Abstract
Although substrate stiffness has been previously reported to affect various cellular aspects, such as morphology, migration, viability, growth, and cytoskeletal structure, its influence on cell adherence has not been well examined. Here, we prepared three soft, medium, and hard polyacrylamide (PAAM) substrates and utilized AFM to study substrate elasticity and also the adhesion and mechanical properties of endothelial cells in response to changing substrate stiffness. Maximum detachment force and cell stiffness were increased with increasing substrate stiffness. Maximum detachment force values were 0.28 ± 0.14, 0.94 ± 0.27, and 1.99 ± 0.59 nN while Young’s moduli of cells were 218.85 ± 38.73, 385.58 ± 131.67, and 933.20 ± 428.92 Pa for soft, medium, and hard substrates, respectively. Human umbilical vein endothelial cells (HUVECs) showed round to more spread shapes on soft to hard substrates, with the most organized and elongated actin structure on the hard hydrogel. Our results confirm the importance of substrate stiffness in regulating cell mechanics and adhesion for a successful cell therapy.
Introduction
Tissue engineering aims at providing biological constructs to restore physiologic functions of diseased or injured tissues and organs. While numerous studies have confirmed the importance of surface chemistry in cell-substrate interaction for successful performance of implants or tissue engineered constructs, the impact of mechanical parameters of substrate, such as stiffness on cellular functions has only been studied in the past 10 years (Wang et al. Citation2000; Yeung et al. Citation2005; Saha et al. Citation2008; Byfield et al. Citation2009; Stroka & Aranda-Espinoza Citation2011a; Yeh et al. Citation2012; Huang et al. Citation2013; Ataollahi et al. Citation2015).
Extracellular matrix provides chemical and mechanical signals to influence cellular behaviors. On the other hand, diseased tissues exhibit altered mechanical properties which can disrupt the normal cellular function, such as ECM synthesis and cause further progression of the disease (Mason et al. Citation2012). Cells sense mechanical cues from their surrounding environment and use various mechano-transducers, such as integrins, stretch-activated ion channels, primary cilia, and cytoskeleton to convert such signals to chemical stimuli which regulate their function (Wang Citation2006).
Therefore, to achieve success in cell therapy strategies, it is necessary to take the native mechanical conditions of cells into account so as to attain the desired cellular responses and functional tissue engineered constructs. Unlike chemical cues, the effects of mechanical stimuli produced by extracellular environment on different cellular functions have not yet been fully studied. In vitro studies have reported that cells alter their cytoskeletal organization, mechanical properties, cell-substrate adherence properties and other features to maintain, and regulate their functionality in response to changing substrate stiffness (Mason et al. Citation2012).
Cells apply tension on their surroundings including ECM and adjacent cells through their cytoskeleton. On the other hand, the cytoskeleton remodels cell body in response to mechanical signals from the environment. Such interaction defines the state of homeostasis within the cell and tissue scales. The mutual effects of cell-environment interaction are key factors in morphogenesis, growth, remodeling, and healing of injured tissues. ECM synthesis and maintenance are set through functional cells, and in return cellular functions are set by the microenvironment of cells including ECM and other cells (Geiger et al. Citation2001; Berrier & Yamada Citation2007). In vitro studies are limited in simulation of such synergy. In the majority of studies, cells are cultured on predefined substrates and scaffolds with limited ECM synthesis during initial days of culture. However, even for such conditions, study of the cell-substrate interaction can broaden the scope for optimization of tissue engineering approaches. Among different physical and chemical characteristics of substrates, study of the effects of mechanical properties of substrates on cell behavior has attracted a great deal of scientific attention.
Some studies have examined cellular responses to the stiffness of different substrates, such as polyacrylamide (PAAM) (Lo et al. Citation2000; Wang et al. Citation2000; Engler et al. Citation2004a; Stroka & Aranda-Espinoza Citation2011a; Galie et al. Citation2015), Polydimethylsiloxane (PDMS) (Sarkar et al. Citation2005; Ataollahi et al. Citation2015) or alginate (Huang et al. Citation2013), as such materials are biocompatible and can be fabricated using suitable methods to achieve desired stiffness values.
Depending on cell structure and cytoskeleton, different cell phenotypes respond to substrate stiffness in different ways. For instance, fibroblasts and smooth muscle cells were rounded on soft surfaces and exhibited more extensions on hard substrates (Engler et al. Citation2004a), while motor neurons derived from spinal cord of embryonic mouse showed extended neurites on soft substrate and not on the hard ones (LA et al. Citation2002). In another study on neutrophils, fibroblasts and endothelial cells using PAAM gels with controlled elasticity, Yeung et al. showed that substrate stiffness resulted in different morphological responses among the three cell types and that cellular response to stiffness was dependent on the adhesion ligand attached to the substrate. They also reported that altering substrate stiffness can upregulate the adhesion receptors (Yeung et al. Citation2005). In a recent study, the required shear stress level for affecting the inflammatory response and morphology of endothelial cells along with their response to TNF-α treatment have been reported to depend on substrate stiffness (Galie et al. Citation2015).
In summary, substrate elasticity has been generally reported to affect different cellular behaviors, such as cell morphology and migration (Lo et al. Citation2000), viability and growth (Wang et al. Citation2000) as well as cytoskeletal structure (Pelham & Wang Citation1998). But the effect of surface stiffness on cell adherence has not been well examined.
Cell adherence to neighboring cells or ECM is among major characteristics of cell behavior. Within the vasculature, adhesion of endothelial cells to intima membrane is of particular importance since in clinical conditions, such as atherogenesis and cancer, the passage of macromolecules, and metastatic cells through endothelial junctions is a major determinant.
In order to examine cell adherence to another cell or a substrate, several single-cell force spectroscopy (SCFS) methods, such as those based on micropipette or atomic force microscopy (AFM) have been introduced. Since AFM-based assays make it possible to measure a wide range of forces applicable in cell studies (5 pN–100 nN), they are considered as useful tools for studying cell–cell or cell-surface interactions (Helenius et al. Citation2008). AFM-based SCFS are also associated with a high precision, which allows spatial and temporal manipulation of cells during the experiment (Friedrichs et al. Citation2010).
Vascular endothelial cells are important elements for maintaining the integrity of vessels and transferring environmental signals. Disruption in the normal functions of these cells is associated with some vascular diseases (Wood et al. Citation2010).
The underlying basement membrane of endothelial cells provides them with different signaling cues through its chemical components and biophysical properties. Such biophysical signals provided by this membrane (due to its topography and stiffness) modulate different cellular functions, such as adhesion, proliferation, migration, and differentiation (Wood et al. Citation2010). Hence, understanding the impact of surface elasticity on endothelial cell function will result in optimization of scaffolds for vascular tissue engineering. While the effects of different substrate properties on endothelial behavior have been examined, the effect of substrate stiffness on adhesive behavior of ECs has not been well determined yet.
Therefore, in this study, we prepared three soft, medium, and hard PAAM substrates with unique surface properties and utilized AFM to study not only the stiffness of substrates but also the adherence behavior and mechanical properties of endothelial cells in response to such alterations in substrate stiffness.
Methods and materials
Three PAAM hydrogels with different stiffness values, namely soft, medium, and hard substrates, were synthesized and analyzed for biocompatibility. Human umbilical vein endothelial cells (HUVECs) were cultured on the three hydrogels for 24 or 48 h and their morphology, elastic modulus, and adhesion force were examined. AFM was used to measure substrate stiffness as well as the mechanical and adhesive properties of cells. The resultant values were compared to quantify effects of substrate elastic modulus on endothelial behavior. All data were expressed as mean ± standard deviation. One-way ANOVA was used to compare the results of three groups, assuming the significance level of p = 0.05.
Materials
For cell culture and evaluation assays, low glucose Dulbecco’s Modified Eagle’s Medium (LG-DMEM), phosphate buffer saline (PBS), trypsin/EDTA, 3-[4, 5-dimethyltriazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT), penicillin, streptomycin, and collagen type I (all from Sigma, St. Louis, MO) as well as fetal bovine serum (FBS) (Gibco, Carlsbad, CA) were used.
For hydrogel synthesis, ammonium per sulfate (APS) (FisherBiotech, Fair Lawn, NJ) and tetramethylethylenediamine (TEMED) (Invitrogen, Carlsbad, CA) were used as the initiator and catalyst, respectively. Acrylamide and bis-acrylamide were both obtained from FisherBiotech (Fair Lawn, NJ).
Biotin-bovine serum albumin (BSA), streptavidin, biotin-conjugated concanavalin A (all from Sigma, Freiburg im Breisgau, Germany), Tipless Arrow-TL1 (Nanoworld, Neuchatel, Switzerland), and HYDRA6R-200NG (AppNano, Santa Clara, CA) were used for AFM experiments.
Preparation of PAAM hydrogels
A total of 40 wv% Acrylamide and 2 wv% bis-acrylamide stock solutions were separately prepared by adding 40 g of Acrylamide and 2 g of bis-acrylamide to PBS, respectively. The two mixtures were continuously stirred until the complete dissolution of each powder in the buffer and then passed through a 0.2-micron filter. Thereafter, three different mixtures of Acrylamide and bis-acrylamide solutions, namely soft, medium, and hard gels, were prepared according to a previously published protocol for different compositions of acrylamide and bis-acrylamide mixtures (Yeung et al. Citation2005). These values are shown in .
Table 1. Compositions of soft, medium and hard PAAM hydrogels.
After sufficient stirring, an amount of 10 wv% APS, equal to 0.01 of the total volume, was added to each solution and mixed until a homogeneous and clear solution was obtained.
Then the catalyst, TEMED (99%), as 0.001 of the total volume was added. After the solution was sufficiently mixed, it was transferred to an empty die (a 12-well plate). Gel surface was covered by a layer of ethanol (96%) in order to prevent oxidation of surface layer of gel. The gel was solidified after 30 min. After three times washing with PBS, each gel was placed in a culture medium and was incubated at 37 °C and 5% CO2 for 72 h, to reach equilibrium. Then, it was further washed by PBS. Gel surface was covered with 133 μl of type I collagen and left to dry for 4 h. Following gentle washing with PBS, the prepared gel was kept at 4 °C for 24 h and then sterilized by UV radiation for 30 min (Yeung et al. Citation2005).
Evaluation and calculation of substrate elasticity
Atomic force microscopy was used to evaluate substrate elasticity. A fresh CSC17/noAl conical cantilever (Mikromasch, Watsonville, CA) was used to measure the Young’s modulus of synthesized substrates utilizing Nanowizard2 AFM head (JPK Instruments AG, Berlin, Germany). The cantilever indented the substrate for 1.0 μm with applying maximum force of 0.5 nN. In each experiment the tip – surface interaction force was plotted versus indentation depth. In order to convert the vertical deflection of cantilever into the force, the spring constant and sensitivity of the cantilever were multiplied (Hutter & Bechhoefer Citation1993; Butt et al. Citation2005). Using published protocols ( Hutter & Bechhoefer Citation1993; Butt et al. Citation2005), the thermal noise method was employed by first indenting a rigid surface to ensure that only the elasticity of the cantilever is sensed, then after a thermal fluctuation the spring constant was calculated as 0.03 ± 0.015 N/m. The values of maximum force and indentation were set to 1.0 nN and 0.5 μm, respectively. Almost 250 force-distance curves for different points of each substrate were analyzed.
The young’s modulus of each substrate (Es) was calculated using the modified Hertz model for the conical-shaped tip of cantilevers (Lin et al. Citation2006):
(1)
in which F indicates force, δ shows the depth of indentation and ϕ is the half-angle of conical tip (set to 17.5°). Parameter νs indicates the Poisson’s ratio and was set to 0.5 due to the assumption of incompressibility for hydrogels (Sunyer et al. Citation2012).
A previously published algorithm (Guo & Akhremitchev Citation2006; Nikkhah et al. Citation2011) was used to obtain the young’s modulus of substrates based on δ parameter in Hertz’s model. The values of AFM head movement (z) were subtracted from deflection (d) of the cantilever to obtain indentation depth (δ). Writing Eqn. (1) as a linear expression for δ results in:
(2)
Here, d0 and z0 indicate cantilever deflection and piezo position at the time contact between cell and tip contact, respectively. In Eqn. (2), the first bracket, being the slope of (F1/2 versus z − d) curve, gives the young’s modulus of substrate (Es).
Cytotoxicity analysis
To evaluate the cytotoxicity of hydrogels, HUVECs provided from National Cell Bank of Iran (Pasteur Institute of Iran, Tehran, Iran) were cultured in the presence of synthesized polymer extract. The MTT assay was used to analyze cell proliferation based on a previously published protocol (Qu et al. Citation2014). Briefly, HUVECs were seeded into a 96-well plate (5 × 103 cells per well) and then incubated with culture medium at 37 °C and 5% CO2 for 24 h. Then polymer extract was added to each well, except for control samples, and cells were incubated for another 24 h at 37 °C and 5% CO2. After removal of the liquid phase, each well was added with 100 μl of 5 mg/ml MTT solution. The plate was then incubated at 37 °C for 4 h. Then the liquid phase in each well was replaced by 100 μl of isopropanol. After shaking for 20 min, cell growth was analyzed by measuring the optical absorbance of each well at 495 nm by means of an Elisa reader (Awareness, Palm City, FL). Cell viability was calculated by dividing the absorbance value of each experimental well by the absorbance value of the control well.
Cell culture
HUVECs were cultured in LG-DMEM supplemented with 10% FBS, 100 IU/ml penicillin, and 100-μg/ml streptomycin. The medium was changed every 4 days and cells with 90% confluency were passaged using trypsin/EDTA. Passage 4 HUVECs were cultured on prepared hydrogels at a cell density of 5000 cells/cm2.
Staining of actin fibers
To observe changes in cell cytoskeleton, actin staining was performed for cells cultured on soft and hard substrates. This was carried out since alterations in cell adherence in terms of surface proteins in response to substrate elasticity might correlate with changes in cell cytoskeleton and consequently cell mechanical properties. After removal of culture medium and three times washing with PBS, cells were fixed by 3.7% formaldehyde in PBS for 5 min. Followed by removing the liquid phase and further washing with PBS for several times, cells were exposed to 0.1% Triton X-100 in PBS for 10 min. After removal of the liquid, cells were washed three times with PBS and then incubated with 4 μg/ml phalloidin in PBS for 45 min at room temperature in darkness, after which, cells were further washed and observed using florescence microscope.
Single cell force spectroscopy (SCFS)
Based on a previously published protocol (Friedrichs et al. Citation2010), Tipless AFM cantilevers (Arrow-TL1) with spring constant of 3.072 ± 0.015 N/m were coated by concanavalin A. To clean the cantilevers, UV-radiation for 45 min was used followed by incubation in 0.5 mg/ml solution of biotin-conjugated BSA in NaHCO3 for an overnight. After three times washing with PBS for removing unbounded biotin molecules, cantilevers were placed in 0.5 mg/ml solution of streptavidin in PBS for 30 min. Then, they were washed three times with PBS and incubated in 0.4 mg/ml solution of biotin-labeled concanavalin A in PBS for 30 min and washed three times more with PBS.
A CellHesion 200 device (JPK Instruments AG, Berlin, Germany) was used to perform SCFS examinations. Cells, which were cultured on soft, medium, or hard substrates for 24 h, were trypsinized and resuspended in culture medium. In order to prepare probe cells, cell suspension was incubated with 0.5% trypsin-EDTA (Gibco, Carlsbad, CA) for 2 min at 37 °C according to a published protocol (Friedrichs et al. Citation2010) and then centrifuged at 1400 rpm for 5 min to form a cell pellet. After removal of the supernatant, cell pellet was resuspended in fresh culture medium, and 2 × 104 cells were transferred to a Petri dish. After several minutes for reaching equilibrium, cells were ready to be used as probe cells for SCFS experiments.
The petri dish was placed on the stage with temperature maintained at 37 °C and the functionalized cantilever was mounted on the device head. After adjusting the position of the laser spot on the cantilever apex within the culture medium, and leaving the cantilever for several minutes to reach a thermal equilibrium, the cantilever was moved down toward the spotted cell and captured it using back and forth cycles. The retract speed was set to less than 5 μm/s (Friedrichs et al. Citation2010).
After cell attachment followed by a 10-min recovery, the cell attached to the AFM cantilever was placed above the substrate and moved down to contact the (polystyrene) substrate until a preset contact force was reached. After keeping stationary for a predefined duration of contact time (10 s), the cell-attached cantilever was retracted from the substrate with a constant speed, leading to the breakage of cell-substrate bonds and subsequently the complete separation of cell, and substrate. Meanwhile the force, being proportional to the vertical deflection of the cantilever was plotted versus the head displacement. Experimental SCFS parameters are illustrated in .
Table 2. Parameters used for SCFS experiments.
Each SCFS test resulted in a force-displacement curve, containing approach, contact, and retract regions. A typical force-displacement curve is illustrated in . Each jump in the retract curve indicates the separation of adhesive bonds or cell membrane tethers. With the complete separation of the cell, the force returns to its initial value.
Figure 1. A typical force-displacement curve for a SCFS test: approach curve (black) and retract curve (grey). In the approach region, initially there is no cell-surface contact (Approach 1), then the cell meets the bottom of petri dish and squeeze between the cantilever, and dish surface (Approach 2). In the retract part, Retraction 1 contains several information about cell-substrate adhesion, including maximum detachment force, detachment work, and unbinding force of molecular bonds. Finally, there is no more cell-surface contact (Retraction 2).
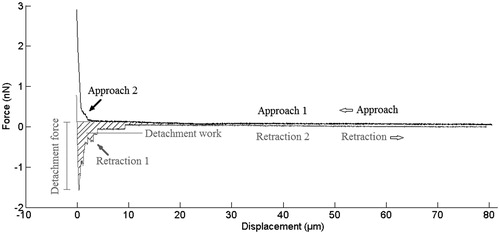
In the approach region (black curve in ), initially there is no cell-surface contact (Approach part 1) until the cell meets the bottom of petri dish and squeeze between the cantilever and dish surface (Approach part 2), where the elastic response of this cell is observable. This is similar to the force-indentation test performed to measure substrate elasticity, except that here cell acts as the cantilever tip. Therefore, the hertz equation must be rewritten for such spherical cantilever tip. Since the control mode of system was set to constant height during contact section, the retract curve is being started at lower values of vertical deflection (force). It is observable as a vertical difference between black and grey curves at zero displacement in . No more features can be seen from contact section in F-D curves.
The retract curve (grey curve in ) (Retraction part 1) contains several information about cell-substrate adhesion, including maximum detachment force which is related to the number and amplitude of the unbinding events (Weder et al. Citation2009), detachment work which is the required work for detaching cell from the surface and is related to the number, amplitude, and the position of unbinding events during cantilever retraction (Weder et al. Citation2009), as well as unbinding force of molecular bonds. Finally, there is no more cell-surface contact (Retraction 2).
Calculation of cell elastic modulus
Cell elastic modulus was determined from approach curve of SCFS tests via fitting with Hertz equation. Same calculation used for substrate stiffness was used to determine the cell elastic modulus, except that for the Hertz model corresponding to a spherical tip (the probe cell), the following formula was used (Lin et al. Citation2006):
Where F, δ, and R are force, depth of indentation (displacement in ), and the radius of probe cells that was determined through optical images of cantilever adhered cells during SCFS experiment described in 2.7. Parameter νc which indicates the Poisson’s ratio of the cell was set to 0.5.
Calculation of cell adhesion
To determine potential of cells to adhere to the substrate, the work and maximum force of detachment as well as the amplitude of unbinding event (unbinding force), and the resulting force-distance curves were analyzed. Sharp steps in the retract curves, which have been considered as a result of breakage of molecular bonds, can be used to obtain the strength of bonds between molecules and cellular membrane (Friedrichs et al. Citation2010). The amplitude of such unbinding steps is indicative of the required forces for breaking one or a few molecular bonds. The unbinding events were located and quantified by JPK DP version 4.2.61 software (Berlin, Germany) on the resultant curves. Then the magnitude of each unbinding step was obtained in force unit. These data were used to generate the histogram of unbinding events. Using the Expectation-Maximization algorithm the Gaussian distributions from the population of amplitudes were generated and the average values of such plots were reported (Lekka et al. Citation2006).
Statistical analysis
All tests were carried out in triplicate and the results were averaged. Data were presented as mean ± SD. One-way ANOVA was used to compare the results between experimental groups, by assuming a significance level of 0.05.
RESULTS
Substrate elasticity
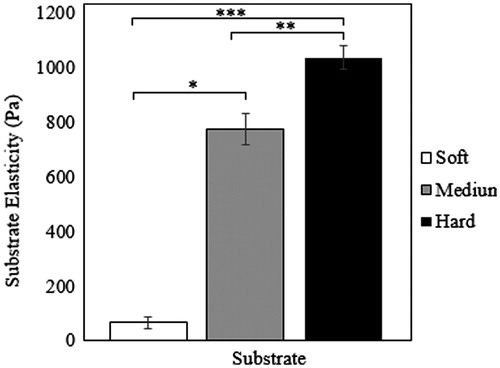
Since changing the acrylamide-bis-acrylamide ratio alters substrate elasticity (Yeung et al. Citation2005), in this study, hydrogels with 8 wv% acrylamide and three different bis-acrylamide contents were prepared (see ). The average values for Young’s modulus corresponding to the soft, medium, and hard substrates based on AFM tests were calculated to be 65.01 ± 21.14, 770.83 ± 67.32, and 1032.61 ± 82.78 Pa, respectively (see ).
Cytotoxicity analysis
The cytotoxicity of prepared hydrogel substrates was studies using MTT assay, which is a quantitative measure for cell metabolism. All samples exhibited high viabilities of about 100%. These results indicated that the prepared hydrogels with different stiffness values were not toxic.
Cell morphology and structure
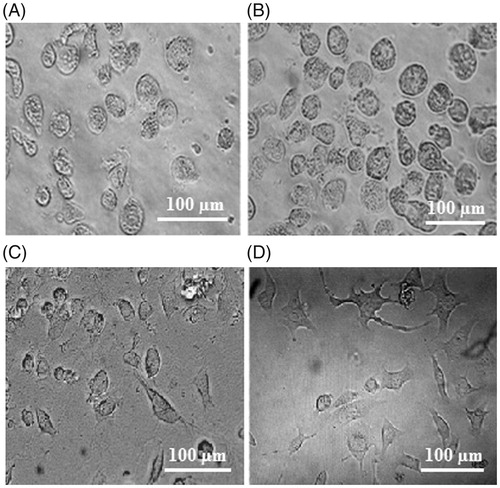
Results were indicative of morphological changes in HUVECs is response to changing substrate stiffness. A typical image is illustrated in ) for cells cultured on three substrates after 24 h of culture. A dependency of cell shape on substrate elasticity can be observed, as HUVECs showed round to more spread resting shapes on soft to stiff substrates. More cell spreading on the substrates were observed over time. For instance, indicate the morphology of cells 24 and 48 h after culture on the hard substrate.
Figure 3. Morphology of cells cultured on (A) soft, (B) medium and (C) hard polyacrylamide substrates after 24 h, and (D) hard substrate after 48 h. Experiments were performed in triplicate.
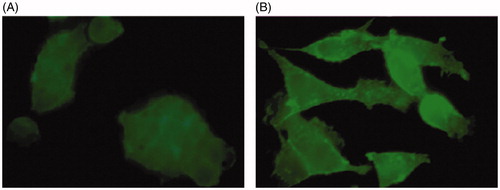
Actin staining was performed to study cell organization for samples cultured on soft and hard substrates after 24 h. A typical image is shown in . Cells cultured on the hard substrate exhibited spread shapes with dense actin structure especially within focal adhesion sites, while cells are round on the soft substrate.
Endothelial cell adhesion and stiffness
A total number of 120 SCFS experiments were performed using 11 cells (3, 4, and 4 cells were used for the soft, medium, and hard substrates, respectively). For each cell, at least 10 SCFS experiments were carried out. The values obtained for cell elastic modulus and detachment force corresponding to the soft, medium, and hard substrates are illustrated in . According to the results, both cell stiffness and detachment force were significantly increased (all p values <0.05) by increasing substrate stiffness (from 65.01 for soft substrate to 770.83 Pa for medium substrate and 1032.61 Pa for hard substrate). Maximum detachment force values were 0.28 ± 0.14, 0.94 ± 0.27, 1.99 ± 0.59 nN (p values <0.05) while Young’s moduli of cells were 218.85 ± 38.73, 385.58 ± 131.67, and 933.20 ± 428.92 Pa (p values <0.05) for soft, medium, and hard substrates, respectively. These values along with those obtained for unbinding force and work of detachment are listed in .
Figure 5. Cell elasticity and detachment force values corresponding to the soft, medium, and hard polyacrylamide substrates. *, **, and *** indicate significant difference (p < 0.05).
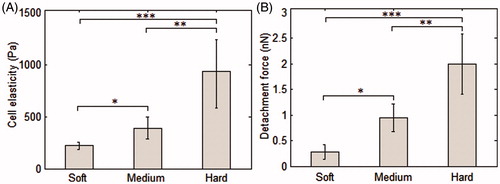
Table 3. Force spectroscopy results for soft, medium, and hard substrates based on 120 SCFS experiments using 11 cells.
Discussion
Endothelial cells which cover the inner walls of blood vessels play major roles in controlling homeostasis and vascular permeability. On the other hand, dysfunction of such cells may result in vascular disorders or facilitate tumor angiogenesis (Yeh et al. Citation2012). The biophysical environment of endothelial cells has been considered as an important factor in the normal homeostasis of these cells. Vascular endothelial cells are supported by their underlying basement membrane which plays major roles in their normal function. Alterations in different characteristics of such extracellular environment including its stiffness have been observed in the case of some vascular diseases (Baluk et al. Citation2003; Liliensiek et al. Citation2009; Stroka & Aranda-Espinoza Citation2011a; Yeh et al. Citation2012). Such changes in the stiffness of the underlying substrate of endothelial cells can affect their function.
Mammalian cells are commonly attached to their surrounding cells or extracellular matrix, which are associated with young’s modulus in the range of 10 Pa–10 kPa (Yeung et al. Citation2005; Saha et al. Citation2008). Therefore to assess cellular responses, previous studies have used different elastic moduli of substrates (for instance, 42 Pa–280 kPa (Stroka & Aranda-Espinoza Citation2011a) and 10 Pa–10 kPa (Saha et al. Citation2008)). The choice of substrate stiffness depends on the substrate material, too. For instance, hydrogels, such as PAAM gels, or those based on polyethylene glycol, hyaluron, gelatin, or other natural polymers have been previously utilized to prepare substrates with stiffness levels between 0.1 and 100 kPa, covering the stiffness values of many soft tissues. However, some tissues are stiffer and associated with dense ECM structures, such as some basement membranes (with elastic moduli around 1 MPa), arterial walls (with elastic moduli around 800 kPa), or cardiac muscle (left ventricle with stiffness in the range of 30–400 kPa, during peak systole). Rubber-like elastomers, such as PDMS, or polyesters are better able to provide such stiffness values (Palchesko et al. Citation2012). Here, we prepared PAAM hydrogel substrates with elastic moduli of around 65, 770, and 1032 Pa.
Several cell features, such as viability and growth (Wang et al. Citation2000), migration, and morphology (Lo et al. Citation2000), as well as cytoskeleton organization (Pelham & Wang Citation1998) have been previously reported to be altered in response to changing substrate stiffness. In this work, the effect of substrate stiffness on cell adhesion as well as morphology and cell stiffness was examined.
We used HUVECs, human umbilical vein/vascular endothelium cells (instead of primary endothelial cells), as they have been commonly used in relative studies and have been well characterized. Such endothelial cells exhibited rounded to more spread morphologies on soft to hard PAAM substrates, respectively. This is in agreement with previously published data on the morphological dependence of endothelial cells on substrate stiffness, where cells showed rounded to more spread resting shapes, as substrate stiffness was increased (Yeung et al. Citation2005; Ataollahi et al. Citation2015).
Actin cytoskeletal organization and alignment is important in determining cell shape (Mason et al. Citation2012). Increasing substrate stiffness has been generally reported to enhance cytoskeletal organization and result in the formation of more stable focal adhesions in association with cell shape alterations (Mason et al. Citation2012). In accordance to this, we observed rounded cell morphologies with diffuse actin fibers on the soft substrate while cells cultured on the hard substrate exhibited more spread resting shapes with organized actin fibers. This is in agreement with published data on the increase in spread area of endothelial cells in response to increasing substrate stiffness (Reinhart-King et al. Citation2005; Califano & Reinhart-King Citation2008).
It should be noted that while such behavior has been reported for cells, such as endothelial cells (Yeung et al. Citation2005), fibroblasts (Lo et al. Citation2000; Yeung et al. Citation2005), and smooth muscle cells (Engler et al. Citation2004a), no dependence of cell shape on substrate elasticity has been observed in the case of neutrophils (Yeung et al. Citation2005), implying that not all cell types utilize substrate stiffness as a signal for morphology and such spreading on harder substrates cannot be extended to all phenotypes (Yeung et al. Citation2005). Since both morphology and actin structure interplayed in response to substrate stiffness, it might imply that substrate stiffness triggers cellular response through cell cytoskeleton, which determines mechanical properties of cells. Hence, the behavior of cells with defined actin structure depends on substrate stiffness, while other cell types might not be influenced as so.
In this study, adherence behavior of HUVECs to the prepared hydrogels was examined. Cell adherence to other cells and to its substrate affects cell shape and cellular organization in the tissue (Thompson et al. Citation2010). Adhesion of a cell to its ECM starts with the recognition of particular domains in adhesive proteins of the matrix, such as laminin, fibrinogen, fibronectin, vitronectin, and some collagens, by structurally related-plasma membrane receptors, called integrins. After this initial cell-substrate attachment, cytoskeletal elements reorganize and cause cell spreading and flattening and subsequently, focal adhesion formation (Bidanset et al. Citation1992).
Study of the adherence of endothelial cells might be important in clinical situations since one of the major functions of endothelial cells is the selective passage of blood components through their junctions which is partly controlled by endothelial-membrane adhesion. While passage of nutrients and vital blood components with small size molecules is in demand, the passage of unwanted macromolecules, such as lipoproteins or metastatic cancer cells through endothelial junctions account for dysfunctioning.
Alterations in substrate stiffness caused changes in both adhesion and mechanical properties of cells. Changes in cell cytoskeleton results in altered cell elastic properties. The adhesive proteins in cell membranes are connected to cytoskeletal fibers through a network of protein complex. Alterations in cell adhesion in terms of either number of proteins or their binding forces may result in changes in the structure of internal proteins. The adhesive proteins in the cell membrane are directly prone to external mechanical stimuli, such as substrate elasticity. Hence by activation of mechanotransductive cascades both adhesive proteins and cytoskeleton are prone to remodeling. It has been well observed that by application of mechanical forces, cells respond by alterations in cytoskeletal organization (Geiger et al. Citation2001). It is of great interest to find that change in cell adherence due to mechanical stimuli is correlated to mechanical properties most probably through change in the cytoskeletal actin fibers. For instance, actin reorganization has been shown to be influential on the mechanical behavior of cancerous cells (Suresh Citation2007; Pachenari et al. Citation2014; Lekka Citation2016).
Cells respond to their mechanical environment including substrate stiffness by altering their physical features, including cell adhesion and elastic modulus. Mechanical stimuli can be passed on from integrins as the surface adhesive proteins into the cell body through physical or chemical responses by the cytoskeleton (Wang Citation2006). Vinculin, talin, and alpha-actinin are among major proteins that interconnect integrins to F-actin structure of cell cytoskeleton (Davies & Tripathi Citation1993). Actomyosin contractility, which is directly related to the actin structure of cytoskeleton, affects cell organizations through force generation at cell adhesion sites (Liu et al. Citation2010).
It has been reported that increase in the force on adhesion site, either by cell body (cell contractility) or external loading reinforces the adhesion (Schwartz & DeSimone Citation2008). Moreover, it has been reported that the size of focal adhesions is proportional to the force applied on them by the cell (Bershadsky et al. Citation2006). Hence by altering substrate stiffness, both cell adhesion and structure are expected to change.
Cells sense the stiffness of their substrates through adhesion sites and respond by altering their cytoskeletal structure which in turn affects their adhesion to the substrate. Stiffer substrates cause enhanced cytoskeleton through enrichment of actin structure and generation of new stress fibers (Geiger et al. Citation2001), as an early study suggested that stress fibers represent an increased need for adhesion to the substrate (Sumpio et al. Citation1988). Here, we observed cell stiffening and also enhanced adhesiveness of HUVECs when interacted with stiffer substrates, consistent with a recent study which reported the increased adhesion of endothelial cells with increasing the stiffness of substrates (Ataollahi et al. Citation2015).
According to our results, maximum force of detachment was almost 3.36 and 7.12 times higher for medium and hard substrates compared to the soft substrate, respectively. The maximum force of detachment has been considered as a dependent parameter, which is related to the number and amplitude of unbinding events (Weder et al. Citation2009) due to molecular bonds breakage (Friedrichs et al. Citation2010). Detachment work also depends on the number, amplitude, and position of unbinding events (Weder et al. Citation2009). Here, the lowest value of this parameter was corresponding to the soft substrate which indicates the least cell adherence on the soft gel. The resulting average values for medium and hard gels were close to each other.
It has been reported that the nature of molecules bound to the substrate affects cellular response to surface stiffness. For instance, fibroblasts have been reported to exhibit more spreading on substrates coated with fibronectin compared to those with collagen coating (Yeung et al. Citation2005). Here, each substrate was coated with a collagen layer which was thin enough to allow cells sense the mechanical properties of substrate while providing an identical surface chemistry for all surfaces.
Findings of previous studies indicate a relationship between cellular traction forces, actin cytoskeleton, and focal adhesions which contribute to cell adherence. Low-stiffness substrates have been shown to result in irregular and dynamic focal adhesions (Pelham & Wang Citation1998) while stiffer substrates lead to the formation of more stable and elongated focal adhesions which can consequently alter cell adherence to the substrate (Engler et al. Citation2004b).
Mechanical properties of endothelial cells depend on several factors including shear stress, substrate ligands, and stiffness, as well as soluble molecules (Stroka & Aranda-Espinoza Citation2011b). Here, we focused on the effect of substrate stiffness, and similar to a previous study (Stroka & Aranda-Espinoza Citation2011b) utilized AFM to evaluate the stiffness of HUVECs. We observed the dependence of HUVECs stiffness on the elasticity of their substrate. Analyzing the positions of sharp steps in the retract phase revealed that stiffness of these cells was increased with increasing surface stiffness. This is in consistence with a previously published work on the dependency of endothelial cell stiffness on the stiffness of 2D and 3D matrixes (Byfield et al. Citation2009).
Conclusion
Our results imply the influence of substrate stiffness on adhesive behavior and elasticity of vascular endothelial cells. Achieving a successful tissue engineering strategy requires utilization of substrates with proper chemical and mechanical properties which facilitate cell adhesion and function. The findings of this study imply the importance of substrate stiffness in regulating cell-substrate adhesion as well as cell stiffness and can be used to optimize the scaffolds of vascular tissue engineering.
Disclosure statement
The authors report no declarations of interest.
Funding
This study was performed in AmirKabir university of technology and National Cell Bank of Iran (NCBI). These supports are greatfully acknowledged.
References
- Ataollahi F, Pramanik S, Moradi A, Dalilottojari A, Pingguan-Murphy B, Wan Abas WA, Abu Osman NA. 2015. Endothelial cell responses in terms of adhesion, proliferation, and morphology to stiffness of polydimethylsiloxane elastomer substrates. J Biomed Mater Res A. 103:2203–2213.
- Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. 2003. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 163:1801–1815.
- Berrier AL, Yamada KM. 2007. Cell-matrix adhesion. J Cell Physiol. 213:565–573.
- Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, Kozlov MM. 2006. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur J Cell Biol. 85:165–173.
- Bidanset DJ, LeBaron R, Rosenberg L, Murphy-Ullrich JE, Hook M. 1992. Regulation of cell substrate adhesion: effects of small galactosaminoglycan-containing proteoglycans. J Cell Biol. 118:1523–1531.
- Butt H-J, Cappella B, Kappl M. 2005. Force measurements with the atomic force microscope: technique, interpretation and applications. Surf Sci Rep. 59:1–152.
- Byfield FJ, Reen RK, Shentu TP, Levitan I, Gooch KJ. 2009. Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. J Biomech. 42:1114–1119.
- Califano JP, Reinhart-King CA. 2008. A balance of substrate mechanics and matrix chemistry regulates endothelial cell network assembly. Cell Mol Bioeng. 1:122–132.
- Davies PF, Tripathi SC. 1993. Mechanical stress mechanisms and the cell. An endothelial paradigm. Circ Res. 72:239–245.
- Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. 2004a. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 86:617–628.
- Engler A, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE. 2004b. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 166:877–887.
- Friedrichs J, Helenius J, Muller DJ. 2010. Quantifying cellular adhesion to extracellular matrix components by single-cell force spectroscopy. Nat Protoc. 5:1353–1361.
- Galie PA, Oosten AV, Chen CS, Janmey PZ. 2015. Application of multiple levels of fluid shear stress to endothelial cells plated on polyacrylamide gels. Lab Chip. 15:1205–1212.
- Geiger B, Bershadsky A, Pankov R, Yamada KM. 2001. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2:793–805.
- Guo S, Akhremitchev BB. 2006. Packing density and structural heterogeneity of insulin amyloid fibrils measured by AFM nanoindentation. Biomacromolecules. 7:1630–1636.
- Helenius J, Heisenberg CP, Gaub HE, Muller DJ. 2008. Single-cell force spectroscopy. J Cell Sci. 121:1785–1791.
- Huang X, Hang R, Wang X, Lin N, Zhang X, Tang B. 2013. Matrix stiffness in three-dimensional systems effects on the behavior of C3A cells. Artif Organs. 37:166–174.
- Hutter JL, Bechhoefer J. 1993. Calibration of atomic-force microscope tips. Rev Sci Instrum. 64:1868–1873.
- Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. 2002. Neurite branching on deformable substrates. Neuroreport. 13:2411–2415.
- Lekka M, Laidler P, Labedź M, Kulik AJ, Lekki J, Zajac W, Stachura Z. 2006. Specific detection of glycans on a plasma membrane of living cells with atomic force microscopy. Chem Biol. 13:505–512.
- Lekka M. 2016. Discrimination between normal and cancerous cells using AFM. BioNanoScience. 6:65–80.
- Liliensiek SJ, Nealey P, Murphy CJ. 2009. Characterization of endothelial basement membrane nanotopography in rhesus macaque as a guide for vessel tissue engineering. Tissue Eng Part A. 15:2643–2651.
- Lin DC, Dimitriadis EK, Horkay F. 2006. Robust strategies for automated AFM force curve analysis-I, non-adhesive indentation of soft, inhomogeneous materials. J Biomech Eng. 129:430–440.
- Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. 2010. Mechanical tugging force regulates the size of cell–cell junctions. Proc Natl Acad Sci USA. 107:9944–9949.
- Lo C, Wang H, Dembo M, Wang Y. 2000. Cell movement is guided by the rigidity of the substrate. Biophys J.79:144–152.
- Mason BN, Califano JP, Reinhart-King CA. 2012. Matrix stiffness: a regulator of cellular behavior and tissue formation. In: Bhatia SK, editor. Engineering biomaterials for regenerative medicine. New York (NY): Springer; p. 19–37.
- Nikkhah M, Strobl JS, Schmelz EM, Agah M. 2011. Evaluation of the influence of growth medium composition on cell elasticity. J Biomech. 44:762–766.
- Pachenari M, Seyedpour SM, Janmaleki M, Babazadeh Shayan S, Taranejoo S, Hosseinkhani H. 2014. Mechanical properties of cancer cytoskeleton depend on actin filaments to microtubules content: investigating different grades of colon cancer cell lines. J Biomech. 47:373–379.
- Palchesko RN, Zhang L, Sun Y, Feinberg AW. 2012. Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve. PLoS One. 7:e51499.
- Pelham RJ, Wang YL. 1998. Cell locomotion and focal adhesions are regulated by the mechanical properties of the substrate. Biol Bull. 194:348–350.
- Qu Y, Wu Y, Gao Y, Qu S, Yang L, Hua J. 2014. Diketopyrrolopyrrole-based fluorescent conjugated polymer for application of sensing fluoride ion and bioimaging. Sensor Actuat B Chem. 197:13–19.
- Reinhart-King CA, Dembo M, Hammer DA. 2005. The dynamics and mechanics of endothelial cell spreading. Biophys J. 89:676–689.
- Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. 2008. Substrate modulus directs neural stem cell behavior. Biophys J. 95:4426–4438.
- Sarkar S, Dadhania M, Rourke P, Desai TA, Wong JY. 2005. Vascular tissue engineering: microtextured scaffold templates to control organization of vascular smooth muscle cells and extracellular matrix. Acta Biomater. 1:93–100.
- Schwartz MA, DeSimone DW. 2008. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 20:551–556.
- Stroka KM, Aranda-Espinoza H. 2011a. Effects of morphology vs. cell-cell interactions on endothelial cell stiffness. Cell Mol Bioeng. 4:9–27.
- Stroka KM, Aranda-Espinoza H. 2011b. Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction. Blood. 118:1632–1640.
- Sumpio BE, Banes AJ, Buckley M, Johnson G. 1988. Alterations in aortic endothelial cell morphology and cytoskeletal protein synthesis during cyclic tensional deformation. J Vasc Surg. 7:130–138.
- Sunyer R, Jin AJ, Nossal R, Sackett DL. 2012. Fabrication of hydrogels with steep stiffness gradients for studying cell mechanical response. PLoS One. 7:1–9.
- Suresh S. 2007. Biomechanics and biophysics of cancer cells. Acta Biomater. 3:413–438.
- Thompson O, Moore CJ, Hussain SA, Kleino I, Peckham M, Hohenester E, Ayscough KR, Saksela K, Winder SJ. 2010. Modulation of cell spreading and cell-substrate adhesion dynamics by dystroglycan. J Cell Sci. 123:118–127.
- Wang JH. 2006. An introductory review of cell mechanobiology. Biomech Model Mechanobiol. 5:1–16.
- Wang H, Dembo M, Wang Y. 2000. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 279:C1345–C1350.
- Weder G, Vörös J, Giazzon M, Matthey N, Heinzelmann H, Liley M. 2009. Measuring cell adhesion forces during the cell cycle by force spectroscopy. Biointerphases. 4:27–34.
- Wood J, Liliensiek SJ, Russell P, Nealey PF, Murphy CJ. 2010. Biophysical cueing and vascular endothelial cell behavior. Materials. 3:1620–1639.
- Yeh YT, Hur SS, Chang J, Wang KC, Chiu JJ, Li YS, Chien S. 2012. Matrix stiffness regulates endothelial cell proliferation through septin 9. PLoS One. 7:1–13.
- Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. 2005. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 60:24–34.