It has become clear that miRNA processing plays a pivotal role in fundamental aspects of cell behavior. As recently reported by Viswanathan et al. (Citation2008), miRNA processing by Lin28 plays an important role in cancer progression. These data are consistent with the promotion of tumor cell growth by Lin28B as reported by Guo et al. (Citation2006). In addition, studies by Yu et al. (Citation2007) indicate that Lin28 plays a critical role in enabling stem cell pluripotency.
Although Lin28 can promote tumor cell migration and differentiation potential, underlying factors that control Lin28 expression have not been defined. The Src kinase also plays pivotal roles in early embryonic development and cancer progression (Yeatman Citation2004). This led us to hypothesize that Lin28 expression can be regulated by the Src tyrosine kinase.
The test this hypothesis, we examined Lin28 expression in Src transformed and nontransformed mouse embryonic fibrobasts. As shown in a, Lin28 expression was several fold higher in cells transfected with v-Src than control transfectants. Thus, Src kinase activity seemed to increase Lin28 expression in transformed cells.
Figure 1. Src induces Lin28 expression. (a) Mouse embryonic fibroblasts transfected with v-Src or the empty parental vector were analyzed by Western blotting for Lin28 expression, β-actin expression, and Src activity. Lin28 expression levels are shown as the percent of nontransformed cells (mean + SEM; n=2). (b) Nontransformed cells were EGF deprived by growth in serum-free medium overnight. Cells were either unstimulated or stimulated with 100ng/ml of EGF for 12 h. Lysates were analyzed for Lin28 expression, Src activity, phosphorylated Akt, and β-actin. Lin28 expression levels are shown as a percent of unstimulated cells (mean + SEM; n=2).
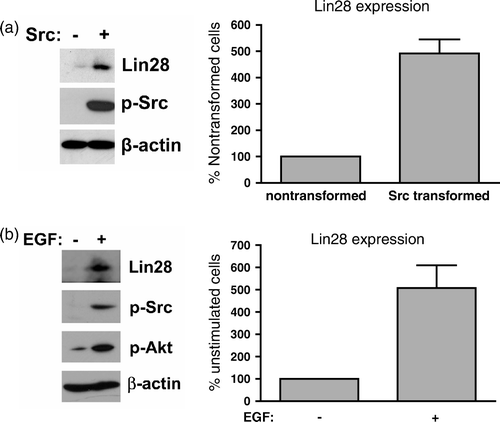
Epidermal growth factor (EGF) stimulation has been shown to increase cellular Src (c-Src) activity (Osherov and Levitzki Citation1994). Therefore, nontransformed cells were also treated with EGF to determine if mitogenic kinase activity could increase Lin28 expression in nontransformed cells. As shown in b, EGF stimulation increased c-Src activity and Lin28 expression by about 4-fold. In addition, we analyzed Akt activity to verify EGF stimulation (Jordon et al. Citation2004). These data support the hypothesis that Src kinase activity can increase Lin28 expression in transformed and nontransformed cells.
Src is a clinically relevant tyrosine kinase that has been implicated in many types of cancer (Yeatman Citation2004). It should also be noted that Src can affect cell communication and migration by directly modifying junctional proteins including integrins and connexins (Pahujaa et al. Citation2007; Yeatman Citation2004). For example, integrin signaling can activate Src, which not only can augment Cx43 transcription and protein production (Goldberg and Lau Citation1993), but also phosphorylate Cx43 to block gap junctional communication (Lampe and Lau Citation2004; Pahujaa et al. Citation2007). Thus, Src tyrosine kinase activity may provide a molecular link between miRNA processing and the control of nonanchored cell growth, migration, intercellular communication, embryogenesis, and cancer. The role of such mitogenic kinase activity on miRNA processing and cell junction proteins should be carefully considered when evaluating many biological processes, including the utility of stem cell production from nonembryonic sources.
METHODS
Mouse embryonic fibroblasts were transfected with v-Src or the parental vector (pBabePuro) and maintained as previously described (Shen et al. Citation2007). Cells were lysed in buffer containing 62.5 mM Tris-HCl (pH 6.8), 50 mM dithiothreitol (DTT), 2% sodium dodecyl sulfate (SDS), and 10% glycerol as previously described (Shen et al. Citation2007). Proteins were resolved by gel electrophoresis, transferred to Immobilion-P membranes (Millipore IPVH00010), and immunoblotted with antiserum specific for Lin28 (Moss and Tang Citation2003), Src phosphorylated at Y416 (Cell Signaling Technology, 2101), Akt phosphorylated at S473 (Cell Signaling Technology, 4060), or β-actin (Sigma, A1978). Signal was detected with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies and enhanced chemiluminescence (ECL) reagents (Millipore), and quantitated with ImageJ software (NIH, version 1.38x).
References
- Goldberg GS, Lau AF. Dynamics of connexin43 phosphorylation in pp60v-src- transformed cells. Biochem J. 1993; 295: 735–742
- Guo Y., Chen Y., Ito H., Watanabe A., Ge X., Kodama T., Aburatani H. Identification and characterization of lin-28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene 2006; 384: 51–61
- Jordon N.J., Gee J.M.W., Barrow D., Wakeling A.E., Nicholson R.I. Increased constitutive activity of PKB/Akt in tamoxifen resistant breast cancer MCF-7 cells. Breast Cancer Res Treat. 2004; 87: 167–80
- Lampe P.D., Lau A.F. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004; 36: 1171–1186
- Moss E.G., Tang L. Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev Biol. 2003; 258: 432–442
- Osherov N., Levitzki A. Epidermal-growth-factor-dependent activation of the Src-family kinases. Eur J Biochem. 1994; 225: 1047–53
- Pahujaa M., Anikin M., Goldberg G.S. Phosphorylation of connexin43 induced by Src: Regulation of gap junctional communication between transformed cells. Exp Cell Res. 2007; 313: 4083–4090
- Shen Y., Khusial P.R., Li X., Ichikawa H., Moreno A.P., Goldberg G.S. Src utilizes Cas to block gap junctional communication mediated by connexin43. J Biol Chem. 2007; 282: 18914–18921
- Viswanathan S.R., Daley G.Q., Gregory R.I. Selective blockade of microRNA processing by Lin28. Science 2008; 320: 97–100
- Yeatman T.J. A renaissance for SRC. Nat Rev Cancer. 2004; 4: 470–80
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., Slukvin I.I., Thomson J.A. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318: 1917–1920