ABSTRACT
By constant surface tension molecular dynamics simulation method, we show that the structural transformation of smectic liquid crystals can be caused by surface tension. Comparison with systems under hydrostatic pressure is discussed.
1. Introduction
Macroscopically, the surface is treated as a rigid or free boundary surrounding a substance. In macroscopic scale, surface tension is described in relation to the spontaneous curvature of the free boundary. In mesoscopic scale, it is described by the anisotropy in the internal stress tensor
(1) where Pz and Pxy are the normal and transverse pressure profiles across the interfacial zone of width lz [Citation1]. When the surface tension γ is positive, at least a part of the interfacial zone Pxy is less than Pz. There can be regions of tension, Pxy < Pz, and of compression Pxy > Pz, in the interfacial zone. Interface stability requires only the integral of (Pz − Pxy) across the zone to be positive.
The effect of a rigid surface against the alignment of liquid crystals, with/without substrate, has been intensively studied because of it’s importance to industries. In addition, many interesting phenomena appearing in free standing films (FSF) are reported. In FSFs, phases which do not appear in bulks have been observed. There are compounds that only show the HexB state for relatively thin FSFs [Citation2]. It is also observed in many compounds that surfaces and interior layers behave differently. For instance, the transition temperature for the interior layers shifts to higher values when the number of layers of the FSF film decrease [Citation3]. Many thermotropic materials are reported that their surface layers show different phase sequences [Citation4].
Whether the surface is rigid or free, the physical properties are different compared to that of bulk. Microscopically, the interfacial zone can extend to a length scale much larger than the size of the constituent molecules. The influence of a surface extends far beyond the region where two materials are directly in contact, i.e. the length scale of intermolecular interaction.
2. Method and model
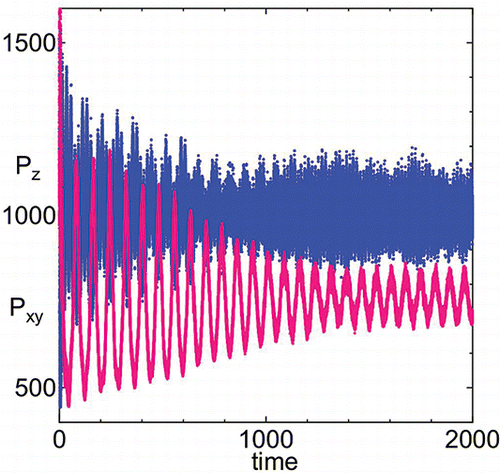
Molecular dynamics simulation methods designed for soft matter [Citation5, Citation6] control the anisotropic fluctuation of the simulation cell to obtain a desired state of the stress tensor, i.e., under hydrostatic pressure [Citation7] or under surface tension [Citation8]. The Hamiltonian is explicitly integrated by a symplectic method [Citation5]. It has been shown that when the value of the mass of the thermostat K is relatively small (i.e. when there is strong coupling to the thermostat), physical properties show large fluctuations which do not decay [Citation6], especially at low temperatures. In this study, we use larger values of masses of the barostat M and the thermostat K compared to Ref. [Citation9]. The simulations reported in this work employ the constant surface tension method with M = K = 1.0 × 104. As an example, we show how the values of stress evolve at the beginning of the simulation to attain the given values of normal pressure Pn, and surface tension γ. shows the instantaneous values of the stress in normal Pz and transverse Pxy directions at the beginning of a run under γ = 4000 at T = 80. Fluctuations in both normal z and transverse direction xy of the stress are large at the beginning, Pmaxz = 3236 and Pmaxxy = 2074, which are not shown in .
Figure 1. Time evolution of pressure in normal Pz and transverse Pxy directions in system of size N = 1344, temperature T = 80 under surface tension γ = 4000 and normal pressure Pn = 1000.
The potential energy of the surface which is usually denoted by Aγ, (surface area A and surface tension γ) is expressed in intensive and extensive quantities by anisotropy of the stress tensor ΔP = Pz − Pxy and the volume V = lzA, respectively, where lz is the total thickness of the system. Although these simulation methods can be used with or without a surface, we report simulation results obtained under periodic boundary conditions applied in x, y and z-directions. Thus, we do not include interactions of any contacting materials at the surface, such as excluded volume effects and wetting/dewetting effects. The results of the present simulations are the consequence of the pure effect of surface tension. The system size is kept rather small (number of molecules N = 1344 with initial layer number equal to 6), to allow systematic investigations. System size effects of our method are much smaller than conventional methods (for instance, see inset of Fig. 6 of [Citation9]). Since our simulation do not include a surface (contacting materials), the results can be interpreted as a part of larger scale interfacial zone, where the local values of anisotropy of stress tensor, ΔP = Pz − Pxy, vary depending on the positions, or it can be interpreted as interior layers of FSFs.
The model particles are parallel soft spherocylinders interacting through purely repulsive pairwise potential expressed by the minimum distance between the particles [Citation9, Citation10]. The molecular axis (hard line of the core) of the spherocylinder is parallel to the z-axis. The results of molecular length L = 2 are shown in this paper. Reduced simulation units, where the mass, diameter, and energy describing the interatomic potential are unity, are employed throughout this paper. Time step for integration is δt = 1.0 × 10− 4. All the data are taken from independent calculations which started from the same initial configuration prepared at T = 14 and γ = 0. Each datum is an average of Δt = 1.0 × 102 at the end of calculations with the total time of 2.0 × 104 unless otherwise stated. A wide range of surface tension is explored in these simulations.
3. Simulation results
3.1. Structural transformation under surface tension
In , the radial distribution functions in transverse direction for different surface tension and temperature are shown.
Figure 2. Radial distribution function in transverse direction gxy versus intermolecular distance rxy under surface tension γ = 500 or 1000 (blue) and γ = 4000 (magenta) at temperatures (a) T = 80, (b) T = 90, and (c) T = 100. The intermolecular distance rxy is normalized by the value at γ = 0.
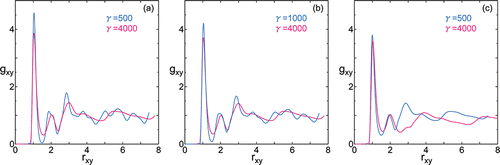
shows that for larger surface tension γ = 4000, fine structures at larger distance become vague. At temperature T = 100, notably, the 3rd and 4th large peaks are shifted to larger distance. The reason will be cleared later on.
In , pair distribution functions in normal direction gz are plotted against the relative distance of the pair for γ = 500 and 4000. The relative distance is normalized by the layer thickness at γ = 500.
Figure 3. Pair distribution function in normal direction gz versus relative distance in z-direction under surface tension γ = 500 (blue) and γ = 4000 (magenta) at temperatures T = 100. The relative distance in z-direction is normalized by the layer thickness at γ = 500.
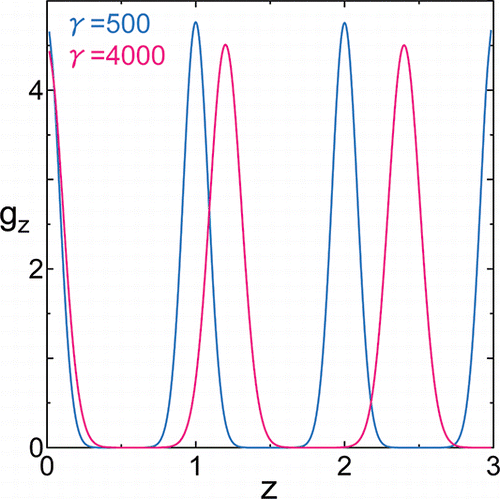
shows that the number of layers is 6 for γ = 500 and 5 for γ = 4000 at T = 100; spontaneous change in layer number occurred. For systems with γ = 4000 at temperatures T ⩾ 95, the layer number is five. Physical property data of γ = 4000 at T = 95, 100, 110, 120, and 125 shown in the following subsection are systems with 5 layers. For systems with γ = 3000 at temperatures T ⩾ 105, the number of layers is 5.
3.2. Physical properties under constant surface tension γ ⩾ 0
To understand why the structural transformation occurs under surface tension, temperature dependencies of various properties are calculated under various surface tensions.
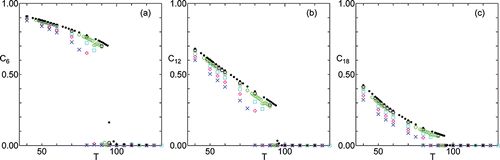
First of all, temperature dependence of 6n-fold bond orientational order parameters for various surface tension are shown in , where systematic dependence on the surface tension γ can be discerned; when γ becomes larger the values of C6n become smaller and the transition to SmA, where C6n vanish, shifts to lower temperatures.
Figure 4. Temperature dependence of 6n-fold bond orientational order parameter (a) C6, (b) C12, and (c) C18 under hydrostatic pressure γ = 0 (•), and different surface tension γ = 500 (○), γ = 1000 (green ○), γ = 2000 (cyan □), γ = 3000 (magenta ⋄), γ = 4000 (blue ×).
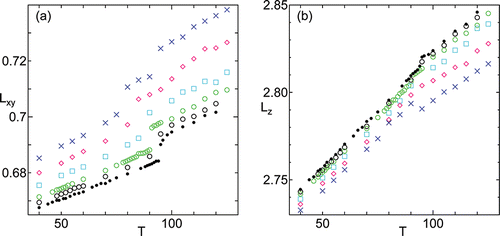
Temperature dependence of intermolecular distance Lxy in transverse direction and layer thickness Lz are shown in . The intermolecular distance Lxy clearly shows the nature of 1st order transition between HexB and SmA. When the surface tension increases the transition temperature decreases. The change of the layer thickness Lz at the HexB-SmA transition is quite small compared to Lxy. There is a notable change in both Lxy and Lz at T ⩾ 95 for the largest surface tension γ = 4000, where spontaneous change in layer number occurred. The transition of HexB to SmA occurs at 75 < T < 80 for this system. The change in layer number only occurs in systems γ = 3000 and γ = 4000 for the shown temperature range. The layer number is 5 at T ⩾ 95 for γ = 4000 and at T ⩾ 105 for γ = 3000. Since we initially have only 6 layers, the increase in Lxy (especially drastic for γ = 4000) at these temperatures might be an artifact of the small number of layers. Similarly, the notable change in Lz for γ = 4000 at T ⩾ 95 might be caused by the same reason. System with larger number of initial layers, which has more choices in number of layers, should be investigated to clarify this. The notable change in the 3rd and 4th large peak of the transverse radial distribution function in (c), should be related to the increase of the intermolecular distance Lxy because the peak shifting will be more prominent at larger distance in such situation.
Figure 5. Temperature dependence of (a) intermolecular distance Lxy in transverse direction and (b) layer thickness Lz under hydrostatic pressure γ = 0 (•), and different surface tension γ = 500 (○), γ = 1000 (green ○), γ = 2000 (cyan □), γ = 3000 (magenta ⋄), γ = 4000 (blue ×).
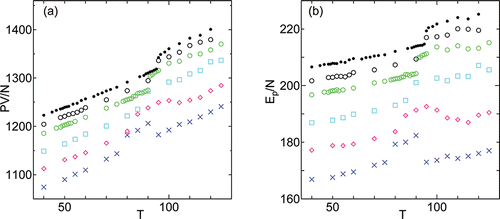
Temperature dependence of pressure times volume (PV) and potential energy (Epot) per molecule for different values of γ are shown in . As other physical properties, PV/N and Epot/N show systematic dependence on the values of γ. It is interesting to point out that PV/N and Epot/N of the systems with 5 layers show values close to those extrapolated from the lower branch at lower temperatures. This can not be explained by the increase in intermolecular distance Lxy alone (which might be an artifact of small number of layers), since the phenomenon is also clear in γ = 3000 case with five layers (T ⩾ 105) where Lxy is not drastically different from values of system with 6 layers (). It looks as if the layer thinning occurs to sustain the branch of the lower values of the thermodynamic quantities.
3.3. Effect of surface tension on elastic moduli
Elastic modulus in normal direction (layer compression modulus B) were experimentally measured for racemic binary mixture which show the tilted hexatic smectic I (HexI) phase [Citation13]. Sharp decrease in the value of the layer compression modulus, called softening, is observed near the HexI-SmC transition for low frequencies.
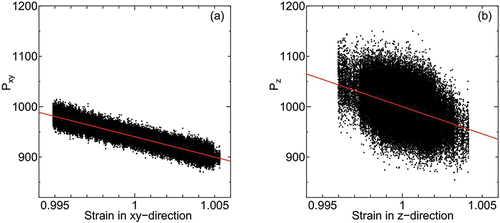
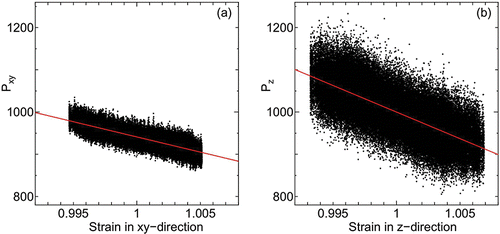
By analyzing the spontaneous fluctuations of the stress tensor and strain, the elastic moduli normal and transverse to the smectic layers are calculated. In , internal stress versus strain of system of parallel spherocylinders of L = 2 under surface tension γ = 1000 and T = 80 is shown. Each datum point (dot) is an instantaneous value of internal stress and strain taken at interval Δt = 1.0 × 10− 2 for time duration Δt = 1.0 × 103 (1.0 × 107 time steps) after t = 2.0 × 104. The cell length averaged for the same time duration of the elastic moduli measurements is employed to calculate the strain from the instantaneous values of cell length. Since the internal stress is taken as vertical axis (the change in stress is positive in contraction), the sign of inclination of the stress and strain plot is the opposite compared to usual experiments where the material is externally stressed.
Figure 7. Internal stress and strain of (a) transverse and (b) normal directions under surface tension γ = 1000 at temperature T = 80. Red line show the linear fitting.
For a higher temperature T = 110, where the hexatic nature disappears, the internal stress-strain plot is given in .
Figure 8. Internal stress and strain of (a) transverse and (b) normal directions under surface tension γ = 1000 at temperature T = 100. Red line show the linear fitting.
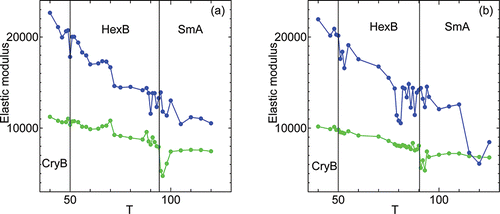
In , we show the temperature dependence of elastic moduli in both normal and transverse directions calculated by a linear fitting of the internal stress-strain plot as shown above. The elastic modulus in normal direction corresponds to the layer compression modulus B measured in experiments. In these simulations, the elastic modulus in transverse direction is a two dimensional bulk modulus in nature since we only have an anisotropic factor α = lz/lxy in the simulation cell dynamics; the transverse surface area changes isotropically. For the elastic modulus in transverse direction to show the visco-elastic characteristics of liquids, another anisotropic factor θ = ly/lx must be introduced in the simulation cell dynamics to allow shapes to be changed also in the transverse direction. For higher temperatures, the layer compression modulus B becomes smaller than the elastic modulus in transverse direction in our simulation (not shown) because of the two dimensional bulk modulus nature of the transverse one. Since all the data has been calculated independently from the same initial configuration, does not include thermal histories, i.e. cooling/heating rate dependencies. Softening of layer compression modulus near both transitions, CryB-HexB and HexB-SmA, are observed. Surface tension enhances the softening near the HexB-SmA transition and shifts the temperature to lower values. The maximum softening in HexB occurs at T = 81 for system under surface tension γ = 1000, and at T = 90 for system under hydrostatic pressure. Elastic modulus in transverse direction only shows some softening at temperatures slightly above the transition from HexB to SmA.
Figure 9. Temperature dependence of elastic modulus in normal (blue) and transverse (green) directions under (a) hydrostatic pressure γ = 0 and (b) surface tension γ = 1000.
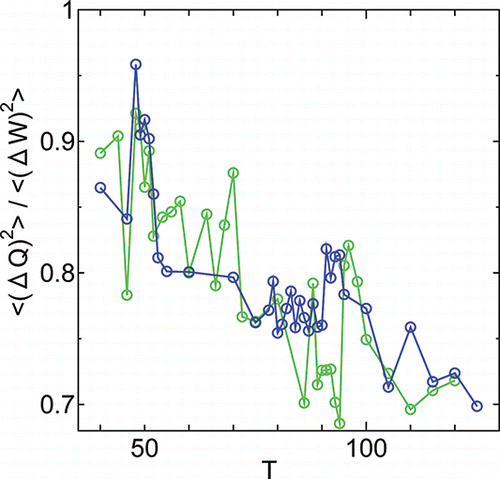
For the same time duration where the data for elastic modulus was taken, the fluctuations of various quantities are calculated. If , the fluctuation of heat against work is plotted. For the system under the surface tension γ = 1000 (blue), the values of the fluctuation ratio (ΔQ)2/(ΔW)2 show a large change near the transitions, and show some changes where the softening of layer compression modulus occurs in HexB (T ⩾ 79). This is in contrast to the 1st order thermodynamic quantities such as those reported in the previous subsection, which do not show any anomaly at the softening of B. However under hydrostatic pressure, the values show much larger fluctuations. This seems to be in agreement with the fact that surface tension usually suppress perturbation in liquids [Citation14].
4. Concluding remarks
By using the constant surface tension simulation method designed for soft matter [Citation5], molecular dynamics studies have been conducted for a large range of surface tension. Structural transformation of Sm LC has been observed. Physical properties show that those structural transformations have been occurred because of the shift in transition temperatures and layer thinning which occur for systems under surface tension. Systematic dependencies on surface tension γ are observed in the investigated physical properties. It has been shown that surface tension enhances the softening of the layer compression modulus. To fully reveal the nature of layer thinning which occurs at high values of surface tension, it is necessary to conduct simulations of systems with larger number of layers.
References
- Davis, H. T., & Scriven, L. E. (1982). Adv. Chem. Phys., 49, 357.
- (a) Moncton, D. E., Pindak, R., Davey, S. C., & Brown, G. S. (1982). Phys. Rev. Lett., 49, 1865; (b) Collett, J., Pershan, P. S., Sirota, E. B., & Sorensen L. B. (1984). Phys. Rev. Lett., 52, 356; (c) Sirota, E. B., Pershan, P. S., Sorensen, L. B., & Collett, J. (1987). Phys. Rev. A, 36, 2890; (d) Amador, S., Pershan, P. S., Stragier, H. Swanson, B. D. Tweet, D. J., Sorensen, L. B., Sirota, E. B., Ice, G. E., & Habenschuss, A. (1989). Phys. Rev. A, 39, 2703.
- Geer, R., Stoebe, T., & Huang, C. C. (1992). Phys. Rev. B, 45, 13055.
- Oswald, P., & Pieranski, P. (2006). Smectic and columnar liquid crystals, Taylor and Francis. and references therein.
- Aoki, K. M. (2008). J. Phys. Soc. Jpn., 77, 044003.
- Aoki, K. M. (2014). JPS Conf. Proc., 1, 016009.
- Aoki, K. M., Yoneya, M., Yokoyama, H. (2004). J. Chem. Phys., 120, 5576.
- Aoki, K.M., Yoneya, M., & Yokoyama, H. (2006). J. Chem. Phys., 124, 064705.
- Aoki, K. M., Yoneya, M., & Yokoyama, H. (2010). Phys. Rev. E, 81, 021701.
- Aoki, K. M., & Yoneya, M. (2011). J. Phys. Soc. Jpn., 80, 124603.
- Aoki, K. M., & Ohnishi, S. (2015). Mol. Cryst. Liq. Cryst., 612, 64.
- Aoki, K. M. (2015). Mol. Cryst. Liq. Cryst., 612, 72.
- Rogez D., Benguigui, L. G., & Martinoty, P. (2005). Eur. Phys. J. E, 16, 193-198.
- Chandrasekahar S. (1981). Hydrodynamic and Hydromagnetic Stability, Dover Publications: New York.