Abstract
Recruitment of leukocytes into inflamed tissue requires migration of leukocytes from the blood stream across the endothelial lining and the basement membrane of the local blood vessels. CD99 in humans is a 32-kDa highly O-glycosylated cell surface protein expressed on most leukocytes. The authors recently found CD99 to be expressed in leukocytes and at human endothelial cell contacts. Human CD99 is involved in homophilic interaction between the two cell types and participates in the transendothelial migration of monocytes and polymorphonuclear neutrophils (PMNs) in vitro. To test the role of CD99 in vivo, the authors cloned murine CD99 (muCD99), expressed it in vitro, and generated a blocking monoclonal antibody against it. We first showed that muCD99 is expressed on mouse leukocytes as well as enriched at the endothelial cell borders. Transfection of cells with muCD99 imparts on them the ability to aggregate in a CD99-dependent homophilic manner. Cells expressing muCD99 did not bind to cells expressing murine or human platelet endothelial call adhesion molecule (PECAM) or human CD99. In the thioglycollate peritonitis model of inflammation, anti-CD99 monoclonal antibody blocked the recruitment of neutrophils and monocytes by over 40% and 80%, respectively, at 18 h. Microscopy showed that this blocking occurred at the luminal surface of venules. The authors conclude that CD99 plays a major role in the emigration of leukocytes in vivo.
Introduction
Extravasation of leukocytes from blood into inflamed tissue is a critical step in the innate immune response. Endothelial cells at the site of inflammation recruit leukocytes locally. Leukocytes are captured out of the flowing bloodstream and roll along the luminal wall predominantly using selectin molecules and their counter-receptors. G protein–coupled receptors on the leukocytes sense their cognate chemokines presented on the endothelial surface. This triggers inside-out activation of leukocyte integrins to stop the rolling and induce firm adhesion. The adherent leukocytes crawl along the endothelial cell surface to the junction where they squeeze themselves between two or three cells at the borders (diapedesis) and transmigrate to the basal lamina and the tissue beneath.
Whereas the first steps in leukocyte emigration are reversible, diapedesis is a point of no return. Therefore, it represents a unique and crucial step in inflammation and a potential target to prevent unwanted inflammation. Compared to the earlier steps of rolling and adhesion, relatively little is known about the diapedesis process. However, several molecules expressed at the endothelial borders, including intercellular adhesion molecule (ICAM)-1 and -2, junctional adhesion molecule (JAM-A), the polio virus receptor (PVR), platelet endothelial cell adhesion molecule (PECAM), and CD99 (reviewed in (Muller Citation2002, Citation2003; Vestweber Citation2007) and CD99L2 (Bixel et al. Citation2007; Schenkel et al. Citation2007), have been implicated in diapedesis. This large array of molecules may underscore the complexity of diapedesis. On the other hand, specific molecules may be relatively more important in the response to a specific stimulus or the recruitment of selected leukocyte subsets.
Among these molecules, PECAM and CD99 play a major role in humans. Both have been reported to form homophilic interactions in vitro between endothelial cells and leukocytes. PECAM is involved in the initiation of transmigration (Muller et al. Citation1993), whereas CD99 is involved in the completion of the diapedesis for both monocytes (Schenkel et al. Citation2002) and neutrophils (Lou et al. Citation2007). The role for PECAM in diapedesis of neutrophils, monocytes, (Bogen et al. Citation1994; Liao et al. Citation1997, Citation1999; Schenkel et al. Citation2004; Vaporciyan et al. Citation1993), and activated murine T cells (Qing et al. Citation2001; Reinke et al. Citation2007) has been clearly confirmed in vivo. However, only one report, using a polyclonal antibody, has investigated a role for CD99 (Bixel et al. Citation2004) in vivo. Furthermore, these studies were restricted primarily to T-cell emigration.
In order to analyze the relevance of CD99 for diapedesis of myeloid cells in vivo, we used a blocking monoclonal antibody against the homologue mouse CD99. Our results demonstrate in a peritonitis model that CD99 is necessary for extravasation of both monocytes and neutrophils.
Materials and Methods
Mouse Strains
FVB/n wild-type mice were raised and housed at Weill Cornell Medical College. All procedures were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College.
Production of Stable Transfectants Expressing CD99-EGFP
A full-length murine CD99 cDNA (kindly provided by Drs. Cheryl Guyre and Johanne Kaplan, Genzyme, Framingham, MA) was inserted in the pEGFP-N1 plasmid. L-cell fibroblasts were transfected with the CD99-EGFP (enhanced green fluorescence protein) cDNA as described (Muller et al. Citation1992). Briefly 20 µg of a full-length CD99-EGFP cDNA construct in the pEGFP-N1 vector was mixed with 107 L-cells in 0.5 ml of RPMI medium and subjected to electroporation at 250 mV, 960 µF. After 2 days of culture in nonrestrictive medium (RPMI 1640 + 10% fetal bovine serum (FBS); Gibco-BRL, Grand Island, NY), transfected cells were selected by the addition of 0.5 mg/ml of geneticin (G418; Gibco). Colonies representing single clones were selected, tested for EGFP expression and subcloned by limiting dilution.
Western Blot
Clones expressing mCD99 were harvested either by trypsin or using 10 mM EDTA in Hanks’ balanced salt solution (HBSS). Cells were washed twice in phosphate-bufered saline (PBS) and lysated with 0.1% NP40 saline buffer on ice, centrifuged at 14,000 rpm for 5 min at 4°C, and the clarified lysates were collected. Lysates were first fractionated on 4% to 16% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), then proteins immobilized on a polyvinylidene difluoride membrane (Millipore, Bedford, MA) and incubated with an anti-EGFP antibody (Santa Cruz sc-8334) diluted in PBS + 5% bovine serum albumin (BSA). The membrane was then washed and incubated in horseradish peroxidase–conjugated goat anti-mouse immunoglobulin G (IgG) and detected by enhanced chemiluminescence (Amersham Pharmacia, Piscataway, NJ).
Flow Cytometry
Adherent clones expressing mCD99 were resuspended for flow cytometry by incubation in 10 mM EDTA in HBSS. Cells were washed in PBS + 2.5% FBS and analysed for EGFP expression. Alternatively, cells were incubated with a rat anti-mCD99 primary antibody, clone EJ2 (Park et al. Citation2005), or isotype control, diluted in PBS + 2.5% FBS, incubated for 30 min on ice, washed by repeated centrifugation in PBS + 2.5% FBS, then incubated for 30 min on ice in phycoerythrin (PE) -labeled F(ab′)2 fragments of goat anti-rat IgG (Beckman Coulter PN IM1623). After extensive washing, cells were analyzed on a FACScalibur (Becton Dickinson, Mountainview, CA) flow cytometer using Cell Quest Software.
Flow Cytometry of Mouse Leukocytes
Blood was collected from mice by cardiac puncture into heparinized syringes. After preblocking with 2.5% FBS, anti-muCD99 antibody was incubated with 105 leukocytes on ice, washed twice in cold PBS, incubated with fluorescein isothiocyante (FITC)-labeled F(ab′)2 fragments of goat anti-rat IgG (Beckman Coulter, PN IM1623). PE-labeled antibodies against T cells, B cells, and myeloid cells (anti-CD3, anti-CD19, and anti-Ly6C/G, respectively; BD Biosciences) were added to assess the different expression for each of the subsets.
Murine Endothelial Cell Isolation and CD99 Staining
Murine endothelial cells were isolated based on published procedures (Mason et al. Citation2007). Six mice were euthanazied and the hearts removed. Cardiac tissue was submitted to two enzymatic digestion rounds with collagenase II and trypsin. Endothelial cells were purified from cells in suspension using an anti-murine endoglin (CD105) monoclonal antibody (MJ7/18; BD Biosciences Pharmingen, San Diego, CA) on a magnetic column (miniMACS, Miltenyi Biotech, Auburn, CA). Then endothelial cells were cultured for 3 to 4 days in Dulbecco's modified Eagle's medium (DMEM) + 10% fetal calf serum (FCS). For anti-CD99 staining, endothelial cells were fixed with 2% paraformaldehyde and labeled with the EJ2 anti-muCD99 for 1 h, followed by fluorescent secondary antibody.
Aggregation Assay
Aggregation of L-cell transfectants was performed and quantified as described (Muller et al. Citation1992; Schenkel et al. Citation2002). Briefly, L-cells transfected with muCD99, huCD99, huPECAM-1, muPECAM-1, or vector alone were nonenzymatically resuspended to 106 cells/ml in HBSS with or without divalent cations, transferred in 1-ml aliquots to 24-well tissue culture plates, and incubated at 37°C on a gyrotory shaker at 90 rpm. In some experiments, the transfectants were preincubated with antibody (20 µg/ml), then washed free of unbound antibody before incubation. The aggregation reaction was stopped by adding 5% glutaraldehyde to a final concentration of 2%. In experiments to determine whether aggregation was heterophilic or homophilic, distinct populations of cells were prelabeled before mixing with 5- (and 6-)carboxyfluorescein (CFSE) (Molecular Probes, Eugene, OR). For these experiments, the cells were examined live at the end of the aggregation period by fluorescence microscopy (Muller et al. Citation1992; Schenkel et al. Citation2002).
Thioglycolate-Induced Aseptic Peritonitis
We used the sterile peritonitis model of acute inflammation to define the role of CD99 in vivo (Bogen et al. Citation1994; Schenkel et al. Citation2004). Mice were injected intravenously (i.v.) with 100 µg of a rat anti-muCD99 Ab (clone EJ2) or a rat IgG as a control 1 h before intraperitoneal (i.p.) injection of 1 ml of 4% thioglycollate broth (Difco Laboratories, Sparks, MD). We also simultaneously injected age- and sex-matched controls with PBS as control for trauma. Experiments were conducted as described previously (Bogen et al. Citation1994; Schenkel et al. Citation2004). Briefly, at 18 h after thioglycolate injection, mice were sacrificed. The peritoneal cavity was washed with 5 ml of 10 mM EDTA in HBSS. Cytospin preparation and cell counts were done immediately. Blood was drawn by cardiac puncture into heparinized syringes for white blood cell and differential counts. All internal organs were harvested and fixed in 10% buffered formalin for later examination.
Microscopy
Evaluation of the positions of leukocytes relative to the venular wall were performed as previously described (Bogen et al. Citation1994; Liao et al. Citation1997). Sections of mesentery were embedded in paraffin and coded hematoxylin and eosin (H&E) sections were examined by an experienced pathologist blinded to the identity of the specimens. Ten representative profiles of postcapillary and small venules were examined for each slide. The number of leukocytes apparently adherent to the vessel wall and the total number of leukocytes in the profile were recorded.
Statistics
All data were analyzed by pairwise comparison using a two-tailed t test assuming unequal variances.
Results
Subcloning muCD99 and Transfection
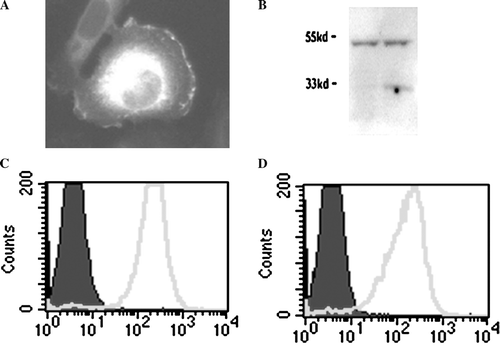
The muCD99 cDNA sequence we identified was identical to the NM.025584.2 Genbank sequence (not shown). We reamplified the muCD99 sequence and subcloned it into the pEGFP-N1 vector. The cDNA encoding the fusion protein was 1086 base pairs long. The muCD99-EGFP sequence was then transfected into L-cell fibroblasts. Expression of the fusion protein was assessed by microscopy, Western blot, and flow cytometry. Microscopy to reveal the location of the EGFP showed the fusion protein at the plasma membrane as well as in the cytoplasm (A). The EGFP-fusion protein ran at 50 kDa on SDS-PAGE, as predicted (B). Comparing trypsinized cells to nonenzymatically harvested cells, we confirmed the presence of about 1/3 of the fusion protein on the plasma membrane surface (B). Flow cytometry allowed us to quantify muCD99-EGFP expression on the FITC channel and show that that clones had uniform expression (C). Flow cytometry performed on intact cells using an anti-muCD99 antibody confirmed the presence of muCD99 at the plasma membrane surface (D).
Figure 1. Murine CD99-EGFP is expressed at the cell surface. (A) L cells transfected with muCD99-EGFP fusion protein were visualized by fluorescence microscopy. muCD99-EGFP fusion protein is expressed on the plasma membrane at the edge of the cell as well as perinuclearly (the latter presumably representing newly synthesized protein in Golgi). (B) Western blot against EGFP showing native muCD99-EGFP (left lane) and trypsin digested muCD99-EGFP (right lane). A shorter band around 33 kDa represents ∼1/3 of the muCD99-EGFP that is present on the cell surface and susceptible to extracellular proteolysis. (C and D) Flow-cytometric analysis of a clone expressing muCD99-EGFP (light line) shows a uniform population expressing EGFP (C), whereas staining of the same clone with a monoclonal antibody against muCD99 (light line; detected with a PE-labeled secondary antibody) shows uniformity of muCD99 expression (D). In C and D filled histograms represent nontransfected cells and isotype control, respectively.
MuCD99 Is Expressed on Leukocytes and Endothelial Cells
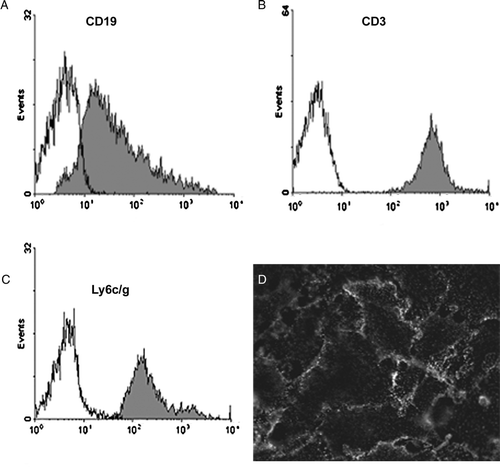
The monoclonal antibody EJ2 was previously shown to bind to CD99 on thymocytes and endothelial cells in situ (Park et al. Citation2005). To determine if its expression were potentially relevant to inflammation, we investigated its expression on peripheral blood leukocytes and localization on endothelial cells. We found that mouse peripheral blood leukocytes stain strongly for CD99. B cells expressed a lower and broader expression (A), whereas T cells (B) and the myeloid cells (C) have a high and relatively homogenous expression. In cultured mouse heart endothelial cells CD99 expression was found to be enriched at the cell borders (D), as expected based on results with human cells (Schenkel et al. Citation2002).
Figure 2. Murine CD99 is expressed on leukocytes and at endothelial cell borders. (A–C) Mouse blood was stained with anti-muCD99 and gated by other fluorescent antibodies into leukocyte subpopulations. Expression of muCD99 is shown on leukocytes gated for subpopulations: (A) B cells (CD19 positive). (B) T cells (CD3 positive). (C) Myeloid cells (Ly6 C/G positive cells). (D) Cultured murine endothelial cell monolayers were fixed and stained with anti-muCD99 mAb, which was detected with fluorescent secondary antibody. MuCD99 is restricted to cell borders. Data are representative of three independent experiments.
muCD99 Supports Homophilic Cell Aggregation in Dose-dependent Manner
CD99 mediates homophilic cell aggregation as demonstrated with human CD99 in L-cells (Schenkel et al. Citation2002). We tested the ability of muCD99 to support cell adhesion by generating L-cells that stably express the muCD99-EGFP fusion protein. We performed the aggregation assay using two different clones expressing different levels of muCD99-EGFP expression. L-cells expressing PECAM were used as positive control for homophilic aggregation, whereas L-cells transfected with EGFP or an inverted PECAM sequence were used as negative control.
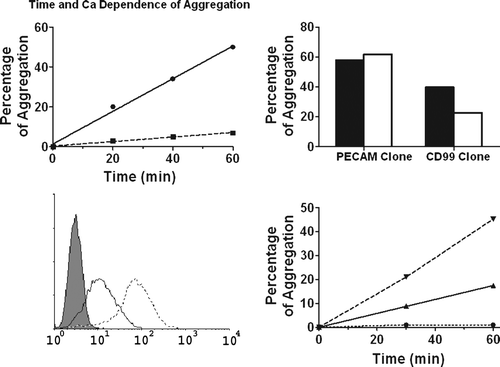
L-cells expressing muCD99-EGFP aggregated in suspension in a calcium-dependent manner (A). Aggregation increased with time over 60 min as previously seen for PECAM and huCD99 (Muller et al. Citation1992; Schenkel et al. Citation2002) and muCD99L2 (Bixel et al. Citation2007; Schenkel et al. Citation2007). Moreover, the monoclonal antibody against mCD99 blocked aggregation (B), consistent with the idea that aggregation depended on surface expression of CD99. Furthermore, L-cells aggregated in a muCD99 dose-dependent manner, because the low level expressing cells consistently aggregated slower (C, D).
Figure 3. Expression of muCD99 imparts on cells the ability to aggregate. (A) L-cells expressing muCD99 aggregate with themselves in a time and calcium-dependent manner, whereas L-cells transfected with a vector only do not aggregate (see D). Solid line, with 1 mM Ca+2; dashed line, no calcium. (B) A monoclonal antibody against muCD99 blocks aggregation by 50% (white bar) compared to control IgG (black bar). The same antibody does not affect aggregation of clones expressing PECAM. (C and D) Two clones expressing muCD99-EGFP at different levels (solid line for lower expression, dashed line for higher expression) are shown by FACS (C). The L-cell aggregation is muCD99 concentration dependent (D), because the clone with the higher expression aggregates two times faster in the presence of calcium. High expressor (▾), low expressor (▴), transfected with EGFP vector only (▪). The data shown are experiments representative of three to six independent experiments. Experimental errors were always less than 10% of the absolute value and are omitted for clarity.
Because aggregation occurred only in the presence of muCD99, we tested whether these cell-cell interactions were due to heterophilic or homophilic muCD99 adhesion. We performed mixed aggregation assays as previously described (Muller et al. Citation1992; Schenkel et al. Citation2002). When muCD99 transfectants were mixed with equal number of CFSE-labeled control cells, they aggregated only with other cells that were displaying muCD99 (A, C). Identical results were obtained when muCD99 transfectants were labeled with CFSE and mixed with unlabeled control cells (data not shown).
Figure 4. L-cell aggregation induced by muCD99 is due to homophilic interactions. (A) muCD99 transfectants mixed with an equal number of CFSE-labeled control cells aggregated only with other cells that expressed muCD99. Note only unlabeled cells in aggregates (arrowheads). (B) Unlabeled muCD99 expressing cells (arrowheads) were mixed with CFSE-labeled L-cells expressing huPECAM. Both cell types aggregated, but only in a homophilic manner. (C to F) The y-axis indicates the number of aggregates counted containing the percentage of muCD99 expressing cells (indicated on the x-axis) when muCD99 transfectants are mixed with an equal number of cells expressing empty vector (C), or transfectants expressing huPECAM (D), huCD99 (E), or muPECAM (F). The U-shaped pattern on D to F is expected if each cell-type aggregates homophilically. (See text for explanation.) Thus, most aggregates have either a very low percentage of muCD99+ cells in them or a very high percentage of muCD99+ cells. See diagram above D, where filled circles represent muCD99-expressing cells. Data shown are representative of at least three independent experiments.
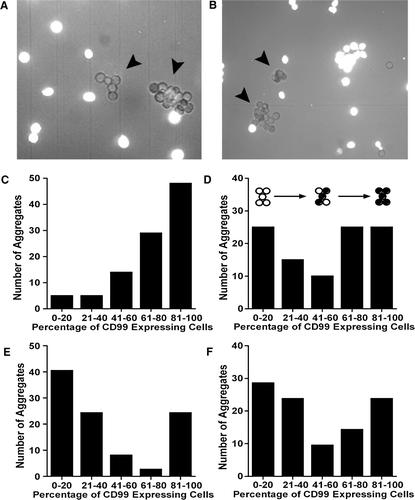
We also demonstrated that L-cells expressing muCD99 do not aggregate with cells expressing human PECAM (B, E). In the mixed aggregation studies, PECAM-bearing cells adhered to PECAM-bearing cells only, whereas muCD99 bound only to other cells expressing muCD99. The resulting histograms therefore are U-shaped, showing that most aggregates have no, or very few, CD99 expressing cells (i.e., aggregates composed of PECAM-expressing cells) or many CD99 expressing cells. In contrast, heterophilic adhesion would produce a histogram where the greatest number of aggregates had about 50% CD99 expressing cells (Muller et al. Citation1992). Furthermore, we found that cells expressing muCD99 aggregated preferentially with themselves when mixed with cells expressing huCD99 (D) or murine PECAM (F).
Blocking Diapedesis In Vivo
We have previously shown that CD99 is involved in the diapedesis process in vitro on human cells, where homophilic interaction between CD99 on endothelial cells and CD99 on neutrophils or monocytes is required (Lou et al. Citation2007; Schenkel et al. Citation2002). In order to determine if CD99 is really involved in vivo, we tested the ability of our monoclonal antibody to block extravasation in a peritonitis model in FVB mice. As shown in , peritoneal thioglycollate injection stimulated a robust inflammatory response. After 18 h, abundant neutrophils were still present in the peritoneal cavity and the number of monocytes/macrophages had increased twofold compared to control.
Figure 5. Anti-muCD99 blocks diapedesis in vivo. FVB mice received anti-muCD99 or control IgG i.v. 1 hour before they were injected i.p. with PBS or thioglycolate (Thio) and were sacrificed 18 h later for peritoneal lavage. Cell count for neutrophils (A) or monocytes/macrophages (B) showed that anti-muCD99 blocked leukocyte emigration. Data are mean±SEM for three independent experiments and are representative of a total of six independent experiments. *p<.01.
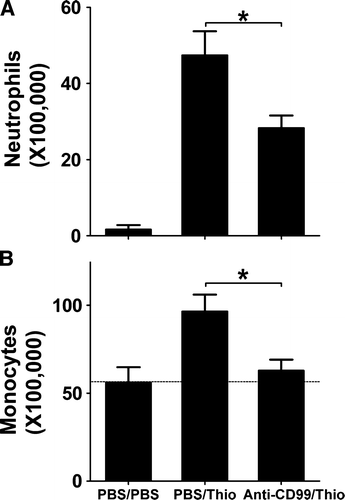
However, when mice were pretreated with an anti-muCD99 antibody, the neutrophil influx was reduced by 40% (A) and the influx of monocytes was blocked almost down to baseline levels (B). The total number of circulating leukocytes was not affected by this treatment (data not shown). Therefore, the failure of leukocytes to accumulate in the peritoneal cavity in response to thioglycollate was not due to leukocyte sequestration or elimination.
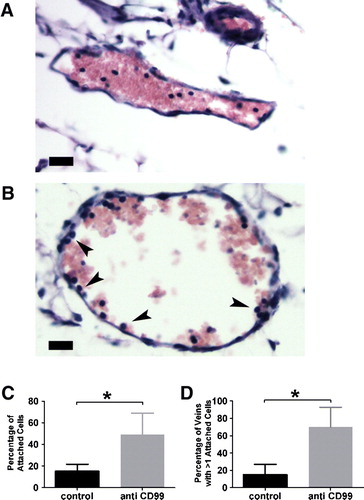
To determine the site of leukocyte blockade, we examined small venules in the mesenteries of the mice following the experiments. These were fixed in formalin immediately after harvesting of the peritoneal cells. We noticed that in the mice that had received the anti-CD99 monoclonal antibody (mAb), a significant number of leukocytes appeared to be arrested on the surface of venules at sites of inflammation (A, B). This is similar to the appearance of leukocytes in vessels in which PECAM activity had been blocked, selectively blocking diapedesis (Bogen et al. Citation1994; Liao et al. Citation1997). In those studies, the percentage of leukocytes adherent to the vascular wall and the number of vessels with more than one leukocyte adherent correlated well with blockade of leukocyte emigration and demonstrated that PECAM arrested leukocytes on the apical surface of the endothelium in this model of inflammation. Using the same method of quantification, we found that within all vessels examined, 15% of the leukocytes appeared in contact with the wall in control mice, whereas 50% appeared adherent when the mice had been treated with anti-CD99 (C). In addition, whereas 15% of vessel profiles in control mice had more than one leukocyte apparently adherent, 70% of vessel profiles in mice that received anti-CD99 mAb had more than one leukocyte adherent (D). Thus, blockade of CD99 appears to block leukocyte transmigration in vivo as it does in vitro (Schenkel et al. Citation2002).
Figure 6. The block in diapedesis is at the level of the endothelial wall. Mice were pretreated with control rat IgG (A) or anti-muCD99 (B) and injected with thioglycollate as in . Venules from mice pretreated with control rat IgG showed few leukocytes attached the venular wall (A) whereas venules from mice treated with an anti-muCD99 (B) displayed numerous leukocytes apparently interacting with the endothelial cells (arrowheads). C) Percentage of attached leukocytes in each group. (D) Percentage of venules with more than one leukocyte attached. Scale bar = 20 µm. *p<.02
Discussion
Diapedesis is a critical step in inflammation, and CD99 as been shown to be involved in vitro in this process. CD99 is an integral membrane glycoprotein with no homology with any other protein family, and has been reported involved also to be involved in apoptosis (Bernard Citation1997; Pettersen et al. Citation2001), differentiation of thymocytes (Alberti et al. Citation2002), T-cell activation (Waclavicek et al. Citation1998; Wingett et al. Citation1999), and in migration in tumor cells (Kreppel et al. Citation2006; Manara et al. Citation2006). This receptor is broadly expressed on cells of the hematopoietic system and at the junction of endothelial cells (Schenkel et al. Citation2002). Several studies showed that two splice variants of CD99 are found in humans (Hahn et al. Citation1997). A long form of 32 kDa is the most abundant whereas a short form of 28 kDa is only expressed in immature thymocytes. The short form seems to have the opposite effect of the long one on adhesion and migration (Bernard et al. Citation1997; Scotlandi et al. Citation2007). Interestingly, in mouse, only one cDNA sequence has been found and shows a highest homology to the short huCD99. Therefore, it was not clear whether the muCD99 would behave the same way as huCD99.
We generated cells expressing murine CD99. This allowed us to show that muCD99 is involved in cell-cell contact and in vitro cell aggregation as previously found for human CD99 (Schenkel et al. Citation2002) and in mouse (Bixel et al. Citation2004). Moreover, experiments with CFSE clearly demonstrated that muCD99 is involved in a homophilic adhesion similar to huCD99. MuCD99 expressing cells did not adhere to muPECAM-expressing cells, suggesting that the extracellular domains of the two proteins do not interact with each other. This is consistent with the idea that they act at two distinct steps in the diapedesis process as suggested by microscopy and blocking assays (Schenkel et al. Citation2002). Interestingly, in this experiment, cells expressing muCD99 did not aggregate with cells expressing huCD99.
A previous report concluded that the role of muCD99 in transendothelial migration in vitro did not involve homophilic interactions (Bixel et al. Citation2004). This was based on the inability of Fab fragments of a polyclonal antibody to inhibit this interaction. Although we have not attempted to reproduce their data, we find that the same monoclonal antibody that inhibits homophilic CD99 interactions in vitro also inhibits leukocyte transmigration in vivo. It seems simplest to conclude that like human CD99 (Schenkel et al. Citation2002), murine CD99 homophilic interactions govern leukocyte transendothelial migration.
Aggregation in L-cells or COS cells may not reflect the behavior of endothelial cells or leukocytes, because protein partners downstream of the receptor may differ. However, aggregation occurred only with cells expressing the same receptor, CD99 or PECAM, strongly suggesting that it is relevant at least for homophilic aggregation. We cannot rule out that muCD99 binding by itself is insufficient to promote adhesion, but instead homophilic interaction of CD99 activates an adhesion signaling cascade and/or recruits other molecules into the zones of cell-cell interaction to promote adhesion.
To study the role of CD99 in vivo, we used a specific monoclonal antibody against muCD99 that worked for immunocytochemistry, Western blot, and flow cytometry. This antibody inhibited homophilic CD99-mediated adhesion in our aggregation assay (). We then used it in vivo in a peritonitis model, since studies using a monoclonal antibody against murine CD99 had not been reported. After 18h, neutrophil transmigration was reduced by 40% (A). Bixel et al. showed using an affinity purified polyclonal antibody that transmigration of neutrophils was blocked in vivo by 60% after 4 h, a time corresponding to the peak of neutrophil extravasation (Bixel et al. Citation2007). Our data show that long after their recruitment peak, 40% of the neutrophils were still blocked by anti-muCD99. This demonstrates the importance of muCD99 for continued neutrophil extravasation. Moreover, we demonstrate in this report that blocking muCD99 also blocked monocyte transmigration to baseline levels. This was the same dramatic response of monocytes to anti-CD99 mAb that observed in vitro (Schenkel et al. Citation2002).
Microscopy showed that many leukocytes in the mice treated with anti-CD99 mAb appeared to be stuck at the luminal surface of venule, similar to the appearance of cells blocked by anti-PECAM (Bogen et al. Citation1994), whereas in a absence of any blocking antibody the cells entered the site of inflammation and were not seen on the vessel wall. At the level of resolution of the light microscope, we cannot determine whether the leukocytes are, in fact, trapped part way through the endothelial border, as found in vitro (Lou et al. Citation2007; Schenkel et al. Citation2002). However, the leukocyte nuclei appeared on the luminal surface of the endothelial cells as they did in the previous in vitro studies (Lou et al. Citation2007; Schenkel et al. Citation2002).
Bixel et al. (2004) reported that a polyclonal antibody against muCD99 partially inhibited migration of T cells as well as swelling into inflamed skin. More recently, they showed that polyclonal antibodies against mCD99 inhibit neutrophil extravasation in vivo at an early time (4 h) following inflammatory challenge (Bixel et al. Citation2007). We demonstrate here that a monoclonal antibody blocked neutrophils as well as monocytes over a longer time span. It is interesting to note that blocking CD99 blocks monocyte transmigration more efficiently than neutrophil transmigration in vivo () as well as in vitro (Lou et al. Citation2007). Because monocytes are important for chronic inflammation, CD99 may be a good therapeutic target for blocking chronic as well as acute inflammation. Antibodies against the related molecule CD99L2 have recently been shown to block inflammation in vivo including in the thioglycollate peritonitis model (Bixel et al. Citation2007; Schenkel et al. Citation2007). It will be interesting to see whether these molecules work in series or in parallel to promote transmigration. The mAb used in these studies does not cross-react with murine CD99L2 (data not shown.) Development of mice deficient in these molecules will be useful in this regard.
Further investigation will be necessary to fully understand the exact biological roles of CD99. In addition to a role in leukocyte emigration, this molecule has been reported to be involved in thymocyte development, cell motility, adhesion, and apoptosis. Recently, van Wanrooij et al. reported that immunizing mice against CD99 inhibited atherogenesis in the low-density lipoprotein (LDL) receptor–deficient mouse model (van Wanrooij et al. Citation2008). Fewer leukocytes were found in lesions, as might be expected, but it is not clear why immune response against CD99 on endothelium did not promote vascular inflammation and increase lesion size. Because blocking CD99 blocks the completion of diapedesis, it may also be involved in cell “detachment.” We showed here that despite the fact that muCD99 corresponds to the short form of huCD99, muCD99 can mediate homophilic cell adhesion in vitro and is required for efficient transendothelial migration in vivo.
Acknowledgements
This work was supported by grants from the National Institute of Health (HL046489 and HL064774). The authors would like to thank Drs. Cheryl Guyre and Johanne Kaplan for providing the muCD99 cDNA, Dr. Seong Hoe Park for providing mAb clone EJ2, Ronald M. Liebman for excellent technical assistance, and Dr. Fei Han for help with the figures. Disclosures: The authors have no financial or competing interests to disclose.
References
- Alberti I, Bernard G., Rouquette-Jazdanian A. K., Pelassy C., Pourtein M., Aussel C., Bernard A. CD99 isoform expression dictates T-cell functional outcomes. FASEB J. 2002; 16: 1946–1948
- Bernard, A. (1997). CD99 workshop panel report. In: Leukocyte Typing VI. Proceedings of the VIth International Leukocyte Differentiation Antigen Workshop, Kobe, Japan 1996. Kishimoto T London, Garland Publishers, pp. 75–77.
- Bernard G., Breittmayer J.-P., de Matteis M., Trampont P., Hofman P., Senik A., Bernard A. Apoptosis of immature thymocytes mediated by E2/CD99. J Immunol. 1997; 158: 2543–2550
- Bixel G., Kloep S., Butz S., Petri B., Engelhardt B., Vestweber D. Mouse CD99 participates in T cell recruitment into inflamed skin. Blood 2004; 104: 3205–3213
- Bixel M. G., Petri B., Khandoga A. G., Khandoga A., Wolburg-Buchholz K., Wolburg H., Marz S., Krombach F., Vestweber D. A CD99-related antigen on endothelial cells mediates neutrophil, but not lymphocyte extravasation in vivo. Blood 2007; 109: 5327–5336
- Bogen S., Pak J., Garifallou M., Deng X., Muller W. A. Monoclonal antibody to murine PECAM-1 [CD31] blocks acute inflammation in vivo. J Exp Med 1994; 179: 1059–1064
- Hahn J-H, Kim M. K., Choi E. Y., Kim S. H., Sohn H. W., Ham D. I., Chung D. H., Kim T. J., Lee W. J., Park C. K., et al. CD99 (MIC2) regulates the LFA-1/ICAM-1-mediated adhesion of lymphocytes, and its gene encodes both positive and negative regulators of cellular adhesion. J Immunol 1997; 159: 2250–2258
- Kreppel M., Aryee D. N., Schaefer K. L., Amann G., Kofler R., Poremba C., Kovar H. Suppression of KCMF1 by constitutive high CD99 expression is involved in the migratory ability of Ewing's sarcoma cells. Oncogene 2006; 25: 2795–2800
- Liao F., Ali J., Greene T., Muller W. A. Soluble domain 1 of platelet-endothelial cell adhesion molecule (PECAM) is sufficient to block transendothelial migration in vitro and in vivo. J Exp Med 1997; 185: 1349–1357
- Liao F., Schenkel A. R., Muller W. A. Transgenic mice expressing different levels of soluble platelet/endothelial cell adhesion molecule-IgG display distinct inflammatory phenotypes. J Immunol 1999; 163: 5640–5648
- Lou O., Alcaide P., Luscinskas F. W., Muller W. A. CD99 is a key mediator of the transendothelial migration of neutrophils. J Immunol 2007; 178: 1136–1143
- Manara M. C., Bernard G., Lollini P. L., Nanni P., Zuntini M., Landuzzi L., Benini S., Lattanzi G., Sciandra M., Serra M., et al. CD99 acts as an oncosuppressor in osteosarcoma. Mol Biol Cell 2006; 17: 1910–1921
- Mason J. C., Lidington E. A., Yarwood H. Isolation and analysis of large and small vessel endothelial cells. Methods Mol Med 2007; 135: 305–321
- Muller W. A. Leukocyte-endothelial cell interactions in the inflammatory response. Lab Invest 2002; 82: 521–534
- Muller W. A. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003; 24: 326–333
- Muller W. A., Berman M. E., Newman P. J., DeLisser H. M., Albelda S. M. A heterophilic adhesion mechanism for platelet/endothelial cell adhesion molecule 1 (CD31). J Exp Med 1992; 175: 1401–1404
- Muller W. A., Weigl S. A., Deng X., Phillips D. M. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med 1993; 178: 449–460
- Park S. H., Shin Y. K., Suh Y. H., Park W. S., Ban Y. L., Choi H. S., Park H. J., Jung K. C. Rapid divergency of rodent CD99 orthologs: Implications for the evolution of the pseudoautosomal region. Gene 2005; 353: 177–188
- Pettersen R. D., Bernard G., Olafsen M. K., Pourtein M., Lie S. O. CD99 signals caspase-independent T cell death. J Immunol 2001; 166: 4931–4942
- Qing Z., Sandor M., Radvany Z., Sewell D., Falus A., Potthoff D., Muller W. A., Fabry Z. Inhibition of antigen-specific T cell trafficking into the central nervous system via blocking PECAM1/CD31 molecule. J Neuropathol Exp Neurol 2001; 60: 798–807
- Reinke E. K., Lee J., Zozulya A., Karman J., Muller W. A., Sandor M., Fabry Z. Short-term sPECAM-Fc treatment ameliorates EAE while chronic use hastens onset of symptoms. J Neuroimmunol 2007; 186: 86–93
- Schenkel A. R., Chew T. W., Muller W. A. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol 2004; 173: 6403–6408
- Schenkel A. R., Dufour E. M., Chew T. W., Sorg E., Muller W. A. The murine CD99-related molecule CD99-like 2 (CD99L2) is an adhesion molecule involved in the inflammatory response. Cell Commun Adhes 2007; 14: 227–237
- Schenkel A. R., Mamdouh Z., Chen X., Liebman R. M., Muller W. A. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol 2002; 3: 143–150
- Scotlandi K., Zuntini M., Manara M. C., Sciandra M., Rocchi A., Benini S., Nicoletti G., Bernard G., Nanni P., Lollini P. L., et al. CD99 isoforms dictate opposite functions in tumour malignancy and metastases by activating or repressing c-Src kinase activity. Oncogene 2007; 26: 6604–6618
- van Wanrooij E. J., de Vos P., Bixel M. G., Vestweber D., van Berkel T. J., Kuiper J. Vaccination against CD99 inhibits atherogenesis in low-density lipoprotein receptor-deficient mice. Cardiovasc Res 2008; 78: 590–596
- Vaporciyan A. A., Delisser H. M., Yan H-C, Mendiguren I. I., Thom S. R., Jones M. L., Ward P. A., Albelda S. M. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science 1993; 262: 1580–1582
- Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev 2007; 218: 178–196
- Waclavicek M., Majdic O., Stulnig T., Berger M., Sunder-Plassmann R., Zlabinger G. J., Baumruker T., Stockl J., Ebner C., Knapp W., Pickl W. F. CD99 engagement on human peripheral blood T cells results in TCR/CD3-dependent cellular activation and allows for Th1-restricted cytokine production. J Immunol 1998; 161: 4671–4678
- Wingett D., Forcier K., Nielson C. P. A role for CD99 in T cell activation. Cell Immunol 1999; 193: 17–23