ABSTRACT
In the past few years, research on the fabrication of deep eutectic solvents (DESs)-based supported liquid membranes (SLMs) is increasing tremendously due to their significant advantages such as low mass transport resistance, high performance, easy scale-up, low cost and simple operation. However, fabrication of DES-based SLMs is challenging and highly dependent on the fabrication parameters: DES properties and properties of membrane supports. Subsequently, the fabrication methods, immersion, pressure-based impregnation and vacuum filtration also affect the permeation performance of DES-based SLMs in liquids and gases separation. Furthermore, the SLM durability is influenced by the DES properties and process parameters including: temperature, pressure, and feed composition. Firstly, present work reviews the current progress and fabrication of DES-based SLMs. Subsequently, it presents a perspective on different strategies that use DESs as a unique tunable platform to design advanced SLMs for liquids and gases separation reported in the literature. In the next step, performances of the SLMs membranes for liquids and gases separation are described in details. The effects of process parameters on DES-based SLMs are also highlighted prior to the challenges. Lastly, challenges and future research opportunities are highlighted.
INTRODUCTION
To meet the continuously growing demands of energy, chemicals and materials, the scale of all industries, especially the chemical industry, had to be expanded many folds (Citation1). Accordingly, the demand for separation technologies such as absorption, (Citation2) adsorption, (Citation3) cryogenic distillation (Citation2) and membranes (Citation4) grew simultaneously (Citation2, Citation5, Citation6). The adsorption technology is costly and requires a high amount of energy (Citation3). Meanwhile, membrane separation technologies hold promise of great energy savings, high selectivity, and therefore lower costs when compared to other conventional methods (Citation7–10). This technology combines the membrane separation capability with adsorption activities of adsorbents for gas/liquid molecules separation and thus, it is considered as a most efficient technique compared to solo adsorption (Citation7). Further benefits of membrane systems are smaller footprints and easy to scale-up making this technology an appropriate candidate for industrial applications (Citation11–17).
A membrane is an immiscible barrier between two phases. In the case of liquid membranes, such immiscible barrier is a liquid film (Citation18, Citation19). Usually, liquid membranes are classified into three categories: (i) bulk liquid membranes (BLMs), (ii) emulsion liquid membranes (ELMs), and (iii) supported liquid membranes (SLMs). shows the comparison between BLMs, ELMs and SLMs reported in literature. Referring to , BLMs contain a water-immiscible liquid phase possessing a high stripping speed. BLMs can be used to study the transport properties of Hg(II) ions carriers in aqueous feed solution. However, the BLM issues are a low mass transfer surface area and a huge mass transfer resistance (Citation20). Therefore, BLMs are limited to low batch operations, i.e., low operating temperature and feed pressure. ELMs were invented by Li (Citation20) containing a water-organic-water phase with a higher surface area resulting in better permeation performance. However, ELMs have a low stability, emulsion swelling, membrane leakage, and coalescence, which hinder their applications at industrial scale (Citation21). In order to overcome the aforementioned drawbacks, supported liquid membranes (SLMs) were introduced for separation processes as shown in .
Table 1. Overview, advantages, disadvantages and limitations of liquid membranes of the SLM, BLM and ELM type
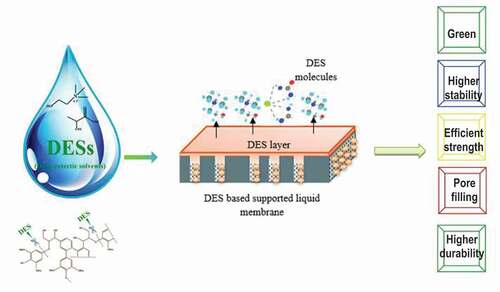
Figure 1. Fabrication and illustration of deep eutectic solvent (DES) based supported liquid membranes (SLMs).
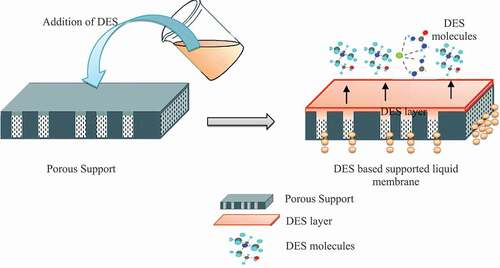
SLMs contain the supported liquid into the pore membrane. SLMs have significant advantages such as a high stability, low cost, high permeability, high mass transfer rate and high extraction efficiency (Citation22). In addition, SLMs possess uphill transport characteristic and a high interfacial surface area (Citation22) They combine extraction and stripping processes, normally performed separately, and therefore they lower the operational and capital costs (Citation22). Furthermore, SLM is a non-dispersive type of membrane with a structural support from microporous hydrophobic polymer (Citation20).
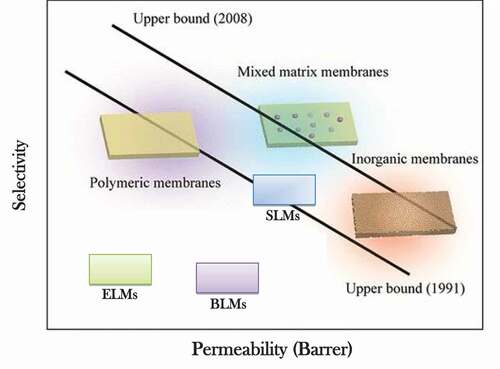
compares the permeation performance of SLMs and other membranes using the Robeson upper bound limit (Citation19). From , the permeation performance of SLMs is higher compared to BLMs and ELMs. However, a lower permeation performance is obtained compared to mixed matrix membranes and inorganic membranes (Citation22). However, SLMs are unstable under high pressure hence limited to low operating conditions: low temperature and pressure (Citation22). In addition, SLMs have a high mass transfer resistance due to the presence of the porous or polymeric support (Citation22).
Figure 2. Comparison of SLM selectivity versus gas permeability for different types of membranes to the 1991 and 2008 Robeson curves. Reprinted (adapted) with permission from from (Citation19).
SLMs contain an immobilized liquid on the top surface playing the role of a liquid membrane. The liquid is embedded in the small membrane pores and kept there by capillary forces. The immobilized liquid, the supported phase, is the most important part of the SLM determining the separation and extraction rate through the membrane (Citation20). Three different supported phases are normally used for the fabrication of SLMs: (i) volatile organic compounds (VOCs), (ii) ionic liquids (ILs), and (iii) deep eutectic solvents (DESs) as displayed in (Citation21). Referring to , VOCs are highly volatile and toxic due to their organic nature. These solvents are not considered good for lab scale experiments. In addition, displays the comparison of different supported phases used for the fabrication of SLMs. VOCs possess a high volatility, which consequently increases operational SLM costs due to solvent losses (Citation23–25). To overcome this issue, researchers replaced the VOC solvents with ILs because of their low vapor pressure and high thermal and chemical stabilities (Citation26–28). The use of ILs enhanced the operational and structural stability of SLMs in separation processes (Citation29–34). Applications of ILs in SLMs significantly enhanced the CO2 permeability and CO2/CH4 and CO2/N2 ideal selectivity (Citation35,Citation36). Besides, ILs also provide high extraction efficiency. However, most ILs are toxic and expensive (Citation28). In addition, they possess high viscosity and polarity, and poor degradability. These drawbacks excluded them from industrial SLMs.
Table 2. Comparison of supported phases used for fabrication of SLMs
Figure 3. Structure and visual comparison of a- VOCs, b-ILs, and c- the choline chloride/urea DES. Adapted from (Citation21).
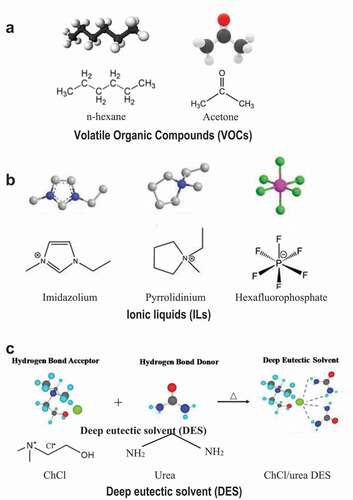
To replace ILs and VOCs, DESs are greener, safer and cheaper for fabrication of SLMs for liquids and gases separation (Citation33, Citation35, Citation36). DESs are sustainable solvents made by mixing a hydrogen bond acceptor (HBA) with a hydrogen bond donor (HBD) (Citation29,Citation30,Citation34). In other words, DES can be defined as “a mixture of pure compounds for which the eutectic point temperature is well below that of an ideal liquid mixture” (Citation34). Most DESs are based on the choline cation ((CH3)3N+CH2CH2OH, trimethylammonium-2-hydroxyethyl cation). ILs and DESs have similar physical–chemical characteristics such as good chemical stability and low vapor pressure (Citation29–32). If they are not as thermally stable as ILs, they possess some additional advantages including, easy availability of raw materials, simple synthesis process and low cost (Citation22, Citation31). Most DESs are nontoxic, biodegradable, recyclable, sustainable, available, and cheap qualifying as a green medium (Citation32).
DES-based SLMs possess unique characteristics including, lower solvent loss, low solvent inventory without flooding or loading limitations which improves efficiency during liquids and gas separation (Citation19). In addition, the mass transfer occurs on both sides of DES-based SLMs due to the presence of a chemical potential gradient (Citation20). Besides, the feed solute does not chemically react with the DES carrier during passive diffusion and thus, DES-based SLMs have a long durability (Citation21). Previous studies (Citation23,Citation24) found that DES-based SLMs show non-Newtonian behavior and medium viscosity, two more advantages for liquid and gas separation processes. Various researchers (Citation20, Citation29) focused on SLM fabrication using DESs as supported phase for the separation of liquids and gases.
Despite the significant advantages of DES-based SLMs, their fabrication is a delicate process that depends on a number of parameters such as type of DES, its composition (Citation20, Citation29), the thickness of the membrane support (Citation35–37), wettability (Citation38), as well as additives (Citation39–41). In addition, different fabrication methods such as immersion (Citation20), pressure-based impregnation (Citation29) and vacuum filtration (Citation42) are influencing factors that affect membrane performances. Besides, process parameters also affect the permeation performance of DES-based SLMs in gases and liquid separation. To date, limited literature has been reported on the fabrication of DES-based SLMs and the influence of fabrication parameters on separation properties. A real breakthrough in the design of DES-based SLM materials in terms of durability is yet to be investigated. For long-term durability, a good match between the DES and its membrane support is a crucial issue and significant effort is required to establish this match reliably (Citation33). Furthermore, the effect of process parameters including, temperature, pressure difference and feed composition on SLM permeation performances also need to be investigated.
The scope of this article includes a critical review of the configuration, transport mechanism and current progress of DES-based SLMs for liquids and gases separation. Subsequently, the effects of fabrication methods, fabrication parameters and process parameters on DES-SLM permeation performances are addressed. Besides, challenges and future research opportunities of DES-based SLMs are also highlighted in this article.
CONFIGURATION AND TRANSPORT MECHANISMS OF DES-BASED SLMS
DES-based SLMs are classified based on their configuration, being flat sheets and hollow fibers the most common (Citation43). Flat sheet DES-based SLMs could offer a mass transfer area per unit volume of 1000 m2/m3. They are normally applied to lab scale experiments (Citation44). Meanwhile, hollow fiber DES-based SLMs could offer a mass transfer area per unit volume up to 200,000 m2/m3 (Citation45). Hollow fiber SLMs are compact and possess high pressure stability which make them an appropriate candidate for industrial applications (Citation46). However, hollow fiber SLMs are not yet commercialized at industrial scale and thus, required more significant research.
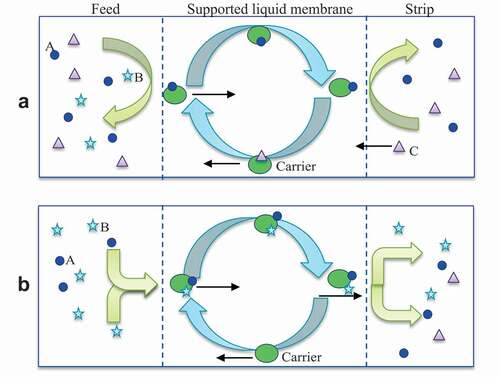
There are two types of transport mechanisms occurring in DES-based SLMs: (i) passive diffusion, and (ii) facilitated mass transport. In passive diffusion, concentration gradients contribute to the feed transportation through SLMs. Meanwhile, facilitated mass transport mechanism occurring in DES-based SLMs is classified into two sub-categories: (ii-a) facilitated coupled counter transport, and (ii-b) facilitated coupled co-transport as shown in (Citation22). Coupled transport mechanism ()-a occurs when charged species “A” from the feed side interact with acidic carriers present in the DES of SLMs. The DES acidic carriers interact with charged species “A”, producing a “A-carrier complex” which is then separated by extraction (Citation22). Usually, metal cations are used as acidic carriers to obtain the coupled counter transport mechanism through the SLMs. However, when acidic carriers are replaced with basic carriers such as amines, it will be classified as a facilitated coupled co-transport mechanism of SLMs (Citation22). In this mechanism, two species “A” and “B” from feed side are extracted by basic carriers and produced the species “A-B-carrier complex” which are then separated by extraction as shown in -b (Citation22).
Figure 4. Facilitated transport mechanism of gases (C2H4 blue circles/C2H6 stars) and liquids (furfural, HMF purple triangles) through DES (green big circles) based SLMs (a) coupled counter transport (b) coupled co-transport. Adapted from (Citation22).
CURRENT PROGRESS OF DES-BASED SLMS
In the past few years, research on the fabrication of DES-based SLMs has significantly increased in the various separation processes such as amino acids, sugar-derived molecules from biomass, removal of heavy metals from water and C2H4/C2H6 separation (Citation29). Recently, Zhuo (Citation20) reported the permeation performance of choline chloride - p-toluenesulfonic acid/aspartic acid (ChCl-PTS-Asp) DES-based SLM for the separation of an amino acid mixture of glutamic acid (Glu), histidine (His), asparagine (Asn), tyrosine (Tyr), arginine (Arg), tryptophan (Trp) and phenylalanine (Phe). This membrane demonstrated the highest permeability and extraction efficiency for Trp with 927 L/h m2 and 73.1%, respectively, as compared to other amino acids separation. This behavior is mainly attributed to the strong interaction of ChCl and Asp with Trp which could enhance the transport mechanism through the DES and resulted in the higher permeability.
Diez investigated thymol-lidocaine (thy-lid), decanoic acid-menthol (deca-men), decanoic acid-thymol (deca-thy), decanoic acid-ɳ-tetraoctyl ammonium (deca-am) DES-based SLMs (Citation37) for separation of sugar-derived molecules such as furfural (FF) and hydroxymethylfurfural (HMF). The thymol-lidocaine DES-based SLM demonstrated high FF and HMF permeabilities of 9.30 and 6.37 cm s−1, respectively, in comparison with other DES-based SLMs. The thymol-lidocaine interaction between FF and HMF might improve the facilitated coupled counter transport mechanism through the SLM.
DES-based SLMs were also applied for the removal of heavy metals from water. Decanoic acid-tetrabutylammonium chloride DES-based SLM had water flux of 143 L/m2h, 4.65 times higher than that of a neat polyethersulfone (PES) membrane (Citation47). Recently, DES-based SLMs have also been applied for C2H4/C2H6 and CO2/N2 separation as shown in (Citation29, Citation39–41, Citation48, Citation49). Trifluoromethanesulfonate/acetamide (AgCF3SO3/CH3CONH2) DES-based SLM showed a C2H4 permeability of 850 Barrer and a C2H4/C2H6 ideal selectivity of 64 (Citation29). Although, silver-based SLMs exhibited higher C2H4/C2H6 permeation performance, the high cost of AgCF3SO3 and potential degradation are serious drawbacks for this DES application. Therefore, researchers focused on the application of Cu salts based DESs for fabrication of SLMs in C2H4/C2H6 separation (Citation50).
Table 3. Single gas permeation performance of DES-based SLMs in C2H4/C2H6 separations
Sun (Citation48) reported the permeation performance of a cuprous monochloride (CuCl) DES/PVDF-based SLM in the C2H4/C2H6 separation. A C2H4 permeability and C2H4/C2H6 ideal selectivity of 8200 Barrer and 5.8, respectively, were obtained. In another work, a C2H4 permeability of 8.1 and a remarkable 9.9 C2H4/C2H6 ideal selectivity were attained over the ChCl-glycerol DES-based SLM (Citation39). The poor long-term performance of this ChCl-glycerol DES-based SLM was mainly due to the low activity of the Ch− carrier ions. Therefore, in their subsequent work, (Citation40) ChCl was replaced with CuCl to prepare a glycerol DES-based SLM in C2H4/C2H6 separation. This CuCl-glycerol DES-based SLM demonstrated a C2H4 permeability of 35 Barrer and a very good 9.6 C2H4/C2H6 ideal selectivity, slightly lower than the values achieved with the ChCl-glycerol DES-based SLM but with a much better long-term performance.
Also using polyol electron donors, Deng (Citation41) reported series of DES-based SLMs including, ChCl/glycerol, ChCl/ethylene glycol, CuCl/glycerol, CuCl/ethylene glycol in C2H4/C2H6 separation. They found that the ChCl/ethylene glycol DES-based SLM showed the best C2H4 permeability of 120 Barrer and C2H4/C2H6 ideal selectivity of 18 better than the values achieved for ChCl/glycerol, CuCl/glycerol and CuCl/ethylene glycol DES-based SLMs. The highest C2H4 permeability of ChCl/ethylene glycol DES-based SLM was mainly attributed to the presence of specific hydrogen bondings between ChCl and ethylene glycol which could facilitate the C2H4 transport mechanism through the SLM.
The C2H4/C2H6 separation was also studied by Dou using TEANO3-glycerol, DMANO3-glycerol and DMANO3-ethylene glycerol DES-based SLMs (Citation49). The TEANO3-glycerol/Nylon demonstrated the C2H4/C2H6 ideal selectivity of 100 higher than the selectivities obtained for DMANO3-glycerol and DMANO3-ethylene glycerol DES-based SLMs. A synergetic regulation of hydrogen bond and coordinate interactions between DES and NO3 carrier were proposed to explain the increase in reaction rate between the silver cation and its counter anion. Therefore, the carrier activity was promoted and subsequently resulted in higher C2H4/C2H6 ideal selectivity (Citation50).
DES-based SLMs have also been reported for CO2/N2 and CO2/CH4 separation (). ChCl-ethylene glycol DES/PVDF-co-PTFE SLM had a CO2/N2 ideal selectivity of 2.0 (Citation51). Lin (Citation42) reported the permeation performance of graphene oxide DES-based composite SLM in CO2/N2 and CO2/CH4 separation. They incorporated graphene oxide into the ChCl/ethylene glycol DES over the polycarbonate support getting permeabilities of 4.5 and 0.1 Barrer for N2 and CH4, respectively. Meanwhile, CO2/N2 and CO2/CH4 excellent selectivities of 450 and 350, respectively, were obtained using this SLM (Citation51).
Table 4. CO2/N2 single gas permeation performance of DES-based SLMs reported in literature
Based on the above discussion, limited applications of DES-based SLMs have been reported in the literature. Therefore, further investigation on fabrication of DES-based SLMs is needed to apply them in various fields such as drugs preparation for medical applications, syngas production, dry methane reforming and propane separation, and more.
Fabrication of DES-based SLMs
According to previous research (Citation20, Citation29, Citation39–41, Citation48, Citation49) on the use of DES-based SLMs for the separation of amino acids, FF, HMF, CO2/N2, CO2/CH4 and C2H4/C2H6, the fabrication of SLMs significantly affected its permeation performance in both liquid and gas separations. Therefore, finding the rationale of such effect and accordingly developing optimal and reproducible methods for DES-SLM fabrication is crucial. A detailed description of the reported fabrication methods is provided in the following section:
Fabrication Methods
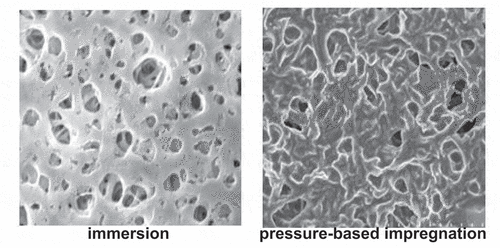
In the recent years, researchers essentially focused on the different strategies to fabricate DES-based SLMs for liquids and gases separation that were: immersion, (Citation20) pressure-based impregnation (Citation29) and vacuum filtration (Citation42) as listed in . Referring to , immersion seems to be a simple and easy method having a low cost, small maintenance and small footprint (Citation20). Therefore, Zhuo (Citation20) fabricated a PTS-Asp DES-based SLM in 24 h using the immersion method. In another work, Dietz (Citation37) fabricated twelve different DES-based SLMs in 5 h also by immersion. However, the immersion method could not fill the pores of SLMs efficiently (Citation20) as shown in ).
Table 5. Methods used for fabrication of DES-based SLMs reported in literature
Figure 5. SEM images of surface morphologies (porous and dense) of CuCl-ChCl DES/PVDF based SLMs fabricated using different methods. Reprinted (adapted) with permission from (Citation20, Citation48).
In order to overcome these issues, a pressure-based impregnation method has been introduced as an efficient method for fabrication of SLMs. In this method, an N2 feed pressure of 1–2 bar is applied along with DESs over the membrane support for 10 to 30 min. This method provides short fabrication duration with maximum pore filling of membrane supports. In addition, it also provides a uniform SLM morphology as revealed in ) (Citation48). Besides, this method allowed controlling the thickness of the DES-based SLM by adjusting the N2 gas pressure forcing DES molecules to penetrate into the pores of membrane support. lists the different works using pressure-based impregnation fabricated DES-based SLMs (Citation29, Citation39, Citation48).
For example, Bin (Citation29) firstly reported the fabrication of an AgCF3SO3/CH3CONH2 DES-based SLM using the pressure-based impregnation method. They fabricated their SLM in 30 min under 1 bar N2 environment. This procedure was repeated 3 times in order to obtain a uniform DES layer over the PVDF polymer support as shown in ). Sun (Citation48) reported the fabrication of a CuCl DES-based SLM in 9 min using the impregnation method reported by Bin (Citation29) under 2 bar N2 feed pressure. Recently, Jian (Citation39) and Deng (Citation41) also used a similar method to fabricate ChCl-glycerol and ChCl/ethylene glycol-based SLMs obtaining better morphologies as compared to the immersion method as shown in .
Vacuum filtration coupled with impregnation was used for the fabrication of graphene oxide-ChCl-ethylene glycol DES-based composite SLMs (Citation42). Lin (Citation42) optimized the fabrication duration between 10 s and 2 h over the graphene oxide-ChCl-ethylene glycol DES-based composite SLM. Although, this method produced SLMs with uniform morphology, its high capital cost and maintenance problems with frequent leakage limit its employment.
Based on the above discussion, it has been found that the pressure-based impregnation is an efficient and fast fabrication method which could produce the SLM with uniform morphology compared to the other fabrication methods (Citation41).
Fabrication Parameters
Along with fabrication methods, the fabrication parameters include DESs properties (Citation20, Citation29) and membrane support properties (Citation35–41). These parameters play an important role in controlling the permeation performance of DES-based SLMs in liquids and gases separation. Therefore, research on DESs properties and membrane support properties for fabrication of DES-based SLMs must and has tremendously increased. Detailed description on DESs properties and membrane support properties is provided in the following sections.
Deep Eutectic Solvent Properties
DES properties including DESs types, composition and additives significantly affect the permeation performance of SLMs in liquids and gas separation (Citation39–41). A detailed description of each DES property is provided in the following subsections as follows.
DESs Type
The DES used plays an important role in obtaining high-quality SLMs in liquids and gases separation (Citation31,Citation32). DESs are classified into four different categories as illustrated by (Citation31,Citation32). Each type of DES exhibits different structure, nature and composition which could affect the facilitated transport mechanism and thus control the SLM permeation performance (Citation32. DES type I contains a metal halide or imidazolium salt which initiates the hydrogen bonding between carriers and feeds over the SLM. Therefore, DES type I could provide a high permeation performance over the SLMs in liquids and gas separation (Citation52). Besides, this type of DES possesses advantages of lower vapor pressure and low viscosity. Therefore, DESs type I have been significantly used by various researchers for SLM fabrication in liquids and gas separation (Citation39).
Figure 6. Classification of DESs in four types on the basis of their structure, Reprinted (adapted) with permission from (Citation31,Citation32).
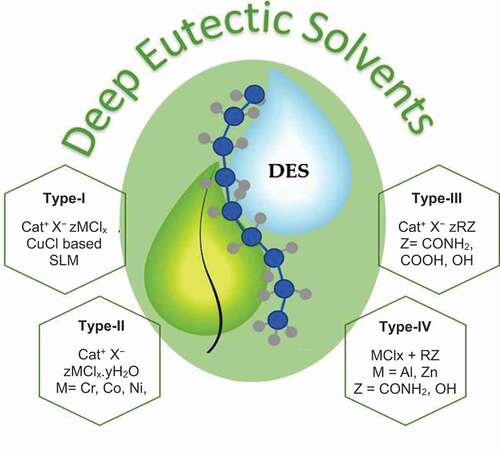
DESs type II contain inherent water in their structure and provide significant resistance during the mass transport mechanism over the SLMs in liquids and gases separation. Besides, these DESs type II exhibit a high viscosity which causes resistance during the filling of the pores inside the membrane support (Citation52). Therefore, this type of DES has not been used for the fabrication of SLMs (Citation41, Citation42). DESs type III contain OH and CONH2 group in their structure and they have been used by Bin (Citation29) for the fabrication of AgCF3SO3-CH3CONH2/PVDF based SLMs in C2H4/C2H6 separation (Citation29). However, DESs type III are not stable and not durable when used for liquids and gases separation. DESs type IV are a combination of types II and III since they possess hydroxyl (OH) groups in their structure. Similar to DESs type II, DESs type IV have also not been used for the fabrication of SLMs due to their thermal fragility.
Overall, among the various DES types, DES type I is considered as the most efficient for the fabrication of SLMs due to its high surface area, low viscosity, high reaction rate and strong hydrogen bonding between membrane support and DES which resulted in high SLM permeation performance in liquids and gas separation (Citation53).
DES Composition
The DES composition is another fabrication parameter that governs SLM permeation performance in gas separation (Citation20, Citation29). presents the effect of the DES composition on single gas permeation performance in C2H4/C2H6 separation. Based on , a variation of CH3CONH2, CuCl and glycerol composition significantly affected the DES-SLM performance in C2H4/C2H6 separation. Bin (Citation29) reported the effect of AgCF3SO3/CH3CONH2 DES composition in the same C2H4/C2H6 separation. By varying the AgCF3SO3/CH3CONH2 DES molar ratio from 1:2.5 to 1:30 over the SLM, they found that the C2H4 permeability was increased from 450 to 850 Barrer and the C2H4/C2H6 ideal selectivity went from 45 to 64. However, further increments of AgCF3SO3/CH3CONH2 molar ratio from 1:30 to 1:40 over the SLM were detrimental with the C2H4/C2H6 ideal selectivity dropping from 64 to 38. This result was mainly attributed to the increment of C2H6 diffusivity due to the saturation of NH2 ions in DES which reduced the C2H4/C2H6 selectivity. Therefore, the AgCF3SO3/CH3CONH2 molar ratio of 1:30 was the optimum composition to achieve the best permeation performance of SLM in C2H4/C2H6 separation (Citation29).
Table 6. Effect of DES composition on the single gas permeation performance of DES-based SLMs in C2H4/C2H6 separation
Sun (Citation48) reported the effect of the CuCl proportion on the permeation performance of CuCl DES-based SLM in C2H4/C2H6 separation. When the CuCl molar ratio in DES was increased from 0.5 to 1, the ethylene permeability and C2H4/C2H6 ideal selectivity of the SLM were enhanced from 2000 to 8200 Barrer and 2.6 to 5.8, respectively. However, further increment of CuCl molar ratio 1 to 2 reduced the C2H4 permeability from 8200 to 2100 Barrer while the C2H4/C2H6 ideal selectivity was further increased up to 20. Jian (Citation40) found that the C2H4 permeability dropped from 55 to 25 Barrer while the C2H4/C2H6 ideal selectivity was also increased by up to 340% by incrementing the CuCl molar ratio from 1 to 2 in DES over the SLM. These coherent results were due to a higher Cu+ content that reduced the C2H4 and C2H6 transport mechanism through the DES.
In another work, the C2H4 permeability and C2H4/C2H6 ideal selectivity of a ChCl-glycerol/Nylon based SLM were dropped from 8.2 to 7.1 Barrer and 11.5 to 8.5, respectively, when the glycerol molar ratio increased from 1 to 3 (Citation39). Dou reported similar observation with TEANO3-glycerol and DMANO3-glycerol based SLMs also varying the glycerol proportion (Citation49). The C2H4 permeability of TEANO3-glycerol and DMANO3-glycerol-based SLMs were reduced from 300 to 135 Barrer and 185 to 27 Barrer, respectively, raising the glycerol molar ratio from 1 to 3 (Citation49). Meanwhile, the C2H4/C2H6 respective TEANO3-glycerol and DMANO3-glycerol based SLM selectivities dropped from 100 to 45 and 68 to 54. It was speculated that glycerol could reduce the activity of NO3 carriers. Therefore, glycerol with the minimum molar ratio of 1.0 was identified as the optimum DES value for fabrication of SLMs with high C2H4/C2H6 separation performance.
Overall, a higher CuCl content in the DES could increase the C2H4/C2H6 SLM ideal selectivity by about 350%, which was the highest as compared to other composition of DES reported in literature. Thus, it can be considered as an efficient composition that can be implemented for fabrication of DES-based SLMs in C2H4/C2H6 separation.
Additives
The presence of additives such as metal chlorides (MCln; M = Cu, Zn or Co), silver salt (AgNO3) and nanoparticles (graphene oxide) into the DES is a crucial factor influencing the SLM permeation performance in gases separation. shows the key advantages to incorporate different additives into DES-based SLMs (Citation54). Incorporating additives could increase the mechanical strength, stability and performance of DES-based SLMs (Citation40). Therefore, various researchers (Citation39–41) reported the effect of additives on the single gas permeation performance of DES-based SLMs in C2H4/C2H6 separation as tabulated in .
Table 7. Effect of additives on single gas permeation performance of DES-based SLMs in C2H4/C2H6 separation
Figure 7. Key advantages of incorporation of different additives into DES-based SLMs. Adapted from. (Citation54).
Jiang (Citation40) reported the effect of CuCl/CoCl2 and CuCl/ZnCl2 additives on the permeation performance of DES-based SLM for C2H4/C2H6 separation. Addition of CuCl/CoCl2 to DES dropped the C2H4 permeability from 25 to 16 Barrer while the C2H4/C2H6 ideal selectivity was increased from 9.6 to 11.4. The improvement of C2H4/C2H6 ideal selectivity was attributed to the viscosity increase due to the presence of Co ions which reduced the C2H4 and C2H6 diffusivities over the SLM (Citation40). The C2H4 permeability and C2H4/C2H6 ideal selectivity of a CuCl/ZnCl2 DES-based SLM were 32 Barrer and 16.8, respectively. These values were respectively 28% and 75% higher than those achieved for the neat SLM.
In another work, Jiang (Citation39) investigated the effect of adding CuCl to a ChCl-glycerol based SLM on the C2H4/C2H6 separation performances. The addition of 3 mol/L CuCl into the DES increased the C2H4 permeability from 1.0 to 13 Barrer. Meanwhile, the C2H4/C2H6 ideal selectivity was enhanced from 2.0 to 20% or 900% higher than what was obtained with the DES-based SLM produced without CuCl addition. These significant improvements were attributed to the presence of cations Cu+ which developed coordination bonds with C2H4 and eventually enhanced its diffusivity through the SLM (Citation39). Similar CuCl increasing trend of C2H4 permeability and C2H4/C2H6 ideal selectivity with ChCl/ethylene glycol based SLM were reported by Deng (Citation41).
Dou (Citation49) reported that the addition of AgNO3 from 0 mol/L to 5 mol/L into TEANO3-glycerol and DMANO3-glycerol DES over the SLMs enhanced the C2H4 permeability from 25 to 308 Barrer and 15 to 190 Barrer, respectively. The C2H4/C2H6 selectivities were increased from 2.2 to 140 and 1.7 to 95, respectively. These results were attributed to Ag+ cations that facilitated the C2H4 transport mechanism through the SLMs. Besides, AgNO3 introduced a structural compactness into the DES which dropped the C2H6 permeability and thus the C2H4/C2H6 SLM selectivities were significantly increased.
Recently, the effect of graphene oxide additive on the performance of DES-based SLMs has been reported by Lin et al. (Citation42). They found that the CO2 permeability and CO2/N2 ideal selectivity of a ChCl/ethylene DES-based SLM were increased by two orders of magnitude from 4.5 to 450 Barrer upon the graphene oxide incorporation. It seems that the graphene oxide can greatly enhance the CO2 dual sorption transport mechanism through the SLM.
Among the various additives, AgNO3 significantly improved the C2H6 permeability and C2H4/C2H6 ideal selectivity. However, it is expensive which could increase the cost of DES-based SLMs. The addition of CuCl to ChCl/glycerol DES-based SLM could increase the C2H4/C2H6 ideal selectivity by 900% which was one of the highest increment compared to what was obtained after the addition of other additives into DES for fabrication of SLMs reported in literature. Therefore, it is considered as an efficient additive for the fabrication of DES-based SLMs in C2H4/C2H6 separation.
Membrane Support Properties
The properties of the membrane support such as its type of polymer, thickness and wettability play an important role to control the transport mechanism of DES-based SLMs which subsequently affect the liquid and gas permeability. Therefore, the selection of an efficient membrane support with minimum thickness is crucial and challenging. A detailed description of each parameter is provided as follows.
Types of Polymer
Based on the literature, polymers widely used as membrane support are selected on the basis of permeation performance, adhesion capacity, porosity, thermal and chemical stability (Citation35, Citation36). Usually, semi-aromatic polymers have a high permeability, perm-selectivity, high chemical resistance, as well as a good thermal stability and mechanical strength compared to other families of polymers (Citation35). Among the various polymers, cellulose, polycarbonate, polyamide, and PVDF are commonly used for the fabrication of DES-based SLMs in liquid and gas separation.
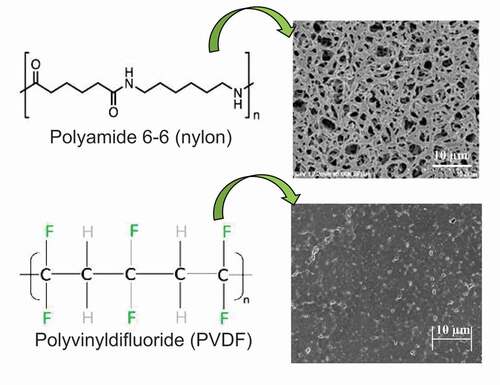
Polyamide and PVDF are effective polymers because they are easy to make and to process (Citation43). In addition, nylon possesses a high solvent solubility, low density, high degree of substitution and high chain stability (Citation54). An extensive use of nylon as membrane support in the market of separation processes is mainly due to its low manufacturing cost and high tensile strength which helps in the formation of film and fibers (Citation55). Besides, PVDF is considered as a predominant material to be used for hydrocarbon separation from gases in industrial-scale applications (Citation36). However, gas permeation performances of nylon and PVDF membranes are under the Robeson upper bound curve (Citation56). Therefore, the application of DES over nylon and PVDF could improve their performance in liquid and gas separation due to the presence of hydrogen bonds in their structures as shown in & b) (Citation57). These hydrogen bonds could initiate interaction between the DES and polymer chains and thus, facilitate the transport mechanism through SLMs. Therefore, PVDF and nylon are considered as efficient polymers for fabrication of SLMs in liquid and gas separation.
Figure 8. Chemical structures and SEM images of surface (porous and dense) morphology of different polymer supports used for the fabrication of SLMs. Adapted from. (Citation57).
Thickness of Support
The thickness of the membrane support influences the permeation performance of DES-based SLMs as it has been reported by Dietz (Citation37) . shows this effect as reported by Dietz (Citation37). Referring to Thymol-lidocaine, decanoic acid-menthol, decanoic acid-ɳ-tetraoctyl ammonium-based SLMs were produced over three different polymer membrane supports including, polyethylene (M320B), polyethylene (16P10A) and poly-propylene (PP2E) with thicknesses of 80 µm, 120 µm and 170 µm, respectively. The respective furfural permeability were 9.30, 5.32 and 2.39 cms−1. Meanwhile, HMF permeabilities of 6.37, 1.20 and 1.18 cms−1 were respectively obtained over these SLMs. Decanoic acid-menthol, decanoic acid-ɳ-tetraoctyl ammonium-based SLMs also revealed a similar decreasing trend of FF and HMF permeabilities that was attributed to the thicker membrane supports which reduced the pore sizes and thus enhanced the resistance to mass transport of FF and HMF molecules through the SLMs.
In summary, the thin polyethylene support demonstrated the highest FF and HMF permeabilities over the SLMs due to its small thickness and higher pore volume. Therefore, a thickness of 80 µm is considered as an efficient thickness for fabrication of DES-based SLMs in liquid separation.
Wettability
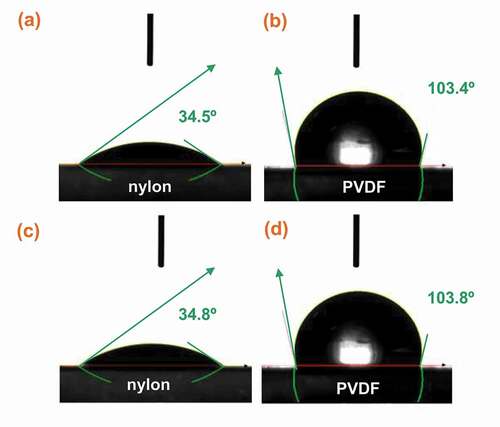
The compatibility between the membrane support and the DES is another important factor in the formation of DES-based SLMs. This compatibility is evaluated in terms of wettability (Citation58). Usually, the wettability is measured using the contact angle of a DES drop on the membrane support. If the contact angle is less than 90°, the DES can be stabilized into the membrane pores supported by capillary forces (Citation38). However, if the contact angle is more than 90°, capillary forces will not be strong enough to hold the DES in the pores, as studied by various researchers (Citation39, Citation42, Citation59).
Jian (Citation39) reported the contact angle of a water droplet on nylon and PVDF-based SLMs as shown in . Using this method, the contact angles of ChCl-glycerol DES droplets on the nylon and PVDF supports were 34.5° and 103.4°, respectively. However, after the addition of CuCl to ChCl-glycerol DES, the contact angle was very slightly higher 34.8º and 103.8º, respectively. The contact angles of CuCl/ChCl-glycerol DES mixture on the nylon and PVDF supports were comparable with the values reported for neat SLMs (Citation39). The 34.5° and 34.8º values of contact angle confirmed that the wettability between nylon and the ChCl-glycerol DES was appropriate. Therefore, the ChCl-glycerol DES was used as a supporting phase for the fabrication of DES-based SLMs. A C2H4 permeability and C2H4/C2H6 ideal selectivity of 8.1 Barrer and 9.9, respectively, were obtained for these membranes.
Figure 9. Contact angle of different DES-based SLMs including (a) ChCl-glycerol DES/nylon, (b) ChCl-glycerol DES/PVDF, (c) CuCl/ChCl-glycerol/nylon, (d) CuCl/ChCl-glycerol/PVDF measured using the water droplet method. Adapted from. (Citation39).
Lin (Citation42) found that the polycarbonate support possesses a good wettability with graphene oxide-ChCl-ethylene glycol DES with a small contact angle of 30°. Therefore, ChCl-ethylene glycol was used as the supporting phase to fabricate graphene oxide-ChCl-ethylene glycol DES-based SLM for CO2 separation.
Considering cost, a natural DES can be prepared for about 1 US$/cm2 and the CA polymer support costs about 2 US$/cm2. Thus, the fabrication cost of the DES-based SLM could be estimated to be less than 4 US$/cm2. Optimization process parameters and large-scale production could further reduce the fabrication cost and manpower requirements for industrial-scale applications.
Effect of Process Parameters on the Performance of DES-Based SLMs
Process parameters including, temperature, pressure difference and feed composition are also important toward the permeation performance of DES-based SLMs in liquids and gas separation (Citation33). However, study on these effects were poorly studied (Citation60). Detailed description on each process parameter is provided in the following subsections.
Temperature
The operating temperature significantly affects the DES viscosity, free volume and flexibility of polymer matrix which subsequently influence the SLM permeability and selectivity (Citation61). Bin (Citation29) reported that raising the temperature from 25°C to 45°C with a AgCF3SO3/CH3CONH2 DES-based SLM increased the C2H4 and C2H6 permeability from 850 to 1200 Barrer and 13 to 24 Barrer, respectively. Meanwhile, the C2H4/C2H6 SLM ideal selectivity was reduced from 64 to 50. The 45°C value was 28% lower than the ideal selectivity obtained at 25°C. Sun (Citation48) also observed that the C2H4 and C2H6 permeabilities of a CuCl/Cl DES-based SLM were increased by 34.2% and 57.2%, respectively, while the C2H4/C2H6 ideal selectivity was slightly decreased from 9.6 to 8.2 raising the temperature from 30°C to 50°C.
Jian et al. (Citation39) investigated the effect of temperature on the performance of a ChCl-glycerol/Nylon-based SLM in C2H4/C2H6 separation. They found that raising the temperature from 25°C to 45°C enhanced the C2H4 and C2H6 permeabilities of SLM from 8.1 to 15.2 Barrer and 0.9 to 2.8 Barrer, respectively. However the C2H4/C2H6 ideal selectivity of the SLM dropped from 9.9 to 4.3. An opposite decreasing trend in C2H4 permeability and increasing trend in C2H4/C2H6 ideal selectivity over the CuCl, CuCl/ZnCl2 and CuCl/CoCl2 based SLMs were observed incrementing the temperature from 30°C to 60°C (Citation40). The ethylene permeability and C2H4/C2H6 ideal selectivity over the ChCl/ethylene glycol based SLM (Citation41), TEANO3-glycerol based SLM (Citation49) and DMANO3-glycerol based SLM (Citation49) showed a similar behavior upon raising the temperature.
The CO2 and N2 permeabilities over the graphene oxide-ChCl/ethylene glycol DES-based composite SLM (Citation42) were increased upon heating. This increment in gas permeabilities was mainly due to the reduction of DES viscosity which resulted in the enhancement of gas diffusivity (Citation48). However, C2H4/C2H6 and CO2/N2 selectivities were reduced due to the deleterious effect of temperature on the interaction between the carrier and C2H4 or CO2 which eventually reduced the C2H4 or CO2 solubility (Citation48).
Pressure Difference
Pressure difference affects the solution diffusion mechanism and SLM solubility coefficient which subsequently affects the morphology and permeation performance of membranes (Citation62). Bin (Citation29) investigated the effect of pressure difference on the permeation performance of AgCF3SO3/CH3CONH2 DES-based SLMs in C2H4/C2H6 separation. He found that a pressure increment from 0.1 to 0.5 bar dropped the C2H4 and C2H6 permeabilities by 41.1% and 3.8%, respectively. Meanwhile, the C2H4/C2H6 ideal selectivity was reduced from 64 to 40, a 37.5% reduction compared to the value of C2H4/C2H6 ideal selectivity obtained at 0.1 bar pressure difference. Sun (Citation48) reported that C2H4/C2H6 ideal selectivity decreased from 5.8 to 5.1 on raising the pressure from 1.1 to 1.4 bar. In another work, (Citation39) when the pressure difference was increased from 0.1 to 0.5 bar, C2H4 and C2H6 permeabilities, and C2H4/C2H6 ideal selectivity on a ChCl-glycerol/Nylon SLM dropped from 8.1 to 2.5 Barrer, 0.9 to 0.3 Barrer, and 9.9 to 8.3, respectively.
Furthermore, both C2H4 permeability and C2H4/C2H6 ideal selectivity on a ChCl/ethylene glycol based SLM (Citation41) a TEANO3-glycerol (Citation49) and a DMANO3-glycerol based SLM (Citation49) also revealed a similar behavior on increased pressure difference. However, raising the pressure did not affect the C2H6 permeability on TEANO3-glycerol and DMANO3-glycerol based SLMs. The reduction of C2H4 permeability was mainly attributed to the dual sorption mechanism of DES-based SLMs (Citation48). In addition, increment in C2H4 solubility also dropped the C2H4 diffusion over the SLMs (Citation63). Furthermore, the saturation of carrier ions may lower the complexation reaction and thus, the C2H4/C2H6 SLM selectivity decreases as the pressure difference across the membranes increases (Citation64).
Feed Composition
The composition of the feed is another challenging parameter which affects the SLM permeation performances. The literature reported that an increment of C2H4 content from 1% to 5%v/v in C2H6 feed composition enhanced the C2H4 permeability of an AgCF3SO3/CH3CONH2 DES-based SLM from 260 to 1340 Barrer, while the C2H4/C2H6 ideal selectivity was improved to 303 (Citation29). This result is due to a better C2H4 interaction rate with the DES carrier which enhanced the C2H4 permeability and C2H4/C2H6 ideal selectivity.
In summary, an elevation of temperature and feed composition could improve permeation performances of DES-based SLMs in C2H4/C2H6 separation. In this case, temperature and feed ratio should be kept at as high as possible values to obtain the best SLM permeation performances. Meanwhile, increment of feed pressure difference reduced the SLM permeation performance in C2H4/C2H6 separation. Hence, DES-based SLMs should be operated at a low value of feed pressure difference. Overall, temperature, feed pressure difference and feed composition could interact over the DES-based SLM and thus, their optimization is necessary to achieve the higher C2H4/C2H6 separation performance. In addition, optimization of SLM process parameters will also help to reduce operating cost and manpower in industrial-scale applications.
Performance Comparison of SLMs with Zeolite, MOFs and Other Materials-Based Membranes
compares the performances of DES-based SLMs with SLMs, zeolite, MOFs and other materials-based membranes reported in literature (Citation42, Citation52, Citation65–72). shows that DES-based SLMs demonstrated the highest CO2/CH4 ideal selectivity compared to the MOFs, zeolites and other nanomaterials-based membranes. The highest CO2/CH4 SLM ideal selectivity was 450 (Citation42). DD3R zeolite membranes followed with a selectivity value of 130. However, the CO2 SLM permeability was lower compared to that of MOFs, zeolite and other nanomaterials-based membranes. These results could be attributed to the presence of DES molecules which could provide extra resistance to large gas molecules and thus, higher values of CO2 permeability (Citation52). Based on this comparison study (), it is concluded that the SLMs are efficient membranes and could be helpful for CO2 removal at industrial scale.
Table 8. Comparison of DES-based SLMs with SLMs, polymer, MOFs, zeolite and other nanomaterials-based membranes in CO2 separation
CHALLENGES AND FUTURE RESEARCH OPPORTUNITIES
DES-based SLMs are facing challenges with their fabrication as well as applications at industrial scale due to the involvement of a significant number of fabrication parameters, fabrication methods and process parameters. Therefore, limited literature has been reported on the fabrication of DES-based SLMs for liquid and gas separation. Detailed description of challenges and future research opportunities of DES-based SLMs is provided in the following subsection.
Challenges with DES-based SLMs
In the recent years, immersion, (Citation20) pressure-based impregnation (Citation29) and vacuum filtration (Citation42) have been adopted for the fabrication of DES-based SLMs. Among these methods, finding an appropriate method for fabrication of SLMs is complicated since, these methods depend on various conditions such as duration of immersion, N2 feed pressure and vacuum pressure, respectively. Besides, the optimum value of these parameters is needed prior to the fabrication of DES-based SLMs. Another problem for fabrication of DES-based SLMs is the loss of carriers and DESs which consequently changes the SLM permeability as well as its selectivity (Citation71). Therefore, closing of mass balance over the DES-based SLM is complicated. Besides, pore blockage of membrane support by precipitation of a carrier complex at the surface is another challenging issue which needs to be addressed prior to its application for gas separation (Citation65,Citation71,Citation72).
Most DES-based SLM studies focused solely on separation performance, improving permeability and selectivity through manipulation of DES composition and incorporation of additives (Citation35–37). However, studies on fabrication of new combination of DES/polymer-based SLMs through manipulation of DES types and membrane support are challenging but needed. Based on reported literature, process parameters including, temperature, pressure difference and feed composition may interact with each other over the SLMs and subsequently, could affect the permeation performance for gas separation. Therefore, studies on these process parameters may be complicated and challenging, but they are needed.
Long-term durability of DES-based SLMs system is another challenging issue which needs to be addressed prior to scale up the material for industrial applications. The durability of DES-based SLMs depends on the DES characteristics, on the membrane support material, and on carrier deactivation and DES poisoning (Citation49). list the DES-SLM long-term durability in C2H4/C2H6 and CO2/N2 separations, respectively.
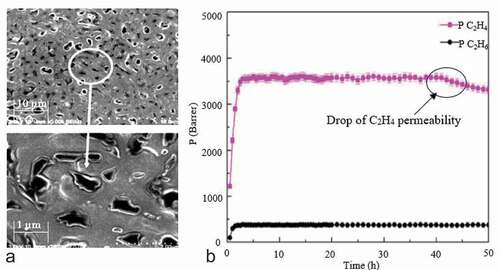
Referring to , Bin (Citation29) studied the durability of AgCF3SO3/CH3CONH2 DES-based SLMs in C2H4/C2H6 separation. They found that the C2H4 permeability of AgCF3SO3/CH3CONH2 DES-based SLMs remained stable for 130 h. But the C2H4/C2H6 ideal selectivity dropped from 64 to 60 and then remained stable for the next 130 h. Sun (Citation48) also reported the durability of CuCl DES-based SLMs in C2H4/C2H6 separations. displays the SEM surface morphology and durability of a CuCl DES-based SLM in C2H4/C2H6 separation (Citation48). The SLM was tested for 50 h showing a uniform morphology with a C2H4 permeability remaining stable for 40 h. After 40 h the C2H4 permeability dropped significantly due to oxidation and quenching of Cu+ which reduced the C2H4 facilitated transport mechanism through the SLM.
Figure 10. CuCl-(Cl) DES/PVDF based SLMs. (a) SEM image of the porous surface view at two magnifications and (b) durability of SLMs tested for 50 h for C2H4/C2H6 separation, Reprinted (adapted) with permission fromd from. (Citation48).
Jian (Citation39) found that the C2H4 and C2H6 permeabilities of ChCl/Glycerol DES-based SLMs slightly decreased from 8.5 to 8.1 Barrer and 1.1 to 0.9 Barrer, respectively, during the first 72 h. But they remained stable for next 336 h (two weeks). In their subsequent work, (Citation40) the C2H4 and C2H6 permeabilities of a CuCl-ZnCl2 DES-based SLM dropped after 40 h due to water evaporation out of the CuCl-ZnCl2 salts. As a consequence, the DES viscosity in the SLM increased which stabilized the C2H4 and C2H6 permeabilities for the next 150 h. A similar trend has been reported by Deng (Citation41) over the ChCl/ethylene glycol DES-based SLM in C2H4/C2H6 separation.
Dou et al. (Citation49) evaluated the durability of TEANO3-glycerol and DMANO3-glycerol based SLMs in the C2H4/C2H6 separation. TEANO3-glycerol and DMANO3-glycerol based SLMs showed a durability higher than 160 h. In the first hour, the C2H4 and C2H6 SLM permeabilities increased significantly and then, become stable during the medium-term run. On the other hand, referring to , the CO2 and N2 permeabilities of graphene oxide/ChCl-ethylene glycol DES-based composite SLMs also dropped with time. The CO2/N2 ideal selectivity of a composite SLM was increased by aging from 450 to 500 (Citation42).
Overall, it has been concluded that the ChCl/glycerol DES-based SLM showed the longest durability up to 336 h as compared to other literature values in C2H4/C2H6 separations (Citation43). Therefore, ChCl/glycerol is a potential DES for fabrication of SLMs for long-term operation in industrial-scale applications. Besides, this type of DES can be used for real breakthroughs in the design of new types of SLMs in gas separations.
Future Research Opportunities
The current trends and contemporary challenges over the DES-based SLMs are critically reviewed to open new perspectives for the fabrication of the next SLM generation for liquid and gas separation. shows possible future research opportunities of DES-based SLMs. The needed future opportunities and research rooms for DES-based SLMs are in the area of applications, process optimization, and DES-SLM preparation.
Figure 11. Classification of future research opportunities of DES/polymer support based SLMs in different fields.
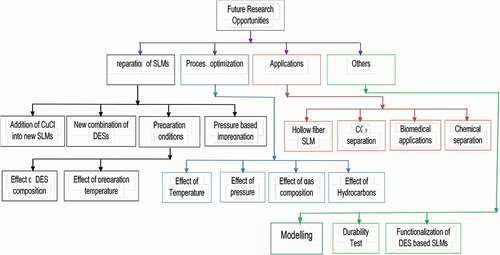
Based on the reported literature, pressure-based impregnation is the more effective and efficient method compared to other conventional methods. It is recommended for fabrication of future DES-based SLMs. Besides, immersion coupled with an ultrasonic irradiation method could also be used for fabrication of DES-based SLMs because it may produce ultrasonic pores and cavities which may help to enhance the penetration of DESs into the membrane support. Based on , the development of DES-based SLMs is significantly affected by fabrication parameters such as the type of DES, its composition, the thickness of the membrane support, wettability and incorporation of additives into the DES. Type I of DES seems to be the best to produce new types of SLMs due to its low viscosity and high reaction rate. The addition of CuCl into ChCl/glycerol SLM increased the C2H4/C2H6 ideal selectivity by up to 900%. Therefore, CuCl can be used for the enhancement of the performance of new types of DES-based SLMs for gas separation. Besides, functionalization of DES with amines or different functional groups could also enhance SLM performances. Also DES-SLM synthesis temperature conditions and molar compositions are yet to be optimized.
Although, few studies have reported the effects of temperature and pressure over the SLMs. Future efforts could be extended to optimize these interactions on process parameters studying temperature, feed pressure and feed composition effects on DES-based SLMs. Central composite design coupled with response surface methodology could be involved. Efforts are also needed to study the effect of hydrocarbon impurities and moisture on the performance of DES-based SLMs. Durability tests for new types of SLMs under real industrial conditions must be explored in order to meet industrial requirements. These investigations may pave the way toward commercialization of DES-based SLMs in various industries. On the other hand, studies on the flat sheet DES-based SLMs can be extended to the hollow fiber SLM configuration for possible further enhancement of ideal membrane selectivities in separation processes. Applications of DES-based SLMs are currently limited to FF, HMF, C2H4 and C2H6 separations. Therefore, DES-based SLMs can be further deployed in different fields such as drugs preparation for medical applications, water purification, syngas production, dry methane reforming and propane separation. A breakthrough application of DES-based SLMs would be the CO2 separation from natural gas and flue gases.
Mathematical modeling is an important tool for optimization and designing of complicated parameters of SLMs based separation processes. Therefore, with the growth of SLMs, new models should be developed to simulate reaction combined with separation processes to achieve lower absolute average error % (AARE%) between experimental and simulated results.
CONCLUSIONS
In the present work, transport properties, current trends and challenges of DES/polymer-based SLMs are critically reviewed. Potential strategies to improve the fabrication of SLMs for liquids and gases separation are suggested. Based on discussion, it has been found that pressure-based impregnation is the most effective and efficient method so far. More specifically, applications of pressure-based impregnation methods could produce more uniform SLMs with minimum thickness and nonselective voids. Subsequently, finding a suitable DES, additive and membrane support with minimum thickness is essential for the enhancement of permeation performance of SLMs in liquids and gases separation. Among the various types of DES, type I is considered as the most efficient due to its higher surface area, low viscosity and higher reaction rate. Contact angle measurements of DES droplets on membrane support should be less than 90° to produce stable SLMs. Moreover, the addition of CuCl into ChCl/glycerol DES-based SLMs produced the 900% highest increase of C2H4/C2H6 ideal selectivity. It may be the best DES additive for SLM fabrication. In addition, ChCl/glycerol-SLM showed the longest durability, up to 336 h for C2H4/C2H6 separation, compared to values reported in literature for other SLMs.
It has also been found that impregnation of DES into polymer support could reduce the SLM fouling. Besides, DES regeneration is significantly easier compared to other solvents and thus, it helps in cost savings and enhanced the SLM life cycle during separation processes. Therefore, DES-based SLMs can be used for real breakthroughs in new SLM designs for gas separation. If increment of temperature and feed composition could enhance the performance of DES-based SLMs, increments in pressure difference reduced SLM permeation performances in C2H4/C2H6 separation. Hence, optimization of process parameters including temperature, feed pressure difference and feed composition over the DES-based SLM is necessary to achieve the higher C2H4/C2H6 separation performance. This work provides some new directions for the development of DES/polymer-based SLMs in liquid and gas separation at industrial scale.
Acknowledgments
The authors duly acknowledge the framework of Chemelot InSciTe Horizontal project and Eindhoven University of Technology, Netherlands, for the support.
Additional information
Funding
REFERENCES
- Bulushev, D. A.; Ross, J. R. H. Towards Sustainable Production of Formic Acid. Chem. Sus. Chem. 2018, 11, 821–836. DOI: https://doi.org/10.1002/cssc.201702075.
- Mubashir, M.; Yeong, Y. F.; Lau, K. K.; Azmi, M. S. Issues and Challenges in the Development of Deca-Dodecasil 3 Rhombohedral Membrane in CO2 Capture from Natural Gas”, Sep. Purif. Rev. 2014, 44, 331–340. DOI: https://doi.org/10.1080/15422119.2014.970195.
- Yu, C. H.; Chung, T. S.; Tan, C.-S. A Review Of CO2 Capture By Absorption And Adsorption “ Aerosol. Air Qual. Res. 2012, 12, 745–769. DOI: https://doi.org/10.4209/aaqr.2012.05.0132.
- Yeo, Z. Y.; Chew, T. L.; Zhu, P. W.; Mohamed, A. R.; Chai, S. P. Conventional Processes and Membrane Technology for Carbon Dioxide Removal from Natural Gas: A Review. J. Nat. Gas Chem. 2012, 21, 282–298. DOI: https://doi.org/10.1016/S1003-9953(11)60366-6.
- Mubashir, M.; Yeong, Y. F.; Lau, K. K. Ultrasonic Assisted Secondary Growth of Deca-dodecasil 3 Rhombohedral (DD3R) Membrane and Its Process Optimization Studies in CO2/CH4 Separation Using Response Surface Methodology”. J. Nat. Gas. Sci Eng. 2016, 30, 50–63. DOI: https://doi.org/10.1016/j.jngse.2016.01.015.
- Irsa, M.; Zahid, M.; Zainab, A.; Basharat, A.; Kiran, K.; Mubashir, M. Optimized Tuning of Rosin Adduct with Maleic Anhydride for Smart Applications in Controlled and Targeted Delivery of Urea for Higher Plant’s Uptake and Growth Efficiency. Ind Crops Prod. 2019, 133, 395–408. DOI: https://doi.org/10.1016/j.indcrop.2019.02.036.
- Galvez, L. E.; Juarez, J. O.; Pacheco, R.; Garzon, I. L.; Paz Borbon, L. O.; Amarillas, A. P. CO2 Adsorption on Gas-phase Cu4−xPtx (X = 0–4) Clusters: A DFT Study. Phys. Chem. Chem. Phys. 2018, 20, 17071–17080. DOI: https://doi.org/10.1039/C8CP00818C.
- Madzarevic, Z. P.; Shahid, S.; Nijmeijer, K.; Dingemans, T. J. The Role of Ortho-, Meta- and Para-substitutions in the Main-chain Structure of Poly(etherimide)s and the Effects on CO2/CH4 Gas Separation Performance. Sep. Purif. Technol. 2019, 210, 242–250. DOI: https://doi.org/10.1016/j.seppur.2018.08.006.
- Muhammad, M.; Yin, F. Y.; Chew, T. L.; Lau, K. K. Effect of Spinning Conditions on the Fabrication of Cellulose Acetate Hollow Fiber Membrane for CO2 Separation from N2 and CH4. Polym. Test. 2019, 73, 1–11. DOI: https://doi.org/10.1016/j.polymertesting.2018.10.036.
- Yang, J.; Fan, W.; Bell, C. M. Effect of Calcination Atmosphere on Microstructure and H2/CO2 Separation of Palladium-doped Silica Membranes. Sep. Purif. Technol. 2019, 210, 659–669. DOI: https://doi.org/10.1016/j.seppur.2018.08.041.
- Mubashir, M.; Yeong, Y. F.; Chew, T. L.; Lau, K. K. Issues and Current Trend of Hollow Fiber Mixed Matrix Membranes for CO2 Separation from N2 and CH4. Chem. Eng. Technol. 2018, 41, 235–252. DOI: https://doi.org/10.1002/ceat.201700327.
- White, L. S.; Wei, X.; Pande, S.; Wu, T.; Merkel, T. C. Extended Flue Gas Trials with a Membrane-based Pilot Plant at a One-ton-per-day Carbon Capture Rate. J. Membr. Sci. 2015, 496, 48–57. DOI: https://doi.org/10.1016/j.memsci.2015.08.003.
- Heidari, M.; Hosseini, S. S.; Omidkhah, M.; Ghadimi, A. Synthesis and Fabrication of Adsorptive Carbon Nanoparticles (Acns)/pdms Mixed Matrix Membranes for Efficient CO2/CH4 and C3H8/CH4 Separation. Sep. Purif. Technol. 2019, 209, 503–515. DOI: https://doi.org/10.1016/j.seppur.2018.07.055.
- Ozen, H. A.; Ozturk, B. Gas Separation Characteristic of Mixed Matrix Membrane Prepared by MOF-5 Including Different Metals. Sep. Purif. Technol. 2019, 211, 514–521. DOI: https://doi.org/10.1016/j.seppur.2018.09.052.
- Castro, R.; Fila, V.; Martin Gil, V.; Muller, C. Enhanced CO2 Permeability in Matrimid® 5218 Mixed Matrix Membranes for Separating Binary CO2/CH4 Mixtures. Sep. Purif. Technol. 2019, 210, 553–562. DOI: https://doi.org/10.1016/j.seppur.2018.08.046.
- Dilshad, M. R.; Islam, A.; Hamidullah, U.; Jamshaid, F.; Ahmad, A.; Butt, M. T. Z.; Ijaz, A. Effect of Alumina on the Performance and Characterization of Cross-linked PVA/PEG 600 Blended Membranes for CO2/N2 Separation. Sep. Purif. Technol. 2019, 210, 627–635. DOI: https://doi.org/10.1016/j.seppur.2018.08.026.
- Davood, M. H.; Chung, T. S. A Novel Crosslinking Technique Towards the Fabrication of High-flux Polybenzimidazole (PBI) Membranes for Organic Solvent Nanofiltration (OSN). Sep. Purif. Technol. 2019, 209, 182–192. DOI: https://doi.org/10.1016/j.seppur.2018.07.026.
- Hao, Z.; Vilt, M. E.; Wang, Z.; Zhang, W.; Ho, W. S. Supported Liquid Membranes with Feed Dispersion for Recovery of Cephalexin. J. Membr. Sci. 2014, 468, 423–431. DOI: https://doi.org/10.1016/j.memsci.2014.06.009.
- Youdong, C.; Zhihong, W.; Dan, Z. Mixed Matrix Membranes for Natural Gas Upgrading: Current Status and Opportunities. Ind. Eng. Chem. Res. 2018, 57, 4139–4169. DOI: https://doi.org/10.1021/acs.iecr.7b04796.
- Li, Z.; Cui, Y.; Shen, Y.; Li, C. Extraction Process of Amino Acids with Deep Eutectic Solvents-Based Supported Liquid Membranes. Ind. Eng. Chem. Res. 2018, 57, 4407–4419. DOI: https://doi.org/10.1021/acs.iecr.7b05221.
- Luciana, I. N. T.; Vanessa, B.; Wanderson, S.; Christopher, M. A. B. Deep Eutectic Solvents for the Production and Application of New Materials. Appl Mater Today. 2018, 10, 30–50. DOI: https://doi.org/10.1016/j.apmt.2017.11.005.
- Kocherginsky, N. M.; Yang, Q.; Seelam, L. Recent Advances in Supported Liquid Membrane Technology. Sep. Purif. Technol. 2007, 53, 171–177. DOI: https://doi.org/10.1016/j.seppur.2006.06.022.
- Bao, L.; Trachtenberg, M. C. Facilitated Transport of CO2 across a Liquid Membrane: Comparing Enzyme, Amine, and Alkaline. J. Membr. Sci. 2006, 280, 330–334. DOI: https://doi.org/10.1016/j.memsci.2006.01.036.
- Teramoto, M.; Sakaida, Y.; Fu, S. S.; Ohnishi, N.; Matsuyama, H.; Maki, T. An Attempt for the Stabilization of Supported Liquid Membrane,” Sep. Purif. Technol. 2000, 21, 137–144. doi:https://doi.org/10.1016/S1383-5866(00)00197-0
- Takeuchi, H.; Takahashi, K.; Goto, W. Some Observations on the Stability of Supported Liquid Membranes. J. Membr. Sci. 1987, 34, 19–31. DOI: https://doi.org/10.1016/S0376-7388(00)80018-6.
- Rios, A. P. L.; Hernandez, F. J.; Alonso, F.; Palacios, J. M.; Gomez, D.; Rubio, M.; Víllora, G. A SEM–EDX Study of Highly Stable Supported Liquid Membranes Based on Ionic Liquids. J. Membr. Sci. 2007, 300, 88–94. DOI: https://doi.org/10.1016/j.memsci.2007.05.010.
- Fortunato, R.; Afonso, C. A. M.; Benavente, J.; Rodriguez, E.; Crespo, J. G. Stability of Supported Ionic Liquid Membranes as Studied by X-ray Photoelectron Spectroscopy. J. Membr. Sci. 2005, 256, 216–223. DOI: https://doi.org/10.1016/j.memsci.2005.02.023.
- Lozano, L. J.; Godinez, C.; Ríos, A. P.; Hernandez, F. J.; Sanchez, S.; Alguacil, F. J. Recent Advances in Supported Ionic Liquid Membrane Technology. J. Membr. Sci. 2011, 376, 1–14. DOI: https://doi.org/10.1016/j.memsci.2011.03.036.
- Jiang, B.; Dou, H.; Wang, B.; Sun, Y.; Huang, Z.; Bi, H.; Zhang, L.; Yang, H. Silver-Based Deep Eutectic Solvents as Separation Media: Supported Liquid Membranes for Facilitated Olefin Transport. ACS Sustain. Chem. Eng. 2017, 5, 6873–6882. DOI: https://doi.org/10.1021/acssuschemeng.7b01092.
- Dai, Y.; Spronsen, J.; Witkamp, G. J.; Verpoorte, R.; Choi, Y. H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta. 2013, 766, 61–68. DOI: https://doi.org/10.1016/j.aca.2012.12.019.
- Roda, A.; Matias, A. A.; Paiva, A.; Duarte, A. R. C. Polymer Science and Engineering Using Deep Eutectic Solvents. Polymers. 2019, 11, 912. DOI: https://doi.org/10.3390/polym11050912.
- Smith, E. L.; Abbott, A. P.; Ryder, K. S. Deep Eutectic Solvents (Dess) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. DOI: https://doi.org/10.1021/cr300162p.
- Mubashir, M.; Yin, F. Y.; Chew, T. L.; Lau, K. K. Prediction of CO2 Permeability in NH2-MIL-53(Al)/Cellulose Acetate Mixed Matrix Membranes Using Theoretical Models. Int J Integr Eng. 2018, 10, 176–180. DOI: https://doi.org/10.30880/ijie.2018.10.05.026.
- Martins, A. R. M.; Simao, P. P.; Coutinho, A. P. J. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solution Chem. 2019, 48, 962–982. DOI: https://doi.org/10.1007/s10953-018-0793-1.
- Ohno, H.; Yoshizawa-Fujita, M.; Kohno, Y. Functional Design of Ionic Liquids: Unprecedented Liquids that Contribute to Energy Technology, Bioscience, and Materials Sciences. Bull. Chem. Soc. Jpn. 2019, 92(4), 852–868. DOI: https://doi.org/10.1246/bcsj.20180401.
- María, J. T.; He, N.; Marcelino, V.; Miranda, N. E.; Israel, D. S.; Jared, L. A. Advances of Ionic Liquids in Analytical Chemistry. Anal. Chem. 2019, 91, 505–531. DOI: https://doi.org/10.1021/acs.analchem.8b04710.
- Dietz, C. H. J. T.; Kroon, M. C.; Di, M.; Stefano, M.; Annaland, S.; Gallucci, F. Selective Separation of Furfural and Hydroxymethylfurfural from an Aqueous Solution Using a Supported Hydrophobic Deep Eutectic Solvent Liquid Membrane. Faraday Discuss. 2018, 206, 77–92. DOI: https://doi.org/10.1039/C7FD00152E.
- Dahi, A.; Fatyeyeva, K.; Langevin, D.; Chappey, C.; Rogalsky, S. P.; Tarasyuk, O. P.; Benamor, A.; Marais, S. Supported Ionic Liquid Membranes for Water and Volatile Organic Compounds Separation: Sorption and Permeation Properties. J. Membr. Sci. 2014, 458, 164–178. DOI: https://doi.org/10.1016/j.memsci.2014.01.031.
- Jiang, B.; Dou, H.; Zhang, L.; Wang, B.; Sun, Y.; Yang, H. Novel Supported Liquid Membranes Based on Deep Eutectic Solvents for Olefin-paraffin Separation via Facilitated Transport. J. Membr. Sci. 2017, 536, 123–132. DOI: https://doi.org/10.1016/j.memsci.2017.05.004.
- Muhammad, Y. F. Y.; Chew, T. L.; Lau, K. K.; Keong, L. K. Enhanced Gases Separation of Cellulose Acetate Membrane Using N-Methyl-1-Pyrrolidone as Fabrication Solvent. Int. J. Automotive Mech. Eng. 2018, 15, 4978–4986. DOI: https://doi.org/10.15282/ijame.15.1.2018.7.0386.
- Deng, R.; Sun, Y.; Bi, H.; Dou, H.; Yang, H.; Wang, B. Deep Eutectic Solvents as Tuning Media Dissolving Cu+ Used in Facilitated Transport Supported Liquid Membrane for Ethylene/Ethane Separation. Energy. Fuel. 2017, 31, 11146–11155. DOI: https://doi.org/10.1021/acs.energyfuels.7b01305.
- Lin, H.; Gong, K.; Ying, W.; Chen, D.; Zhang, J.; Yan, Y.; Peng, X. CO2-Philic Separation Membrane: Deep Eutectic Solvent Filled Graphene Oxide Nanoslits. Small. 2019, 15, 1904145. DOI: https://doi.org/10.1002/smll.201904145.
- Goh, P. S.; Ismail, A. F.; Sanip, S. M.; Ng, B. C.; Aziz, M. Recent Advances of Inorganic Fillers in Mixed Matrix Membrane for Gas Separation. Sep. Purif. Technol. 2011, 81, 243–264. DOI: https://doi.org/10.1016/j.seppur.2011.07.042.
- Wódzki, R.; Sionkowski, G. Recovery and Concentration of Metal Ions. II Multimembrane Hybrid System. Sep. Sci. Technol. 1995, 30, 2763–2778. DOI: https://doi.org/10.1080/01496399508013714.
- Wan, C. F.; Li, B.; Yang, T.; Chung, T. S. Design and Fabrication of Inner-selective Thin-film Composite (TFC) Hollow Fiber Modules for Pressure Retarded Osmosis (PRO). Sep. Purif. Technol. 2017, 172, 32–42. DOI: https://doi.org/10.1016/j.seppur.2016.08.001.
- Baker, R. W.;. Membrane Technology, in Kirk Othmer Encyclopedia of Chemical Technology. ed. United kingdom: John Wiley & Sons, Inc., 2000. doi: https://doi.org/10.1002/0471440264.pst194.
- Jiang, B.; Zhang, N.; Wang, B.; Yang, N.; Huang, Z.; Yang, H.; Shu, Z. Deep Eutectic Solvent as Novel Additive for PES Membrane with Improved Performance. Sep. Purif. Technol. 2018, 194, 239–248. DOI: https://doi.org/10.1016/j.seppur.2017.11.036.
- Sun, Y.; Bi, H.; Dou, H.; Yang, H.; Huang, Z.; Wang, B.; Deng, R.; Zhang, L. A Novel Copper(I)-Based Supported Ionic Liquid Membrane with High Permeability for Ethylene/Ethane Separation. Ind. Eng. Chem. Res. 2017, 56, 741–749. DOI: https://doi.org/10.1021/acs.iecr.6b03364.
- Dou, H.; Jiang, B.; Zhang, L.; Xu, M.; Sun, Y. Synergy of High Permeability, Selectivity and Good Stability Properties of Silver-decorated Deep Eutectic Solvent Based Facilitated Transport Membranes for Efficient Ethylene/ethane Separation. J. Membr. Sci. 2018, 567, 39–48. DOI: https://doi.org/10.1016/j.memsci.2018.09.027.
- Tome, L. C.; Mecerreyes, D.; Freire, C. S. R.; Rebelo, L. P. N.; Marrucho, I. M. Polymeric Ionic Liquid Membranes Containing IL–Ag+ for Ethylene/ethane Separation via Olefin-facilitated Transport. J. Mater. Chem. A. 2014, 2, 5631–5639. DOI: https://doi.org/10.1039/C4TA00178H.
- Amira, M. N.; Irfan, M. D.; Jullok, N.; Syahmie, M.; Alamery, H. R. Synthesis and Preparation of Asymmetric PVDF-co-PTFE/DES Supported Membrane for CO2/N2 Separation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 429, 012067. DOI: https://doi.org/10.1088/1757-899X/429/1/012067.
- Abbott, A. P.; Capper, G.; Davies, D. L.; McKenzie, K. J.; Obi, S. U. Solubility of Metal Oxides in Deep Eutectic Solvents Based on Choline Chloride. J. Chem. Eng. Data. 2006, 51, 1280–1282. DOI: https://doi.org/10.1021/je060038c.
- Li, W.; Su, P.; Zhang, G.; Shen, C.; Meng, Q. Preparation of Continuous NH2–MIL-53 Membrane on Ammoniated Polyvinylidene Fluoride Hollow Fiber for Efficient H2 Purification. J. Membr. Sci. 2016, 495, 384–391. DOI: https://doi.org/10.1016/j.memsci.2015.08.049.
- Lyu, G.; Li, T.; Ji, X.; Yang, G.; Liu, Y.; Lucia, L. A.; Chen, J. Characterization of Lignin Extracted from Willow by Deep Eutectic Solvent Treatments. Polymers. 2018, 10, 869. DOI: https://doi.org/10.3390/polym10080869.
- Kaur, H.; Bulasara, V. K.; Gupta, R. K. Influence of pH and Temperature of Dip-coating Solution on the Properties of Cellulose Acetate-ceramic Composite Membrane for Ultrafiltration. Carbohydr. Polym. 2018, 195, 613–621. DOI: https://doi.org/10.1016/j.carbpol.2018.04.121.
- Robeson, L. M.;. The Upper Bound Revisited. J. Membr. Sci. 2008, 320, 390–400. DOI: https://doi.org/10.1016/j.memsci.2008.04.030.
- Bidsorkhi, H. C.; D’Aloia, A. G.; Fortunato, M.; Fortunato, M.; Fortunato, M.; Fortunato, M.; Ballirano, P.; Bracciale, M. P.; Santarelli, M. L.; Sarto, M. S. Nucleation Effect of Unmodified Graphene Nanoplatelets on PVDF/GNP Film Composites. Mater. Today Commun. 2017, 11, 163–173. DOI: https://doi.org/10.1016/j.compscitech.2019.107678.
- Hopkinson, D.; Zeh, M.; Luebke, D. The Bubble Point of Supported Ionic Liquid Membranes Using Flat Sheet Supports. J. Membr. Sci. 2014, 468, 155–162. DOI: https://doi.org/10.1016/j.memsci.2014.05.042.
- Guo, L.; Gu, C.; Zhang, J.; Wang, X.; Wang, K.; Jin, Y.; Tu, J. A Black Conversion Coating Produced by Hot Corrosion of Magnesium with Deep Eutectic Solvent Membrane. Surf. Coat. Technol. 2019, 357, 833–840. DOI: https://doi.org/10.1016/j.surfcoat.2018.10.062.
- Kusworo, T.; Ismail, A.; Mustafa, A. Experimental Design and Response Surface Modeling of PI/PES-Zeolite 4A Mixed Matrix Membrane for CO2 Separation. J. Eng. Sci. Technol. 2015, 10, 1116–1130.
- Molki, B.; Aframehr, W. M.; Bagheri, R.; Salimi, J. Mixed Matrix Membranes of Polyurethane with Nickel Oxide Nanoparticles for CO2 Gas Separation. J. Membr. Sci. 2018, 549, 588–601. DOI: https://doi.org/10.1016/j.memsci.2017.12.056.
- Zhuang, G. L.; Tseng, H. H.; Uchytil, P.; Wey, M. Y. Enhancing the CO2 Plasticization Resistance of PS Mixed-matrix Membrane by Blunt Zeolitic Imidazolate Framework. J. CO2 Util. 2018, 25, 79–88. DOI: https://doi.org/10.1016/j.jcou.2018.03.009.
- Yu, G.; Deng, L.; Abdeltawab, A. A.; Deyab, S. S.; Chen, X.; Zhang, J. Functional Solution Composed of Cu(I) Salt and Ionic Liquids to Separate Propylene from Propane. Ind. Eng. Chem. Res. 2014, 53, 13430–13435. DOI: https://doi.org/10.1021/ie501522m.
- Ortiz, A.; Galan, L. M.; Gorri, D.; De Haan, A. B.; Ortiz, I. Reactive Ionic Liquid Media for the Separation of Propylene/Propane Gaseous Mixtures. Ind. Eng. Chem. Res. 2010, 49, 7227–7233. DOI: https://doi.org/10.1021/ie100576r.
- Mubashir, M.; Yeong, Y. F.; Chew, T. L.; Lau, K. K. Study on the Effect of Process Parameters on CO2/CH4 Binary Gas Separation Performance over NH2-MIL-53(Al)/cellulose Acetate Hollow Fiber Mixed Matrix Membrane. Polym. Test. 2020, 81, 106223. DOI: https://doi.org/10.1016/j.polymertesting.2019.106223.
- Shakeel, I.; Hussain, A.; Farrukh, S. Effect Analysis of Nickel Ferrite (Nife2o4) and Titanium Dioxide (Tio2) Nanoparticles on CH4/CO2 Gas Permeation Properties of Cellulose Acetate Based Mixed Matrix Membranes. J. Polym. Environ. 2019, 27, 1449–1464. DOI: https://doi.org/10.1007/s10924-019-01442-x.
- Sutrisna, P. D.; Savitri, E.; Gunawan, M. A.; Putri, I. H. F.; Rozari, S. G. B. D. Synthesis, Characterization, and Gas Separation Performances of Polysulfone and Cellulose Acetate-based Mixed Matrix Membranes, Polym. Plast. Technol. 2020, 59, 1300–1307. DOI: https://doi.org/10.1080/25740881.2020.1738471.
- Ayesha, R.; Sarah, F.; Hussain, A.; Khan, I. U.; Noor, T. M. H. D.; Othman, M. H. D.; Yousaf, M. F. Development of high Performance Amine Functionalized Zeolitic Imidazolate Framework (Zif-8)/cellulose Triacetate Mixed Matrix Membranes for CO2/CH4 Separation. Int. J. Energy Res. 2020, 44, 7989–7999. DOI: https://doi.org/10.1002/er.5448.
- Muhammad, M.; Yeong, Y. F.; Chew, T. L.; Lau, K. K. CO2 Adsorption Study Using Deca-Dodecasil 3 Rhombohedral (DDR3) Zeolite Synthesized via Ultrasonic Irradiation Coupled with Hydrothermal Heating Method. Procedia Engineering. 2016, 148, 122–127. DOI: https://doi.org/10.1016/j.proeng.2016.06.492.
- Muhammad, M.; Yeong, Y. F.; Lau, K. K. Methods Comparison for the Synthesis of Deca-Dodecasil 3 Rhombohedral (DDR3) Zeolite Crystals. Appl. Mech. Mater. 2015, 773, 1096–1100. DOI: https://doi.org/10.4028/773-774.1096.
- Neplenbroek, A. M.; Bargeman, D.; Smolders, C. A. Supported Liquid Membranes: Instability Effects. J. Membr. Sci. 1992, 67, 121–132. DOI: https://doi.org/10.1016/0376-7388(92)80020-K.
- Mubashir, M.; Yeong, Y. F.; Chew, T. L.; Lau, K. K.; Norwahyu, J. Nanocomposite Catalytic Membranes for Energy Production: Advances and Challenges. Handbook Nanotechnol. Appl. 2021, 239–254. DOI: https://doi.org/10.1016/B978-0-12-821506-7.00010-7.
