletion of N-cadherin in BPAECs implies that VE-cadherin levels are actually limited in these cells by the large quantity of endogenous N-cadherin. Our overexpression studies are consistent with this idea. As shown in B, exogenous expression of N-cadherin in BPAECs produces a significant decrease in VE-cadherin levels. Likewise, overexpression of VE-cadherin reduced N-cadherin levels (C). These results imply an inverse relationship between levels of the two cadherins in endothelial cells, which suggests competition between the two for a protein that stabilizes each at the membrane.
When speaking of cadherin levels, it is crucial to discuss p120, an intracellular binding partner of cadherins that is required for maintenance of cadherin levels. p120 is a member of the Armadillo family of proteins and a component of the cadherin-based adherens junction. p120 functions in cell-cell adhesion, signal transduction, and regulation of gene transcription. Previous studies from our lab have demonstrated that p120 is essential for the maintenance of VE-cadherin levels and barrier function of the endothelium (Iyer et al. Citation2004), and other studies have shown that this depletion of VE-cadherin following loss of p120 binding is due to clathrin-dependent endocytosis of VE-cadherin (Xiao et al. Citation2003b). These data have established p120 as a point of control for regulation of VE-cadherin levels in endothelial cells, and similar data exist in several adhesive cell types (reviewed in Xiao et al. Citation2007). Note, however, that dependence on p120 for maintained expression of N-cadherin has not previously been shown in endothelial cells. Indeed, p120 is required for maintaining N-cadherin as siRNA targeting p120 resulted in decreases in both VE-cadherin and N-cadherin levels (A). Furthermore, by overexpression of p120 in BPAEC monolayers, we demonstrated that increased availability of p120 allowed for increased VE-cadherin levels (B). Similar results were obtained in HLMECs (data not shown). This suggests that cadherin levels in the endothelial cell are limited by the available amount of p120.
Consistent with previous reports (Navarro et al. Citation1998), p120 coimmunoprecipitates both VE-cadherin and N-cadherin (C) . In addition, we quantified by densitometry the percentage of each cadherin associated with p120 by assessing the amount of N and VE-cadherin remaining in the lysate following immunoprecipitation of p120 (100 - [100×[Post-IP1 + Post-IP2]/Pre-IP]). The vast majority of each cadherin (approximately 83% of the VE-cadherin and 76% of the N-cadherin) was bound to p120. Taken together with our p120 siRNA data, we conclude that N-cadherin, like VE-cadherin, is dependent on p120 association for maintaining levels at the membrane. Therefore, N-cadherin is regulated at the mRNA level by monolayer maturity and at the protein level by p120 availability. Interestingly, when we increased p120 availability in BPAECs via overexpression of p120 in confluent monolayers, we did not see an increase in N-cadherin levels, suggesting protein levels of N-cadherin were likely limited by the level of mRNA expression in confluent monolayers.
Navarro et al. (Citation1998) observed greater p120 band intensity in the VE-cadherin IP compared to the N-cadherin IP. They also observed diffuse surface localization of N-cadherin in contrast to localization of VE-cadherin at the endothelial cell-cell junction (Navarro et al. Citation1998). Their interpretation of these data was that p120 bound more abundantly to VE-cadherin than to N-cadherin due to a greater affinity of p120 for VE-cadherin, and that consequently N-cadherin would be excluded from EC-EC contacts. Although we did not directly test binding affinity, we do establish herein that similar percentages of each cadherin, which constitute the vast majority of each cadherin, coprecipitate with p120, demonstrating that the level of association between each cadherin and p120 is similar. Indeed, under control conditions, approximately 80% of each cadherin is associated with p120 (C). The levels of these two cadherins also showed similar dependence on p120 expression as decreased p120 availability results in a loss of both cadherins at the level of total protein (A). When levels of one cadherin were elevated, we observed a decrease in total protein of the other cadherin (B), presumably due to endocytosis in the absence of p120. When a p120-uncoupled VE-cadherin mutant was expressed in BPAEC, N-cadherin did not decrease (D), thus competition for p120 was the mechanism by which excess levels of one cadherin resulted in down-regulation of the other. This result plus the decreases in total N-cadherin seen in C (top) and 5A suggest that if VE-cadherin sequesters a greater amount of the available p120 in ECs due to it having a greater affinity, then N-cadherin would be endocytosed and degraded. This decrease in N-cadherin level could result in a redistribution of N-cadherin away from the EC-EC junctions, as observed by Navarro et al. (Citation1998). Indeed, we observed a decrease in junctional N-cadherin staining (C, bottom panels) in conjunction with the decrease in total N-cadherin protein (C, top) upon overexpression of VE-cadherin in BPAECs.
Increased expression of N-cadherin has been implicated as part of the angiogenic phenotype, playing a role in the recruitment of pericytes/smooth muscle cells to newly forming vessels (Gerhardt and Betsholtz Citation2003, Paik et al. Citation2004, Tillet et al. Citation2005). Serum factors are known to play a role in inducing angiogenesis, thus we treated confluent monolayers with serum to determine whether this activation of the ECs would induce N-cadherin expression. Indeed, activation of confluent cells by addition of serum increased N-cadherin mRNA expression (C). This expression pattern indicates that N-cadherin is important in disrupted endothelium, e.g., during angiogenesis, which is consistent with the functional roles that have been attributed to N-cadherin in mural cell recruitment (Gerhardt and Betsholtz Citation2003; Paik et al. Citation2004; Tillet et al. Citation2005) and in vascular development as suggested by the endothelial specific N-cadherin knockout (Luo and Radice Citation2005). Our data further supports the idea that N-cadherin is required during development for mural cell recruitment but is not required for vessel maturation. This is supported by the finding that N-cadherin expression decreases with formation of a more mature junction () and that coculture further decreased N-cadherin mRNA expression, in both direct contact and noncontact systems (A, B). Somewhat surprising was the finding that N-cadherin decreased to a greater extent in the coculture system than it did in confluent monocultured HDMECs (A, B; ). However, a change in gene expression of a protein is consistent with studies showing that paracrine signaling between endothelial cells and mural cells is essential for vessel stabilization (reviewed in Gerhardt and Betsholtz Citation2003), and regulates several functions in both cell types including gene expression (Darland et al. Citation2003; Korff et al. Citation2001), survival (Darland et al. Citation2003; Korff et al. Citation2001), endothelial monolayer permeability (Kurzen et al. Citation2002; Morel et al. Citation1997; Nakagawa et al. Citation2007), migration (Hirschi et al. Citation1999; Sato and Rifkin Citation1989), and proliferation (Orlidge and D'Amore Citation1987).
Our data, taken together with these functional data, support the following model: N-cadherin is up-regulated in disrupted endothelial cells, as it is essential for the maturation of newly forming vessels. As the endothelial monolayer begins to mature and is stabilized by mural cells, N-cadherin is no longer required and is consequently down-regulated. Ultimately, down-regulation of N-cadherin may result in increased p120 availability, enabling an increase in VE-cadherin levels and a mature, restrictive endothelial monolayer.
Acknowledgements
This work was supported by grants HL-77870 to P.A.V., HL-68079 to F.L.M., and AR-050501 to A.P.K. from the National Institutes of Health.
References
- Aird WC. Endothelial cell heterogeneity. Crit Care Med. 2003; 31: S221–S230
- Aird WC. Mechanisms of endothelial cell heterogeneity in health and disease. Circ Res 2006; 98: 159–162
- Calkins CC, Hoepner BL, Law CM, Novak MR, Setzer SV, Hatzfeld M, Kowalczyk AP. The Armadillo family protein p0071 is a VE-cadherin- and desmoplakin-binding protein. J Biol Chem 2003; 278: 1774–1783
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 1999; 98: 147–157
- Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D'Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol 2003; 264: 275–288
- Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol 2003; 163: 525–534
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 2005; 123: 903–915
- Fillinger MF, Sampson LN, Cronenwett JL, Powell RJ, Wagner RJ. Coculture of endothelial cells and smooth muscle cells in bilayer and conditioned media models. J Surg Res 1997; 67: 169–178
- Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res 2003; 314: 15–23
- Gerhardt H, Liebner S, Redies C, Wolburg H. N-cadherin expression in endothelial cells during early angiogenesis in the eye and brain of the chicken: Relation to blood-retina and blood-brain barrier development. Eur J Neurosci 1999; 11: 1191–1201
- Gory-Faure S, Prandini MH, Pointu H, Roullot V, Pignot-Paintrand I, Vernet M, Huber P. Role of vascular endothelial-cadherin in vascular morphogenesis. Development 1999; 126: 2093–2102
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A 1998; 95: 2509–2514
- Hirschi KK, Rohovsky SA, Beck LH, Smith SR, D'Amore PA. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res 1999; 84: 298–305
- Isakson BE, Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res 2005; 97: 44–51
- Iyer S, Ferreri DM, DeCocco NC, Minnear FL, Vincent PA. VE-Cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 2004; 286: L1143–L1153
- Kondapalli J, Flozak AS, Albuquerque ML. Laminar shear stress differentially modulates gene expression of p120 catenin, Kaiso transcription factor, and vascular endothelial cadherin in human coronary artery endothelial cells. J Biol Chem 2004; 279: 11417–11424
- Korff T, Kimmina S, Martiny-Baron G, Augustin HG. Blood vessel maturation in a 3-dimensional spheroidal coculture model: Direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J 2001; 15: 447–457
- Kurzen H, Manns S, Dandekar G, Schmidt T, Pratzel S, Kraling BM. Tightening of endothelial cell contacts: A physiologic response to cocultures with smooth-muscle-like 10T1/2 cells. J Invest Dermatol 2002; 119: 143–153
- Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. The molecular organization of endothelial cell to cell junctions: Differential association of plakoglobin, β-catenin, and α-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol 1995; 129(1)203–217
- Leong DT, Gupta A, Bai HF, Wan G, Yoong LF, Too HP, Chew FT, Hutmacher DW. Absolute quantification of gene expression in biomaterials research using real-time PCR. Biomaterials 2007; 28: 203–210
- Liebner S, Cavallaro U, Dejana E. The multiple languages of endothelial cell-to-cell communication. Arterioscler Thromb Vasc Biol. 2006; 26: 14371–8
- Luo Y, Radice GL. N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. J Cell Biol 2005; 169: 29–34
- Morel NM, Xu CB, Hechtman HB, Shepro D. Microvessel mural cell secretions modulate endothelial monolayer permeability. Microvasc Res 1997; 53: 197–200
- Nakagawa S, Deli MA, Nakao S, Honda M, Hayashi K, Nakaoke R, Kataoka Y, Niwa M. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol. 2007; 27: 687–94
- Navarro P, Ruco L, Dejana E. Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J Cell Biol 1998; 140: 1475–1484
- Orlidge A, D'Amore PA. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol 1987; 105: 1455–1462
- Paik JH, Skoura A, Chae SS, Cowan AE, Han DK, Proia RL, Hla T. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev 2004; 18: 2392–2403
- Sato Y, Rifkin DB. Inhibition of endothelial cell movement by preicytes and smooth muscle cells: Activation of a latent transforming growth factor-β1-like molecule by plasmin during co-culture. J Cell Biol 1989; 109: 309–315
- Tillet E, Vittet D, Feraud O, Moore R, Kemler R, Huber P. N-cadherin deficiency impairs pericyte recruitment, and not endothelial differentiation or sprouting, in embryonic stem cell-derived angiogenesis. Exp Cell Res 2005; 310: 392–400
- Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol 2003a; 163: 535–545
- Xiao K, Allison DF, Kottke MD, Summers S, Sorescu GP, Faundez V, Kowalczyk AP. Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. J Biol Chem 2003b; 278: 19199–19208
- Xiao K, Oas RG, Chiasson CM, Kowalczyk AP. Role of p120-catenin in cadherin trafficking. Biochim Biophys Acta 2007; 1773: 8–16
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell 2005; 123: 889–901
- Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol 1998; 141: 779–789
- Yoshinaga K, Inoue H, Utsunomiya T, Sonoda H, Masuda T, Mimori K, Tanaka Y, Mori M. N-cadherin is regulated by activin A and associated with tumor aggressiveness in esophageal carcinoma. Clin Cancer Res 2004; 10: 5702–5707