Abstract
Cadherins are synthesized with a proregion that lies between a short amino-terminal signal sequence and the first extracellular domain. Following synthesis, the proregion is cleaved, an event that is mandatory for the mature cadherin to function in adhesion. The authors have previously reported that catenins coimmunoprecipate with pro-N-cadherin, and that the N-cadherin/catenin complex forms in the Golgi/endoplasmic reticulum. It is clear that N- and E-cadherin confer significantly different characteristics on cells, and it is possible that N- and E-cadherin/catenin complex formation is equally different. To investigate this, the authors generated an antibody against the proregion of E-cadherin and have used it to examine the assembly of the E-cadherin/catenin complex.
Introduction
Classical cadherins, which are the transmembrane component of the adherens junction, mediate cell-cell adhesion via their extracellular domain and connection to the actin cytoskeleton through associations with catenins in their cytosolic domain. Epithelial cells typically express E-cadherin and mesenchymal cells express various cadherins including N-cadherin. Despite significant homology, E-cadherin and N-cadherin promote very different cellular activities. For example, during embryogenesis selected morphogenetic events involve an epithelial to mesenchymal transition (EMT), a process that promotes a migratory phenotype (Affolter et al. Citation2003). EMT is characterized by a loss of E-cadherin and gain of N-cadherin (Thiery Citation2003; Thiery and Sleeman Citation2006; Wheelock and Johnson Citation2003; Wheelock et al. Citation2008). Studies from our laboratory and others have shown that N-cadherin expression can promote motility in epithelial cells, whereas E-cadherin suppresses motility (Hazan et al. Citation2000; Islam et al. Citation1996; Kim et al. Citation2000, Citation2005; Maeda et al. Citation2005; Nieman et al. Citation1999). These significant differences between E-cadherin and N-cadherin prompted us to ask whether the dynamics of adherens junction formation differed in cells expressing E-cadherin versus N-cadherin.
Type I classical cadherins are synthesized with a proregion of approximately 130 amino acids that lies between a short amino-terminal signal sequence and the first extracellular domain (EC1) (Boggon et al. Citation2002). Following synthesis in the rough endoplasmic reticulum and phosphorylation of the cytoplasmic domain, the proregion is cleaved by furin proteases in the trans-Golgi network (Huber and Weis Citation2001; Lickert et al. Citation2000), an event that is mandatory for the mature cadherin to function in adhesion (reviewed in Gooding et al. Citation2004). However, cleavage of the cadherin proregion is not necessary for assembly of the cadherin/catenin complex. Expression of E-cadherin with a mutation in the furin recognition site resulted in nonfunctional immature E-cadherin at the plasma membrane in a complex with α-catenin and β-catenin. Thus, binding of the catenins is not dependent on proteolytic processing of E-cadherin (Ozawa and Kemler Citation1990).
Ozawa and Kemler first suggested that catenins could associate with immature forms of cadherins when they used pulse-chase experiments to show that β-catenin is associated with a high-molecular-weight form of N-cadherin that presumably includes the proregion (Ozawa and Kemler Citation1992). We subsequently reported that α-catenin, β-catenin, p120ctn, and plakoglobin all coimmunoprecipate with pro-N-cadherin in HeLa cells (Wahl et al. Citation2003). For this study, we used an antibody generated against the proregion of N-cadherin to show that α-catenin binds as effectively to immature N-cadherin as it does to mature N-cadherin. As the complexes of pro-N-cadherin and β-catenin showed limited plasma membrane localization, this study challenged the previous interpretation of cadherin/catenin assembly (Wahl et al. Citation2003).
One role for p120ctn is to regulate the levels of cadherin expression, and when cell surface cadherin is not in a complex with p120ctn, it is endocytosed and degraded (Kowalczyk and Reynolds Citation2004; Reynolds and Carnahan Citation2004). However, p120ctn is not required for normal synthesis and trafficking of E-cadherin to the plasma membrane (Davis et al. Citation2003). Indeed, it has recently been reported that, in Madin-Darby canine kidney (MDCK) cells, p120ctn does not colocalize with E-cadherin prior to arrival at the plasma membrane (Miranda et al. Citation2003). In contrast, a previous study from our laboratory showed that p120ctn is in a complex with pro-N-cadherin and that it colocalizes to the ER/Golgi with pro-N-cadherin (Wahl et al. Citation2003). It is clear that N-cadherin and E-cadherin confer significantly different characteristics on cells, and it is certainly possible that N-cadherin/catenin and E-cadherin/catenin complexes display equally different dynamics. We have now produced an antibody against the proregion of E-cadherin and have used it to examine assembly of the E-cadherin/catenin complex.
Methods
Reagents
All reagents were obtained from Sigma Chemical (St. Louis, MO) unless otherwise noted.
Cell Culture
Phoenix (human embryonic kidney) cells were a kind gift from Dr. Albert Reynolds, (Vanderbilt University, Nashville, TN). A431 (human epidermoid carcinoma) cells were obtained from American Type Culture Collection (Rockville, MD). JARPR497 (human gestational choriocarcinoma) cells were a kind gift from Dr. Peter Andrews (Wistar Institute, Philadelphia, PA). SCC22A (human oral squamous carcinoma) cells were a kind gift from Dr. Thomas Carey (University of Michigan Cancer Center, Ann Arbor, MI). The cadherin-null A431D cell line was developed in our laboratory and has been previously described (Lewis et al. Citation1997). Phoenix, A431D, and JAR cells were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) (Hyclone Laboratories, Logan, UT) at 37°C in 5% CO2. SCC22A cells were grown in minimum essential medium (MEM) with 10% FBS at 37°C in 5% CO2. Transfected Phoenix cells were maintained in puromycin (1 µg/ml), and infected A431D cells were maintained in G418 sulfate (1 mg/ml) (Mediatech, Herndon, VA). Expression of the pLK vector–based E-cadherin in transfected A431D cells was induced by addition of 10-7 M dexamethasone to culture medium, and cells were maintained in puromycin (1 µg/ml).
cDNA Constructs and Expression
Full-length human N-cadherin cDNA (GenBank no. S42303; Salomon et al. Citation1992) was a kind gift from Dr. Avri Ben-Ze'ev (Weizmann Institute, Rehovot, Israel). Full-length human E-cadherin was isolated from a JAR cell cDNA library (Johnson Citation1990), inserted into pLK-pac and transfected into A431D cells as previously described (Lewis et al. Citation1997). The LZRS-MS-neo retroviral vector has been described (Ireton et al. 2002). Phoenix cells were plated (5×105 cells) in 100-mm polystyrene plates (Becton Dickinson, Franklin Lakes, NJ) 24 h prior to transfection. Plasmid DNA was prepared as described (Johnson Citation1990), and stable transfections were carried out with a Mammalian Transfection Kit (Stratagene, La Jolla, CA) using 10 µg of plasmid DNA. One day prior to infection, target A431D cells were plated in 6-well polystyrene dishes (2×105 cells per well). Virus conditioned medium from Phoenix cells was filtered (0.45 µm), supplemented with polybrene (4 µg/ml) and added to A431D cells, which were cultured at 32°C for 8 h. Fresh DMEM was added and infected cells were selected in G418.
Cell Extraction
Confluent monolayers were washed three times in phosphate-buffered saline (PBS) at room temperature and extracted on ice with 1.5 mL TNE buffer containing 10 mM Tris acetate (pH 8.0), 1 mM ethylenediamine tetracetic acid (EDTA), 0.5% IGEPAL CA-630, and 2 mM phenylmethylsulfonyl fluoride (PMSF). After 15 min of vigorous scraping and mechanical shaking, insoluble material was removed by centrifugation for 15 min at 14,000×g at 4°C. The supernatant was assayed for protein using the BioRad protein assay (BioRad Laboratories, Hercules, CA).
Antibodies
A polyclonal antibody specific to the proregion of human E-cadherin was produced by Affinity BioReagents (Golden, CO). Rabbits were immunized with a 25–amino acid peptide (RPPPHQASVSGIQAELLTFPNSSPG) corresponding to amino acids 124 to 148 of the E-cadherin proregion (GenBank no. NM_004360) conjugated to keyhole limpet haemocyanin carrier protein. Consecutive bleeds of various rabbits were examined for reactivity. All experiments were performed with bleed 3 of a single rabbit (no. 4320) at 1:100 for immunoprecipitation and immunofluorescence, and 1:1000 for immunoblots. Mouse monoclonal antibodies recognizing human proregion N-cadherin (10A10; Wahl et al. Citation2003), cytoplasmic domain N-cadherin (13A9; Johnson et al. Citation1993), β-catenin (15B8; Johnson et al. Citation1993), and α-catenin (1G5; Johnson et al. Citation1993) have been described. Mouse monoclonal antibody, HECD-1, against the extra cellular domain of human E-cadherin was a kind gift from Dr. Masatoshi Takeichi (Kyoto University, Kyoto, Japan). Monoclonal antibodies (hybridoma supernatant) were used at 1:3 for immunoprecipitation and immunofluorescence, and 1:10 for immunoblotting. The mouse monoclonal antibody pp120 against p120ctn (BD Transduction Laboratories, Lexington, KY) was used at 1:1000 for immunofluorescence and immunoblotting. The mouse monoclonal control antibody recognizing human GAPDH (New England BioLabs, Ipswich, MA) was used at 1:10,000 for immunoblots. Mouse monoclonal anti-calnexin and mouse monoclonal anti-58K Golgi protein (Abcam, Cambridge, MA) were used at 1:200 for immunofluorescence. Rabbit polyclonal anti-E-cadherin (anti Gp-80; Damsky et al. Citation1983) was used at 1:200 for immunofluorescence.
SDS-PAGE and Immunoblotting
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Laemmli (Citation1970). Extract volumes (containing 35 to 50 µg protein) were suspended in 1×Laemmli sample buffer. Kaleidoscope Prestained Standards (BioRad Laboratories) were used as molecular mass standards. Following resolution, proteins were electrophoretically transferred to 0.45-µm nitrocellulose membranes (Whatman, Sanford, ME) for 14 h before immunoblotting. Membranes were then briefly washed in Tris-buffered saline (TBST) containing 10 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 0.05% Tween 20 (Fisher, Kalamazoo, MI) blocked in 5% nonfat dry milk in TBST for 45 min. Primary antibodies diluted in TBST were added for 1 h. Membranes were washed and incubated with species-specific horseradish peroxidase–conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h, and immunoreactive bands were visualized by incubation in Super Signal Pico substrate (Pierce, Rockford, IL) and exposure to Kodak BioMax MR film (Kodak, Rochester, NY). For quantification the secondary antibodies were conjugated to IRDye 680 (LI-COR, Lincoln, NE) and membranes were scanned and analyzed using an Odyssey Imaging System (LI-COR).
Immunoprecipitation
Polypropylene tubes were treated with 0.1% IGEPAL CA-630 and dried prior to immunoprecipitation reactions. Fifty microliters of Sepharose beads coated with Protein A (Pierce) or anti-mouse immunoglobulin G (IgG) (Cappel, Durham, NC) were added and the tubes were incubated with 330 µl HY 20% FBS medium containing the specific antibodies for 1 h on a rocking platform at 4°C. Following this preincubation, beads were pelleted and washed once with 1 ml TBST. Equalized volumes of TNE extracts were added to the beads in quantities corresponding to calculated protein concentrations, and the tubes were incubated again for 1 h on a rocking platform at 4°C. Following the second incubation, beads were rinsed three times with 1 ml TBST. After the final wash, the packed beads were resuspended in 60 µl 2 Laemmli sample buffer, processed, and loaded onto SDS-PAGE gels.
Immunofluorescence
A total of 1.5×105 cells were plated on glass coverslips for 48 h at 37°C. Coverslips were washed in 1×HEPES/Hanks (Hanks’ balanced salt solution, 0.01 M Hepes pH 7.4), fixed with 10% buffered formalin solution for 30 min, washed three times with PBS, and blocked in PBS 10% heat-inactivated goat serum for 30 min. Fixed cells were then incubated with primary antibodies at appropriate dilutions in HY medium with 20% FBS for 1 h in a humid chamber, washed, and incubated in the dark with Alexa Fluor 488–conjugated anti-mouse IgG and/or Alexa Fluor 594 anti-rabbit IgG secondary antibodies (Invitrogen, Carlsbad, CA). Coverslips were washed and mounted in Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Images were captured with a Zeiss Axiovert 200M microscope (Carl Zeiss, Thornwood, NY) equipped with an ORCA-ER digital camera (Hamamatsu, Hamamatsu City, JP) and processed using OpenLab software (Improvision, Boston, MA).
Results
To ask whether catenins interact with E-cadherin before the cadherin is fully processed required that we isolate E-cadherin that still contains the proregion. Ideally, experiments would utilize endogenous pro-E-cadherin to examine molecular associations and localization, as we did for N-cadherin, using endogenous protein in HeLa cells (Wahl et al. Citation2003). However, in our experience, E-cadherin–expressing epithelial cells process the proregion quickly and efficiently and it was not possible to see the immature form of E-cadherin by standard immunoblotting. To circumvent this problem, we stably expressed E-cadherin in the squamous epithelial cell line A431D, which does not express any endogenous cadherins (Lewis et al. Citation1997). We transfected A431D cells with the pLK plasmid vector encoding full-length E-cadherin, because we knew from previous experience that this would lead to significant overexpression, resulting in sizable levels of unprocessed cadherin. A shows A431D cells overexpressing E-cadherin (A431DEplasmid) immunoblotted for E-cadherin using the HECD-1 monoclonal antibody that is directed against EC1 of human E-cadherin and thus detects mature E-cadherin (120 kDa) and E-cadherin retaining the proregion (135 kDa).
Figure 1. Expression of immature E-cadherin in A431DE cells. (A) Fifty microgram of protein from TNE extracts of A431D cells transfected to overexpress E-cadherin (A431DEplasmid) was resolved by 7% SDS-PAGE and immunoblotted with the HECD1 monoclonal anti-E-cadherin antibody. The 135-kDa proregion containing E-cadherin and the 120-kDa mature E-cadherin are pointed out. (B). Fifty microgram of protein from TNE extracts of A431DEplasmid cells was resolved by 7% SDS-PAGE and immunoblotted with preimmune serum (pre) and successive bleeds (bl1, bl2, bl3) from a rabbit immunized with E-cadherin proregion peptide. Lane 5 shows an immunoblot of the same extract using HECD1 to point out mature (120-kDa) and immature (135-kDa) E-cadherin. (C) Fifty microgram of protein from TNE extracts of SCC22A cells, JAR cells, A431DE cells (infected to express physiological levels of E-cadherin), and A431D cells were resolved by 7% SDS-PAGE and immunoblotted with anti-proE-cadherin antiserum or HECD1 monoclonal anti-E-cadherin antibody. GAPDH was used as a loading control. (D) Lysates from A431DE and A431D cells were resolved by 15% SDS-PAGE and immunoblotted using anti-pro-E-cadherin antiserum. GAPDH was used as a loading control.
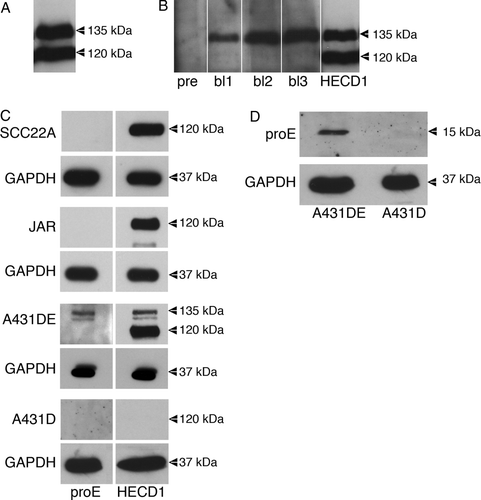
We contracted with Affinity BioReagents to generate a rabbit polyclonal antibody against the proregion of E-cadherin using a peptide comprised of amino acids 124 to 148 of E-cadherin (NP_004351) conjugated to KLH. B shows extracts of A431DEplasmid cells immunoblotted with preimmune serum (lane 1), and successive bleeds from rabbit no. 4320 (lanes 2 to 4). Lane 5 presents the same extract immunoblotted with HECD-1 to identify pro-E-cadherin and mature E-cadherin. The rabbit antiserum clearly recognizes pro-E-cadherin without recognizing mature E-cadherin.
The above cells were appropriate for characterizing the anti-pro-E-cadherin antibody, but we wanted to do experiments on cells that express much lower levels of unprocessed E-cadherin, to mimic the natural state as closely as possible. Thus we infected A431D cells with an LZRS retroviral construct encoding full-length E-cadherin (A431DE), and compared the levels of processed and unprocessed E-cadherin to two cell lines (JAR and SCC22A) that express endogenous E-cadherin (C). These two cell lines do not have sufficient unprocessed E-cadherin to detect on an immunoblot, so it was not possible to use them for our experiments. However, we could show that the expression level of E-cadherin driven by LZRS in A431D cells was comparable to that in SCC22A and JAR cells, and thus not highly overexpressed. In addition, the majority of the E-cadherin was processed, however, sufficient unprocessed protein remained (arrowhead at 135 kDa) to allow us to perform the experiments. Immunoblotting the same extract resolved on 15% SDS-PAGE identified a band of approximately 15 kDa, which was the appropriate size to be the intact proregion, which is most likely removed by the furin class of proteases in the ER/Golgi (D).
Catenin Loading on Pro-E-cadherin
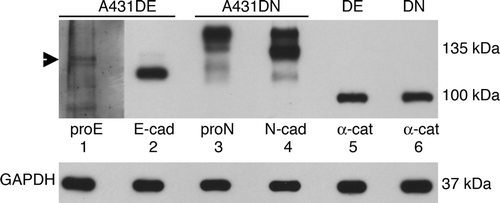
Previous studies from our lab have shown that pro-N-cadherin and mature N-cadherin interact identically with catenins (Wahl et al. Citation2003). The generation of antibodies against the proregion of E-cadherin made it possible for us to determine if pro-E-cadherin behaved similarly. To compare cadherin/catenin complex formation between immature and mature forms of E-cadherin and N-cadherin, it was necessary for us to develop an experimental design with minimal biochemical variables. Accordingly, A431D cells infected with an N-cadherin retroviral construct (A431DN cells) were used in parallel with the A431DE cells for these experiments. Due to inherent differences in antibody affinities, it was necessary to calibrate the signal from four different antibodies: anti-pro-E-cadherin, anti-pro-N-cadherin (10A10), anti-E-cadherin that recognizes both mature and immature forms (HECD1), and anti-N-cadherin that recognizes both mature and immature forms (13A9). A431DE and A431DN cell extracts immunoblotted with the four separate cadherin antibodies showed significantly different band intensities (). The different band intensities do not necessarily reflect different levels of expression of the cadherins. A431D cells do not stabilize catenins unless they express a cadherin (Lewis et al. Citation1997), and when they are transduced to express a cadherin (either E-cadherin or N-cadherin) α-catenin and β-catenin form a 1:1 complex with the cadherin and are protected from proteolytic degradation. Thus, we used the level of α-catenin as a measure of the number of cadherin molecules present in cells expressing different cadherins. We loaded various amounts of extracts from A431DE or A431DN cells and immunoblotted for α-catenin. We then chose volumes of cell extract that contained equivalent levels of α-catenin for the following studies. , lanes 5 and 6, show immunoblots for α-catenin of the same extracts shown in lanes 1 to 4. Equivalent levels of α-catenin (and thus presumably an equal number of cadherin molecules) are present in each lane.
Figure 2. Characterization of cadherin antibodies. TNE extracts (50 µg) from A431DE and A431DN cells were resolved by 7% SDS-PAGE and immunoblotted with anti-pro-E-cadherin antiserum, HECD-1 monoclonal anti-E-cadherin, 10A10 monoclonal anti-pro-N-cadherin, 13A9 monoclonal anti-N-cadherin, or 1G5 monoclonal anti-α-catenin. GAPDH was used as a loading control.
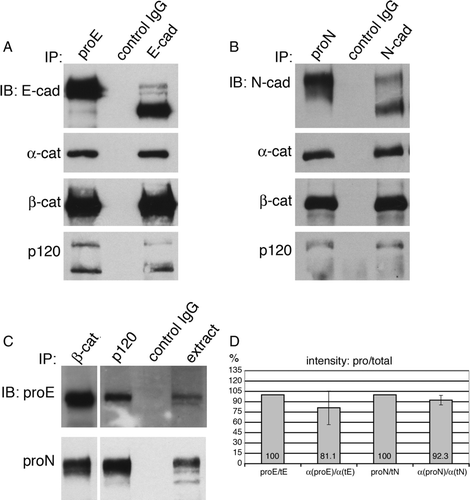
The goal of the next experiment was to immunoprecipitate an equal amount of proE-cadherin or total E-cadherin (immature + mature) using anti-proE-cadherin or HECD1 (which recognizes both mature and immature E-cadherin) and ask whether similar amounts of α-catenin were in the two immunoprecipitation reactions. Again, the experimental design was complicated by the fact that we had to perform the immunoprecipitation reactions with a volume of cell extract that would bring down roughly equivalent amounts of pro-E-cadherin in one reaction and total E-cadherin in the second reaction. A, top panel, shows A431DE cell extracts immunoprecipitated with either anti-pro-E-cadherin (lane 1) or HECD1 (lane 3) and immunoblotted with HECD1, which recognizes the pro-E-cadherin in lane 1 and both the mature E-cadherin and the pro-E-cadherin in lane 3. Roughly equal amounts of cadherin are in the two lanes. Surprisingly, there also are roughly equal amounts of α-catenin, β-catenin and p120ctn in the 2 immunoprecipitation reactions, indicating that each of the catenins is as efficiently incorporated into a complex with immature E-cadherin as it is with mature E-cadherin. As a control experiment, we immunoprecipitated proN-cadherin and total N-cadherin using a previously reported anti-proN-cadherin antibody (10A10) or a previously reported antibody that recognizes the cytoplasmic domain of N-cadherin (13A9) and thus brings down both mature and immature N-cadherin (B). As previously reported for HeLa cells (Wahl et al. Citation2003), each catenin was as efficiently incorporated into the pro-N-cadherin complex as it was in the mature N-cadherin complex. To further test the hypothesis that pro-E-cadherin is associated with catenins, we immunoprecipitated A431DE extracts with antibodies against β-catenin or p120ctn and immunoblotted for pro-E-cadherin. Each antibody brought down a measurable amount of pro-E-cadherin (C, top panel). Immunoprecipitation of A431DN extracts with antibodies against β-catenin or p120ctn and immunoblotted for pro-N-cadherin (C, bottom panel) confirmed that we obtained the same results we have previously reported for endogenous N-cadherin in HeLa cells (Wahl et al. Citation2003). Quantification of three experiments like that shown in A and B showed that an average of 81% of pro-E-cadherin molecules and 92% of pro-N-cadherin molecules were in a complex with α-catenin (D).
Figure 3. Catenins associate with immature E-cadherin. Various quantities of TNE extracts from A431DE (A) or A431DN (B) cells were immunoprecipitated with anti-pro-E-cadherin antiserum (800 µg protein from TNE extract), HECD-1 anti-E-cadherin monoclonal antibody (50 µg protein from TNE extract), 10A10 anti-pro-N-cadherin monoclonal antibody (700 µg protein from TNE extract), 13A9 anti-N-cadherin monoclonal antibody (30 µg protein from TNE extract), or control IgG (800 µg protein from A431DE TNE extracts or 700 µg protein from A431DN TNE extracts). The immunoprecipitation reactions were resolved by 7% SDS-PAGE and immunoblotted with HECD-1 anti-E-cadherin, 13A9 anti-N-cadherin, 1G5 anti-α-catenin, 15B8 anti-β-catenin, or pp120 anti-p120ctn. Both pro-E-cadherin and pro-N-cadherin were efficiently associated with α-catenin, β-catenin, and p120ctn. (C) Extracts from A431DE cells (top panel) or A431DN cells (bottom panel) were immunoprecipitated using anti-β-catenin, or anti-p120ctn and immunoblotted with anti-pro-E-cadherin antiserum (top panel) or anti-pro-N-cadherin monoclonal antibody 10A10. (D) TNE extracts from A431DE or A431DN cell lines were immunoprecipitated with anti-pro-E-cadherin, anti-E-cadherin, anti-pro-N-cadherin, or anti-N-cadherin as above and the immunoprecipitates were resolved by SDS-PAGE and immunoblotted with anti-E-cadherin, anti-N-cadherin, or anti-α-catenin. Bands were visualized by infrared fluorescent secondary antibodies using the Odyssey Imaging System to yield quantitative results. Shown is a bar graph with normalized ratios comparing intensity readings of α-catenin bound to pro-cadherin or total cadherin molecules. The data represent averages of three separate experiments.
Localization of Immature Cadherin/Catenin Complexes
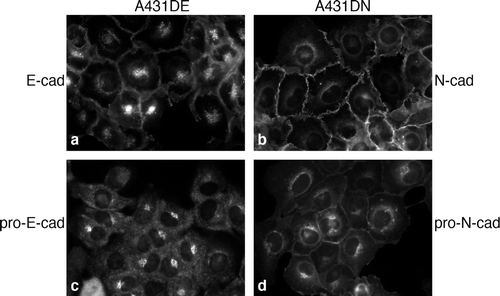
We next asked whether pro-E-cadherin was localized to the endoplasmic recticulum (ER)/Golgi region of cells using immunofluorescence microscopy. We have previously shown using HeLa cells, which express endogenous N-cadherin, that pro-N-cadherin is localized to the ER/Golgi. For the present study, we wanted not only to investigate the localization of pro-E-cadherin, but also to compare its localization to that of pro-N-cadherin. Thus we used A431D cells expressing either E-cadherin or N-cadherin so that the cellular context would be identical for the two cadherins. Antibodies that recognized all forms of E-cadherin or N-cadherin showed staining at both the plasma membrane (as expected) and in cytoplasmic regions reminiscent of ER/Golgi (, panels a and b). In contrast, antibodies against pro-E-cadherin or pro-N-cadherin showed distributions that were more restricted to the cytoplasm, particularly for anti-pro-E-cadherin (, panels c and d). There was a small amount of anti-pro-N-cadherin staining at cell borders; however, the cell border staining was significantly lower than that seen in panel b.
Figure 4. Localization of pro-cadherins in A431DE and A431DN cells. Cells grown on glass coverslips were fixed and stained for immunofluorescence with HECD-1 anti-E-cadherin monoclonal antibody (a), anti-pro-E-cadherin antiserum (c), 13A9 anti-N-cadherin monoclonal antibody (b), or 10A10 anti-pro-N-cadherin monoclonal antibody (d).
The data in show that catenins can associate with pro-E-cadherin very much like they do with pro-N-cadherin. To determine if proregion containing forms of E-cadherin colocalized with catenins in the ER/Golgi region of the cytosol, we did dual-color immunofluorescence microscopy using a rabbit anti-E-cadherin that recognizes all forms of E-cadherin together with mouse monoclonal anti-α-catenin (A, panels a to c). As expected, α-catenin colocalized with mature E-cadherin at cell-cell borders. There was also evidence for colocalization of α-catenin and E-cadherin in the ER/Golgi (A, panels b and c, arrows). To confirm this, we did colocalization with mouse anti-α-catenin antibodies together with the rabbit anti-pro-E-cadherin (A, panels d to f) or mouse anti-β-catenin together with rabbit anti-pro-E-cadherin (A, panels g to i). α-Catenin and β-catenin each colocalized with pro-E-cadherin in a typical ER/Golgi staining pattern, in addition to their cell-cell border localization where they presumably are associated with mature E-cadherin. Thus, we do not see any difference between the ability of catenins to form complexes with immature E-cadherin versus mature E-cadherin.
Figure 5. Colocalization of pro-E-cadherin with catenins. Dual-color immunofluorescence colocalization was performed on A431DE cells grown on glass coverslips. (A) Cells were stained with rabbit polyclonal anti-E-cadherin together with 1G5 mouse monoclonal anti-α-catenin (a–c); rabbit polyclonal anti-pro-E-cadherin together with 1G5 mouse monoclonal anti-α-catenin (d–f); or rabbit polyclonal anti-pro-E-cadherin together with 15B8 mouse monoclonal anti-β-catenin (g–i). B. Cells were stained with rabbit polyclonal anti-pro-E-cadherin together with mouse monoclonal anti-calnexin (a–c), or rabbit polyclonal anti-pro-E-cadherin together with mouse monoclonal anti-58K Golgi marker (d–f). Secondary antibodies were Alexa Fluor 488 anti-mouse IgG (green) and Alexa Fluor 594 anti-rabbit IgG (red).
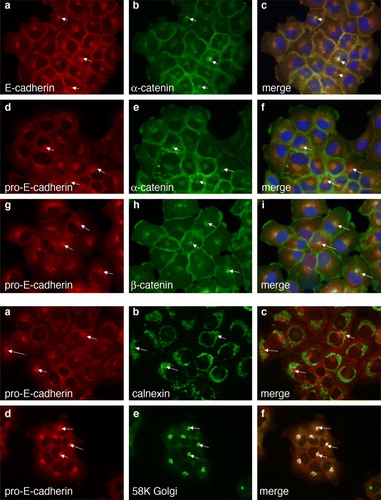
To better define the cytosolic region of the cell where pro-E-cadherin is localized, we did dual-color immunofluorescence colocalization of pro-E-cadherin with calnexin (Wada et al. Citation1991) as a marker for the ER or with 58K (Bloom and Brashear Citation1989) as a marker for the Golgi apparatus (B). Pro-E-cadherin colocalized with calnexin (B, panels a to c), and even more significantly with the Golgi marker (B, panels d to f). Thus, the localization of pro-E-cadherin is very much like that we have previously shown for pro-N-cadherin (Wahl et al. Citation2003).
Discussion
Despite their very different tissue distribution and influences on cell morphology and behavior, E-cadherin and N-cadherin share a functional role in the adherens junction. In addition, each of these classical cadherins is synthesized as a proprotein with an approximately 15-kDa proregion that is cleaved in a single step. Immunoblotting and immunofluorescence of A431D cells expressing either E-cadherin or N-cadherin using antibodies that specifically recognize the proregion showed that immature E-cadherin and immature N-cadherin have similar cytoplasmic distributions.
Much of what we know about cadherin/catenin interactions and junction formation is derived from studies with E-cadherin in polarized epithelial cells. Hinck et al. proposed a model of adherens junction assembly based on three consecutive steps: (1) association of β-catenin with newly synthesized E-cadherin in the endoplasmic reticulum; (2) incorporation of α-catenin into the cadherin/catenin complex concurrent with arrival of E-cadherin/β-catenin at the plasma membrane; and (3) linking of the cadherin/catenin complex to the actin cytoskeleton) (Hinck et al. Citation1994). These authors emphasized that incorporation of α-catenin and β-catenin into the cadherin complex is separated in time and space. Sucrose gradient sedimentation profiles of proteins from [35S]methionine-pulse-labeled MDCK cells showed that, when cells were switched from low-calcium medium to high-calcium medium, E-cadherin and β-catenin cosedimented at all chase times, whereas α-catenin sedimented in a separate fraction that slowly merged over time with the E-cadherin/β-catenin fraction. Furthermore, labeled E-cadherin/α-catenin complexes were formed later than E-cadherin/β-catenin complexes, but immediately prior to titration into the TX-100–insoluble fraction (Adams et al. Citation1996; Hinck et al. Citation1994).
Previous work in our laboratory showed in HeLa cells that α-catenin binds equally well to pro-N-cadherin and mature N-cadherin (Wahl et al. Citation2003). One explanation for this discrepancy is that N-cadherin is fundamentally different from E-cadherin in its synthesis and trafficking. To test this hypothesis, we produced an antibody against the proregion of E-cadherin that could be used in coimmunoprecipitation and colocalization experiments. The data presented in this paper show that pro-E-cadherin is essentially no different from pro-N-cadherin in its ability to form a complex with catenins. Isolated pro-E-cadherin associated strongly with α-catenin, β-catenin and p120ctn in A431DE cells. Importantly, immunoprecipitations using anti-pro-E-cadherin or pro-N-cadherin did not coimmunoprecipitate any of the mature forms of the respective cadherins; thus the catenins were bound to the immature cadherin, and not merely precipitating due to their interactions with an associated mature cadherin. Quantification of pro-cadherin/catenin complexes showed that an average of 81% (± 24%) of pro-E-cadherin molecules and 92% (± 7%) of pro-N-cadherin molecules were bound to α-catenin. This difference between E-cadherin and N-cadherin could simply be experimental variation because we used polyclonal antiserum against pro-E-cadherin and monoclonal anti-pro-N-cadherin. In addition, there was a large difference in the volume of cell extract needed to normalize for pro-E-cadherin. None-the-less, the majority of pro-E-cadherin molecules are bound to α-catenin, as we previously reported for pro-N-cadherin. We presume that the disparity between the results of our study versus those of the Hinck and Adams studies stem from differences in experimental protocols and/or differences intrinsic to the various cell types used in the studies.
An experiment in which we immunoprecipitated β-catenin or p120ctn from A431DE or A431DN cells and immunoblotted for pro-E-cadherin or pro-N-cadherin verified the binding of catenins to the immature cadherins. Immunoblots () of extracts of A431DE cells probed with anti-pro-E-cadherin antibodies or A431DN cells probed with anti-pro-N-cadherin clearly show that both E-cadherin and N-cadherin proregion–containing proteins are present as two to three distinct bands, likely due to differences in phosphorylation or glycosylation, as we have previously published for N-cadherin (Wahl et al. Citation2003). When we immunoprecipitated β-catenin or p120ctn and blotted back for pro-E-cadherin or pro-N-cadherin, it was clear that more than one of these bands are associated with the catenin. A previous study from our laboratory showed that p120ctn can bind the earliest, nonphosphorylated form of pro-N-cadherin in HeLa cells; two bands that correspond to the phosphorylated and nonphosphorylated forms of pro-N-cadherin were distinctly visible with the appropriate monoclonal antibody (Wahl et al. Citation2003). In the current experiments, we could not cleanly detect multiple forms of pro-E-cadherin binding to p120ctn in A431DE. A parallel experiment with A431DN cells, however, confirmed the binding of p120ctn to two forms of pro-N-cadherin. The difference seen in these experiments is likely due to differences in the quality of the anti-pro-cadherin antibodies. The antibody against pro-N-cadherin is a monoclonal antibody that very cleanly binds only to pro-N-cadherin in cell extracts. We have tried numerous times to produce a monoclonal antibody that specifically recognizes the proregion of E-cadherin but have not been able to do so. Thus, we resorted to a polyclonal antipeptide antibody against the proregion of E-cadherin. Although this antibody is very clean, and recognizes pro-E-cadherin but not mature E-cadherin, it does not blot as cleanly as the monoclonal antibody against pro-N-cadherin. These studies provide strong evidence that E-cadherin and N-cadherin are processed in a similar fashion and both interact with catenins before the cadherin is processed and translocated to the plasma membrane, but they do not rule out the possibility for unique functional characteristics between the various classical cadherins.
Immunofluorescence microscopy showed that pro-E-cadherin colocalized with α-catenin and β-catenin in A431DE cells, indicating that the coimmunoprecipitation of pro-E-cadherin with catenins is not an artifact of extracting cells with detergent. The use of subcellular markers indicated that pro-E-cadherin was found in the Golgi apparatus and, to a smaller degree, in the endoplasmic reticulum. In addition, we did not see a signficant signal for pro-E-cadherin at the plasma membrane.
It is becoming clear that although classical cadherins appear to form complexes with the same proteins to form an adherens junction, despite whether the cadherin is E-cadhrein, N-cadherin, R-cadherin, or P-cadherin, the influence of the cadherin on cell morphology and behavior can vary significantly from one cadherin to another (Wheelock and Johnson Citation2003; Wheelock et al. Citation2008). Our study is significant because it makes use of a single cadherin-null cell line (A431D) to carefully examine the formation of E-cadherin/catenin complexes versus N-cadherin/catenin complexes. We could not detect any significant differences in the dynamics of the formation of cadherin/catenin complexes, despite the fact that we know from previous studies that these two cadherins confer very different behaviors on these same cells (Kim et al. Citation2000). It is possible that polarized epithelial cells like MDCK form cadherin/catenin complexes quite differently from a nonpolarized cell, because polarized cells synthesize and sort proteins in a polarized fashion (Alonso et al. Citation1997; Gottlieb et al. Citation1986). Thus, our study, together with others in the literature, points out how important it is to consider cellular context when drawing conclusions about cadherins and their interactions with other proteins.
In summary, we have shown that the majority of the cadherin/catenin complex can be formed prior to cleavage of the E-cadherin proregion. The catenins, including α-catenin, β-catenin, and p120ctn, bind to immature E-cadherin while it is traveling through the endoplasmic reticulum and Golgi apparatus. p120ctn may bind to earlier nonphosphorylated forms of pro-E-cadherin like it does to pro-N-cadherin, but this has not been conclusively shown. In all other respects, the two cadherins display similar dynamics of cadherin/catenin complex formation. Thus, although E-cadherin and N-cadherin promote very different cellular phenotypes and behaviors, it is clear that in the same cellular context, the cadherin/catenin complexes form in a very similar manner. It is likely that the interactions of cadherins with other proteins at the cell surface, such as growth factor receptors, are responsible for the distinct cellular phenotypes observed when a cell expresses different cadherins.
Acknowledgements
This work was supported by NIH R01-DE12308 and NIH R01-GM51188 and by NCI P30 CA36727 to the Eppley Institute.
References
- Adams CL, Nelson WJ, Smith SJ. Quantitative analysis of cadherin-catenin-actin reorganization during development of cell-cell adhesion. J Cell Biol. 1996; 135: 1899–1911
- Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z. Tube or not tube: Remodeling epithelial tissues by branching morphogenesis. Dev Cell 2003; 4: 11–18
- Alonso MA, Fan L, Alarcon B. Multiple sorting signals determine apical localization of a nonglycosylated integral membrane protein. J Biol Chem 1997; 272: 30748–30752
- Bloom GS, Brashear TA. A novel 58-kDa protein associates with the Golgi apparatus and microtubules. J Biol Chem 1989; 264: 16083–16092
- Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science 2002; 296: 1308–1313
- Damsky CH, Richa J, Solter D, Knudsen K, Buck CA. Identification and purification of a cell surface glycoprotein mediating intercellular adhesion in embryonic and adult tissue. Cell 1983; 34: 455–466
- Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol 2003; 163: 525–534
- Gooding JM, Yap KL, Ikura M. The cadherin-catenin complex as a focal point of cell adhesion and signalling: New insights from three-dimensional structures. Bioessays 2004; 26: 497–511
- Gottlieb TA, Beaudry G, Rizzolo L, Colman A, Rindler M, Adesnik M, Sabatini DD. Secretion of endogenous and exogenous proteins from polarized MDCK cell monolayers. Proc Natl Acad Sci U S A 1986; 83: 2100–2104
- Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol 2000; 148: 779–790
- Hinck L, Nathke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: Novel protein interactions and pathways of complex assembly. J Cell Biol 1994; 125: 1327–1340
- Huber AH, Weis WI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell 2001; 105: 391–402
- Islam S, Carey TE, Wolf GT, Wheelock MJ, Johnson KR. Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J Cell Biol 1996; 135: 1643–1654
- Johnson KR. A small-scale plasmid preparation yielding DNA suitable for double-stranded sequencing and in vitro transcription. Anal Biochem 1990; 190: 170–174
- Johnson KR, Lewis JE, Li D, Wahl J, Soler AP, Knudsen KA, Wheelock MJ. P- and E-cadherin are in separate complexes in cells expressing both cadherins. Exp Cell Res 1993; 207: 252–260
- Kim JB, Islam S, Kim YJ, Prudoff RS, Sass KM, Wheelock MJ, Johnson KR. N-Cadherin extracellular repeat 4 mediates epithelial to mesenchymal transition and increased motility. J Cell Biol 2000; 151: 1193–1206
- Kim YJ, Johnson KR, Wheelock MJ. N-cadherin-mediated cell motility requires cis dimers. Cell Commun Adhes 2005; 12: 23–39
- Kowalczyk AP, Reynolds AB. Protecting your tail: Regulation of cadherin degradation by p120-catenin. Curr Opin Cell Biol 2004; 16: 522–527
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–685
- Lewis JE, Wahl JK, 3rd, Sass KM, Jensen PJ, Johnson KR, Wheelock MJ. Cross-talk between adherens junctions and desmosomes depends on plakoglobin. J Cell Biol 1997; 136: 919–934
- Lickert H, Bauer A, Kemler R, Stappert J. Casein kinase II phosphorylation of E-cadherin increases E-cadherin/beta-catenin interaction and strengthens cell-cell adhesion. J Biol Chem 2000; 275: 5090–5095
- Maeda M, Johnson KR, Wheelock MJ. Cadherin switching: Essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci 2005; 118: 873–887
- Miranda KC, Joseph SR, Yap AS, Teasdale RD, Stow JL. Contextual binding of p120ctn to E-cadherin at the basolateral plasma membrane in polarized epithelia. J Biol Chem 2003; 278: 43480–43488
- Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol 1999; 147: 631–644
- Ozawa M, Kemler R. Correct proteolytic cleavage is required for the cell adhesive function of uvomorulin. J Cell Biol 1990; 111: 1645–1650
- Ozawa M, Kemler R. Molecular organization of the uvomorulin-catenin complex. J Cell Biol 1992; 116: 989–996
- Reynolds AB, Carnahan RH. Regulation of cadherin stability and turnover by p120ctn: Implications in disease and cancer. Semin Cell Dev Biol 2004; 15: 657–663
- Salomon D, Ayalon O, Patel-King R, Hynes RO, Geiger B. Extrajunctional distribution of N-cadherin in cultured human endothelial cells. J Cell Sci 1992; 102(Pt 1)7–17
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 2003; 15: 740–746
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006; 7: 131–142
- Wada I, Rindress D, Cameron PH, Ou WJ, Doherty JJ 2nd, Louvard D, Bell AW, Dignard D, Thomas DY, Bergeron JJ. SSR alpha and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J Biol Chem 1991; 266: 19599–19610
- Wahl JK 3rd, Kim YJ, Cullen JM, Johnson KR, Wheelock MJ. N-cadherin-catenin complexes form prior to cleavage of the proregion and transport to the plasma membrane. J Biol Chem 2003; 278: 17269–17276
- Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol 2003; 19: 207–235
- Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci 2008; 121: 727–735