ABSTRACT
The complex mixture of pollutants found in industrial effluents creates a significant environmental challenge. Enzymatic treatment offers a promising alternative to traditional physiochemical techniques. With an emphasis on laccase enzymes, this review examines the potential of both free and immobilized biocatalysts for the treatment of industrial wastewater, especially from pulp and paper mills. Laccase has become one of the most popular options for bioremediation because of its broad substrate specificity and capacity to break down different kinds of organic pollutants. The different methods of laccase immobilization, including adsorption, encapsulation and cross-linking are examined, exploring their advantages and limitations. The benefits of immobilization for enzyme stability, reusability and operational efficiency in wastewater treatment processes are further discussed. By comparing the advantages and limitations of free and immobilized biocatalysts, this review aims to provide valuable insights for the development of efficient and sustainable enzymatic strategies for industrial wastewater treatment. Furthermore, this paper offers future possibilities for using biocatalyst in pulp and paper waste treatment.
INTRODUCTION
One of the most important components of an ecosystem is water[Citation1]. Rapid urbanization and industrialization produce solid and liquid wastes that are challenging to handle and dispose of using standard waste management techniques.[Citation2] Significant amounts of wastewater are produced owing to intensive urbanization and rising water use for household, agricultural, and industrial purposes.[Citation3] Like the majority of developing countries worldwide, South Africa primarily utilizes surface water for residential, recreational, and agricultural applications in its rural areas.[Citation4,Citation5] A significant portion of the population in the rural areas of developing countries continues to obtain their primary domestic water supply from untreated surface water.[Citation5] This is due to either limited potable water supplies or insufficient water supply infrastructures.[Citation5] Wastewater treatment plants become less effective because many new developing contaminants interfere with the separation processes by generating complexes with other common pollutants.[Citation2] Industries like pulp and paper mills are water-intensive and generate wastewater daily containing environmental contaminants like color, lignin and phenolic compounds.[Citation6] Pesticides, toxic heavy metals, fertilizers, pharmaceuticals, personal care items, nano based chemicals and persistent organic pollutants are just a few of the highly complex anthropogenic chemicals that impact modern life worldwide.[Citation2,Citation7,Citation8] Due to the untreated discharge of these sludge chemicals into streams and water bodies, these toxicological pollutants accumulate in soil, surface water, drinking water and other natural resources, posing significant challenges for their estimation, identification, and elimination.[Citation7] Many wastewater treatment plants in South Africa discharge their waste directly into rivers or streams that are used by the neighboring villages for a variety of water purposes. Pindihama et al.[Citation9] demonstrated that wastewater treatment facilities in the Vhembe district of South Africa rarely treat wastewater to acceptable standards. Edokpayi et al.[Citation10,Citation11] confirmed this finding. It seems traditional wastewater treatment techniques are less effective to remove pollutants like pharmaceuticals, personal care products, endocrine disruptors, or paper mill pollutants. Therefore, alternative technologies are necessary to reduce the adverse effects of these pollutants on the environment and also promote waste recycling for industrial purposes.
Modern technologies are being developed and investigated for water treatment processes that are not only economically viable but also environmentally friendly. Nanotechnology is one of the recent developments in water treatment processes, especially in industrial wastewater treatment. Although in its early stages, the use of nanotechnology in the treatment of wastewater has the potential to completely revolutionize the process. Enhancing wastewater treatment technologies requires the use of biocatalysts, primarily enzymes and microbial cells. This is particularly important for effluents originating from the pulp and paper industry, as these effluents contain high amounts of lignin, chlorinated chemicals, and other organic contaminants. Biocatalysts help to break down these complex chemicals into less hazardous and biodegradable molecules, providing a sustainable and effective solution.[Citation12] Wastewater treatment processes can be made more efficient by using enzymes like laccases, peroxidases, and cellulases, which are effective at breaking down many persistent pollutants found in wastewater.[Citation13,Citation14] Employing biocatalysts can result in lower chemical usage and reduced operating costs, which aligns with sustainability goals and environmental regulations. By incorporating biocatalysts into wastewater treatment systems, the pulp and paper industry can remove pollutants more effectively, resulting in cleaner effluent discharge and better environmental health.[Citation14,Citation15] Different strategies like physical, chemical and biological methods have been used by various researchers to remove pollutants from industrial wastewater using free and immobilized biocatalysts.[Citation16–18]
The review aims to promote biocatalysis as a practical and eco-friendly technique of treating wastewater from paper mills. It is focused on the paper industry specifically when other reviews have a more general focus on biocatalysis for wastewater treatment. First, the paper industry effluents and their impacts to the environment are assessed. Next, the treatment techniques are discussed and potential applications of laccase enzymes for the degradation of the pollutants and the different immobilization techniques, as well as their benefits for enzyme stability and reusability are presented. Challenges with biocatalytic treatments must be known, but promising areas for future research to advance the technology for broader application are possible. The review hopes to offer a critical and comprehensive analysis of biocatalysis as a viable technique for the long-term treatment of wastewater from the paper industry.
PAPER INDUSTRY EFFLUENTS
In present day, the pulp and paper industry is recognized as one of the largest consumers of renewable resources such as wood, water and fossil fuels which are required for electricity production.[Citation19] The industry produces significant amounts of pollutants during the pulping and production of paper-based products and these pollutants have varied compositions based on their production method.[Citation19] It also manufactures a wide variety of goods, such as tissue papers and cardboards. The sector supports millions of jobs and contributes significantly to the global economy. Approximately 400 million tons of paper and board products and 188 million tons of raw pulp were produced worldwide in 2015, making the pulp and paper industry among the most important sectors in the world.[Citation20,Citation21]
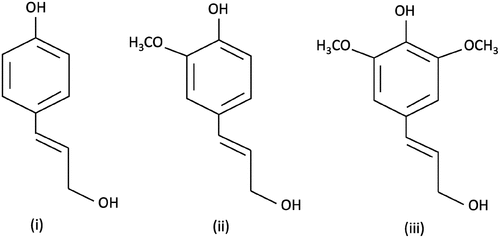
Paper and pulp manufacturing industries contribute to the global economy just as much as the textile industry, but they negatively impact aquatic ecosystems more severely, posing a serious environmental risk. The elimination of lignin and hemicellulose is crucial for creating a higher-quality paper sheet.[Citation7] To carry out these basic paper processing operations, pulp is heated to a temperature between 170 and 180°C and treated with an alkali solution along with various sulfur-based compounds such as sodium bisulfite, sulfide, sulfite, and chlorine dioxide. The lignin and hemicellulose bond link is broken because these chemically assisted digesting processes cause lignin to hydrolyze and transform into hydrophilic compounds.[Citation22] Kraft pulping, often referred to as sulfate-pulping, is the combination of sulfide and alkali pulping.[Citation7] Kraft pulping is the process of dissolving lignin from the hemicellulose and cellulose fibers of wood chips by cooking them at 155–175°C in a pressurized aqueous solution of NaOH and Na2S2, sometimes referred to as white liquor.[Citation23] Low-molecular-weight thiolignin oligomers are released when the aromatic ether linkages of the lignin structure are broken by the resulting OH− and HS− anions.[Citation24] Despite numerous developments in kraft pulping, the fundamentals of this pulping process are still widely used for turning wood into commercial pulp and paper. The pulping process produces sulfonated, soda, kraft and organo lignins as some of the end products. Chlorine dioxide is used as an additional treatment for these lignin residues, resulting in a notable release of organic halogens.[Citation7] Additionally, the main lignin bond cleavers that connect the various monomeric units are organic solvents such as acids, alcohols, ketones, and esters. The primary monomeric units found in lignin are the para-coumaryl alcohol (H-type), coniferyl alcohol (G-type) and sinapyl (S-type) alcohol units as presented in .[Citation25]
Figure 1. Monomeric units constituting the biopolymer lignin: (i) p-coumaryl alcohol, also known as p-hydroxycinnamyl alcohol (H) (ii) coniferyl alcohol, or guaiacyl (G), (iii) sinapyl alcohol, or syringyl (S).
A wide variety of environmentally persistent compounds, such as oligophenols, are produced in the wastewater released by the paper and pulp industries. More aromatic pollutants are produced from the various stages of the papermaking process, such as heavy metals, phenolics, guaiacol, and derivatives based on lignin.[Citation7] Following treatment procedures, lignin experiences modification, which results in the production of large amounts of lignosulfonates as byproducts.[Citation26] Almost all pulp mill stages involve the use of fresh water, including the handling of wood, cooking, screening, washing, and bleaching of pulp. This high amount of water consumption results in wastewater effluents at the different stages as presented in .[Citation27] Untreated wastewater may have significant amount of chemical oxygen demand (COD), biochemical oxygen demand (BOD), suspended particles (mostly fibers), fatty acids, tannins, resins, lignin and its derivatives depending on the raw materials processed.[Citation19]
ENVIRONMENTAL POLLUTANTS IN PULP AND PAPER WASTEWATER
The most prevalent pollutant in effluents from the pulp and paper industry is color. Lignin, its derivatives, and dyes are just a few of the substances that give wastewater from pulp and paper mills its brown color.[Citation28,Citation29] Using traditional treatment techniques, it is challenging to get rid of these substances from wastewater. Since color does not directly affect either human health or the environment, it is classified as aesthetic pollution. However, it might have a variety of unintended consequences.[Citation21] Colored effluents might make it more difficult to spot pollution indications and decrease the recreational value of aquatic bodies.[Citation30] Additionally, the removal of other pollutants may be hampered by colored wastewater. Color can hinder the disinfection of wastewater thereby increasing the difficulty of removing heavy metals.[Citation31] Many researchers concentrate on color reduction strategies at the starting point instead of at the final point of the pipe treatment methods. Different materials for adsorption have also been employed to remove contaminants. These adsorbents include sludge, fly ash, bagasse, saw dust, silica, peat, neem leaf powder, and activated carbon. However, these different techniques have practicable and economic impacts on the treatment process. Coagulation and flocculation procedures cannot remove chromophoric chemicals, resulting in a large volume of chemical sludge.[Citation29]
Pollutants like pesticides, heavy metals, dyes, bleaching agents, pharmaceuticals, personal care products, and many more have been identified as significant dangers to water supplies globally as shown in . [Citation32–136] The accumulation of suspended particles in pulp and paper wastewater is a key environmental issue.[Citation19,Citation40] The spectrum of sunlight that gets to aquatic plants can be diminished by suspended particles, which can also cover the surface of the water.[Citation41,Citation42] This may cause mortality of fishes and lower the productivity of aquatic ecosystems.[Citation43] Additionally, suspended particles may sink to the bottom of water bodies, suffocating benthic organisms.[Citation42] The high concentration of organic materials in pulp and paper wastewater is a significant environmental concern. Decomposing organic material might deplete the water oxygen supply. As a result, this limits the oxygen present for aquatic life to survive.[Citation44] Reduced oxygen levels may result in death of aquatic organisms and harm to aquatic ecosystems.[Citation45] Wastewater from pulp and paper mills may contain a range of inorganic substances, including chlorine and heavy metals, alongside suspended particles and organic materials.[Citation46] These substances have the potential to be hazardous to aquatic life as they bioabsorb into the food chain.[Citation46]
Table 1. Environmental pollutants in industrial sectors and their impacts.
Industrial Wastewater Treatment Techniques
Industrial wastewater treatment technology employs physical, chemical and biological techniques.[Citation47,Citation48] illustrates different wastewater treatment techniques, unit operations and processes. Individual wastewater treatment techniques can be merged into a range of processes to achieve various degrees of contamination removal, which can be categorized as the three stages of wastewater treatment: primary, secondary and tertiary. More stringent wastewater treatment includes removing specific pollutants as well as removing and controlling nutrients.[Citation48]
Several physical and chemical techniques, including sedimentation, membrane filtration, chemical oxidation and ozonation, have been documented, however because of their high operating costs and environmental drawbacks, they are not ideal for the treatment of effluent.[Citation49,Citation50] Apart from being expensive from the perspective of treatment, they also run the risk of escalating pollution and harm.[Citation51] Most contaminants in wastewater can be removed using biological processes, which are more environmentally friendly. For wastewater treatment, these techniques use plants, microorganisms, and enzymes.[Citation46,Citation49] Biological treatment is regarded as one of the best strategies for getting rid of persistent pollutants among the treatment approaches illustrated in . The biological method for the removal of color is particularly desirable because it decreases BOD along with low molecular weight chlorolignins in addition to color and COD.[Citation52] Biological treatment is a successful, economical substitute to chemical and physical alternatives. Research has been done extensively on employing fungi, bacteria, and their enzymes to decontaminate the effluent from pulp and paper industries. Newly isolated Bacillus altitudinis SL7 was employed for effective reduction of color and lignin content from pulp and paper mill effluent.[Citation50] Maximum laccase activity after 5 days of incubation showed that B. altitudinis SL7 reduced the color and lignin content by 26 and 44%, respectively. Degradation was at its best at pH 8.0 and 40°C. The results obtained demonstrated the oxidation of lignin polymer groups like coniferyl (G) and sinapyl (S).[Citation50]
Biocatalysts and Their Industrial Applications
Biocatalysis is the process of catalyzing chemical reactions using enzymes or related biological substances. Proteins called enzymes function as catalysts that accelerate chemical reactions without consuming any energy themselves.[Citation53] Because of their extreme specificity, each enzyme can only catalyze a single kind of reaction. They are therefore perfect for applications in industrial processes that call for high levels of specificity and selectivity.[Citation54] Water typically serves as the media for biocatalysis.[Citation55]
Complete organisms or their specific extracted enzymes can be utilized to biologically eliminate environmental pollutants.[Citation56] Applying entire organisms, plants, or bacteria to wastewater is a slow process.[Citation56] Organisms are susceptible to the severe conditions found in wastewater and may become inactive or disintegrate over time.[Citation56] Conversely, enzymes function effectively in the biocatalytic conversion of water-soluble chemicals of different origins, even when present in minute concentrations. They offer high activity and selectivity as well as rapid catalytic action. Enzymes are less susceptible to inhibition by hazardous chemicals that harm living organisms.[Citation46] Additionally, comparative evaluations of the different wastewater treatment methods reveal that enzymatic removal of pollutants reduces the negative environmental impact of the treatment process and its resulting products.[Citation56] Enzymatic treatment also necessitates the use of less harmful solvents, water and energy. Enzymes can be used as free (crude enzymes or refined enzymes) or immobilized biocatalysts, which affects the effectiveness of the bioremediation as well as the functionality and reusability of the biocatalysts.[Citation57] A well-designed enzymatic system minimizes enzyme consumption during wastewater treatment procedure and makes it easier to use biocatalytic reactors for continuous wastewater treatment.[Citation56]
Biocatalysts and Their Sustainability
A significant challenge in tackling environmental pollutants is ensuring the long-term viability of the method employed to eliminate the pollutants. An optimal methodology wastewater treatment is one that effectively eliminates or breaks down contaminants while preventing the production of hazardous byproducts. A process like this would be ideal if it could be easily modified to accommodate different types of pollutants, be efficient even at low concentrations of contaminants, and have a small amount of sludge or process effluent that is not hazardous.[Citation58] Enzymes have proven to be highly effective in treating both organic and inorganic pollutants, including those that are becoming increasingly concerning such as color, COD, or total organic carbon (TOC).[Citation59] The outcome is the total elimination of pollutants, or their breakdown into simpler compounds with limited hazard potential.[Citation59,Citation60] Enzymes can be used in the biodegradation of a wide variety of molecules with comparable structures because of their broad biocompatibility and specificity.[Citation61]
The elimination of pollutants is mostly accomplished by bioremediation using intracellular accumulation and/or biocatalytic transformation.[Citation61,Citation62] Biocatalysis is appealing because it allows for the engineering of enzymes to have greater durability and efficiency for certain substrates or conditions.[Citation63] Enzyme immobilization on neutral surfaces significantly lowers the possibility that dangerous compounds may develop after biocatalytic treatment.[Citation64] Metal oxides (such as iron, titanium, and aluminum oxides), natural polymers (such as agarose, agar, gelatin, collagen, carrageenan, and alginate), and synthetic polymers (such as polystyrene and polyacrylonitrile) can provide these inert surfaces.[Citation65] The likelihood that biocatalysts can be made insoluble to enable recycling and reusability increases the sustainability of biocatalytic bioremediation.[Citation66] Enzymes are primarily non-hazardous, biodegradable, and may be manufactured from readily available abundant resources. Most enzymatic reactions occur in water at ambient temperature and atmospheric pressure, typically without the need for activation, protection and deprotection stages.[Citation67] Isolated enzyme activities can be performed in traditional multipurpose batch reactors, eliminating the need for investing in high-pressure equipment.[Citation67] It is relatively simple to incorporate several transformations of contaminants into environmentally friendly and commercially successful cascade processes since enzymatic processes often occur under roughly the same pressure and temperature.[Citation58,Citation67]
Economic considerations should be made when thinking about the practical applications of enzymes since enzymes are very expensive. However, in addition to the expenses associated with producing and purifying the biocatalyst, the financial contribution of enzyme immobilization, if applicable, must also be considered.[Citation56] Additionally, consideration must be given to the costs of the biodegradation process, including reactor expenditures, reagent requirements, and energy consumption. Lastly, financial considerations should be made for the separation of resultant products from post-reaction mixtures as well as the expenses associated with using these products and detoxifying them. Predicting the exact monetary impact of the enzymatic treatment is difficult due to the wide range of variables and parameters that vary within a single operation. The overall costs of treating phenol with free manganese peroxidase were approximately 15 times less than those of immobilized enzyme, according to López et al..[Citation68] This is primarily due to the loss of enzyme activity caused by immobilization. Data from Abejón et al.[Citation69] demonstrated that the cost of laccase treatment for 1 m3 of antibiotic-containing wastewater is approximately 13 Euro, which is about 20 times more than the cost of photo-Fenton or electron beam remediation. This financial drawback might be mitigated by the sustainability of the technique and the low environmental impact of the final compounds.[Citation56] The global market demand for enzymes varies among the various nations, with enzyme production market having the largest share from 2015 to 2022.[Citation7] At a compound annual growth rate of 9.1%, the market was projected to increase from $9.4 billion in 2021 to $10.25 billion in 2022.[Citation70] It is anticipated to grow at a compound annual growth rate of 4% from 2023 to 2032, surpassing roughly US$ 15 billion.[Citation71]
Biocatalyst Groups for Environmental Pollutants Removal
Given that using microbes to break down pollutants can be slow and complicated, the last ten years have seen a major shift toward the extraction of microbial enzymes from cells and their applications in bioremediation of water pollutants.[Citation72] By changing the routes that lead to the breakdown of pollutants, biocatalysts accomplish chemical transformations and have an impact on biological reactions. The catalytic activity of the microbial enzyme, rather than the growth of a single bacterium in a contaminated environment, may be the basis for bioremediation, which is dependent on complete and relatively pure enzymes. To accomplish bioremediation in a soil deficient in nutrients, a refined enzyme may be used.[Citation73] Biotechnology uses two different processes, solid-state fermentation (SSF) and submerged fermentation (SmF), to produce a range of biochemical products.[Citation74] Since SmF is the primary method utilized in the commercial production of biocatalysts, most research have concentrated on the design and implementation of this type of bioreactor. On the other hand, SSF is a comparatively less expensive method of biocatalysts production and yields larger amounts of enzymes.[Citation75] SSF involves microorganisms developing on a solid substrate that has a low moisture content.[Citation76] Usually, this substrate is made of waste agricultural materials like rice husk or wheat bran. Benefits of this approach include decreased energy needs, lower chance of contamination, and higher yields of product because of improved control over microbial development. SSF does, however, have certain drawbacks, such as slower fermentation rates, trouble optimizing process variables, and issues increasing output volume.[Citation77] Caroca et al.[Citation78] found that Trametes versicolor and Pleurotus ostreatus exhibited maximum enzymatic activity for fungal growth, with the former producing 1590 U/L of laccase and the latter only 820 U/L of manganese peroxidase in 12 days of SSF. Both extractants demonstrated good enzymatic stability under a temperature condition of 17°C. However, only the enzymes that were recovered with phosphate buffer were able to degrade the micropollutant in the raw bean pod waste used as the growth medium. SSF increased the biodegradability of lignocellulosic residue, increasing the biogas output between 0% and 75%. The amount of biogas per volatile solid mass was reduced prior to treatment, due to high consumption of volatile solids during fungal growth.
On the contrary, SmF gives more control over process variables like pH, temperature, and availability of nutrients because it involves growing microorganisms in a liquid media.[Citation76] Higher biomass concentrations, quicker fermentation rates, simpler production monitoring and scaling up are all common benefits of this approach. The disadvantages to SmF include increased energy consumption, a higher chance of contamination, and the need for a clean environment, which can drive up production costs. Sharma et al.[Citation79] used a fungal strain of Cotylidia pannosa under SmF with wheat bran (2%) supplemented yeast extract peptone dextrose medium and after 56 h at 30°C and pH 5.0, with an agitation rate of 120 rpm and a saccharification value of 50.5%, they produced 8.48 U/mL of endocellulase. The ability to produce endocellulase decreased with additional temperature increase. It has been noted that at higher incubation temperatures, changes in the membrane composition led to protein catabolism and a decrease in the pace at which fungi grow. As a result, the decision between SSF and SmF is based on the needs of the fermentation process as well as the final product.[Citation77]
Biocatalysts have been produced using a variety of agro-industrial wastes as precursors.[Citation80] The immobilization of the source microbes has been the main method utilized to increase biocatalysts production.[Citation81] For benefit in multiple industrial applications, biocatalysts capable of withstanding unfavorable conditions like acidic or alkaline pH, higher temperatures, and high salt concentrations must be synthesized. This is because microbial enzymes are protein-based and therefore susceptible to changes in pH and temperature. Extremophilic microorganisms create biocatalysts appropriate for the hydrocarbon breakdown process.[Citation82] The manufacture of lipases, proteases, and ligninolytic enzymes from filamentous fungus and yeasts has been investigated by Wentzel et al..[Citation83] Numerous sectors currently benefit from the versatile applications of multi-copper oxidase enzymes or laccase produced by microbes like bacteria, fungus, and microalgae. Recombinant protein functions have been shown to increase productivity over shorter times. Using rDNA technology, horseradish peroxidase was isolated from the horseradish plant and introduced into E. Coli BL21. This allowed horseradish peroxidase to be produced in greater quantities and to break down phenolic compounds. Similarly, pesticides and carbamate compounds could be broken down by carboxylesterase that was isolated from human liver and added to E. Coli.[Citation84] The different biocatalyst groups are illustrated in , based on their respective levels of activity in the removal of pollutants.
The most widely utilized groups of enzymes for environmental applications are oxidoreductases including laccases, oxygenases and peroxidases, and hydrolases which include lipases, cellulases and proteases. A wide range of environmental pollutants, such as pharmaceuticals, dyes, personal care items, hormones, pesticides, phenol and its derivatives, can be treated by different oxidoreductases because of their broad substrate specificity, while lipases alongside other hydrolases can effectively convert oils, greases and plastics.[Citation56,Citation85] Because of their limited substrate specificity, laccase and tyrosinase (oxygenase) mostly catalyze the oxidation of monophenols, diphenols, and polyphenols, alongside aromatic amines, and diamines. Their catalytic activity, however, differs greatly. Laccase produces phenoxy free radicals throughout the course of the reaction, while tyrosinase produces quinone intermediates during oxidation. Peroxidases can also convert a wide variety of substances, including monomeric and dimeric phenols, aromatic phenols, and non-phenolic chemicals such as pharmaceuticals, coloring agents, as well as xenobiotics, using free radicals as intermediates.[Citation86] Although free radicals generated during the process of reaction may be more hazardous than the original chemicals, these secondary compounds react with one another promptly. As a result of the reaction, they generate different combinations of C–C and C–O bonds, formin dimers, trimers, and oligomers of the parent compounds.[Citation87] The oligomeric products are distinguished by their markedly reduced toxicity in comparison to the original pollutants. They can be precipitated and centrifuged to separate them from the post-reaction mixture, making it easier to acquire an undiluted stream of the resultant solution.[Citation56] Hydrolases are involved in several related processes, including condensations and hydroxylation, and they accelerate the breaking of C-O, C-C, C-N, S-P, S-S, S-N, C-P, and other bonds by H2O. By using water to break down chemical connections, hydrolases can lessen the toxicity of larger pollutant molecules by reducing their size.[Citation88] Lipases, cellulases, proteases, carboxylesterases, haloalkane dehalogenases, phosphotriesterases, and other enzymes belonging to the hydrolase family are highly useful in the treatment of oil-contaminated soils, biofilm deposits, and the management of wastes from food processing. They can also be used in the degradation of plastic and insecticides.[Citation89]
Laccases and Their Applications
Laccases are a group of multi-copper oxidases which are present in several fungi, bacteria, insects, higher plants, and various microorganisms.[Citation90] They are one of the earliest classes of enzymes (benzenediol: oxygen oxidoreductase, EC 1.10.3.2) identified by Yoshida in 1883.[Citation91] Laccases are composed of four copper atoms that are categorized into three categories based on UV-visible and electron paramagnetic resonance spectroscopy: Type 1 (T1), also known as the blue site, Type 2 (T2), also known as the normal site, and Type 3 (T3), also known as the binuclear site.[Citation91] They function as biocatalysts in the oxidation of various mitigated aromatic as well as reduced phenolic substrates. The biological oxygen degradation to water is a byproduct of their catalytic activity.[Citation90] The mechanism of pollutant removal by laccase involves a catalyzed oxidation reaction in three phases: (i) The substrate is oxidized by binding to the active site of the laccase, losing an electron to form a radical. T1 copper is reduced by accepting the electron from the substrate. The radicals then undergo further reactions like self-coupling or polymerization to form less hazardous and easily removable compounds. (ii) The electrons are afterward transferred from T1 to T2/T3 trinuclear cluster. (iii) Lastly, the electrons at the T2/T3 trinuclear cluster reduces molecular oxygen to water.[Citation91] illustrates a summary of the oxidation of phenolic substrates catalyzed by laccase.[Citation92] Laccases are useful enzymes employed in a variety of industries, including bioremediation, food, pulp and paper, dye decolorization, biocells and biosensors.[Citation93] The most well-known properties of these enzymes are their capacity to break down a variety of phenolic and non-phenolic substrates, as well as long-lasting environmental contaminants by the reduction of molecular oxygen to water.[Citation94] Typically, laccases are found in various isoenzyme forms, each of which is distinguished by a distinct gene.[Citation95] It is typical for the genes to express themselves differently depending on the type of inducer.[Citation95]
Figure 5. Mechanism of phenolic substrates oxidation catalyzed by laccase[Citation92].
![Figure 5. Mechanism of phenolic substrates oxidation catalyzed by laccase[Citation92].](/cms/asset/3aa8ad49-4aba-4d52-b8f5-3a4c35ed5e49/lspr_a_2382313_f0005_oc.jpg)
The oxidation of phenolic compounds like polyphenols, aminophenols, orthodiphenols, paradiphenols, polyamines, aryl diamines, lignin and other inorganic ions can be catalyzed by the intracellular and extracellular laccases secreted by a variety of microorganisms. They are capable of decarboxylation and demethylation in addition to oxidizing phenolic and methoxy-phenolic acids.[Citation92] These enzymes have a role in the depolymerization of lignin, which produces a range of phenols. Moreover, laccase either repolymerizes these substrates to compounds that are humic or uses the substrates as food for microbes.[Citation92] Aniline, hydroxide and cyanide are examples of reagents that impede the catalytic activity of laccases and alter their substrate affinity and specificity in response to pH variations.[Citation96]
A crucial part of oxygen reduction is played by laccase and its active sites involving copper ions. One of the main elements influencing the rate of oxidation is the difference in redox potential (Eo) between the substrate and the T1 copper site. As a result, laccases are divided into three groups based on their redox potential: low (0.4–0.5 V), medium (0.5–0.6 V), and high (0.7–0.8 V).[Citation97] The range of redox potentials correlates with the sources, namely fungi, bacteria, or plants, due to variations in the composition of amino acid residues surrounding the copper of the initial reaction site. The highest potential is exhibited by laccase from white rot fungi, which has an affinity for phenylalanine to function as the unstable axial binding agent at the T1 copper site (0.73–0.79 V).[Citation98] Laccase has been identified in ascomycetes and basidiomycetes fungal species. Several white rot basidiomycetes, like Trametes versicolor, Pleurotus ostreatus and Cerrena unicolor, are also effective lignin degradation enzymes.[Citation3]
Multiple support materials with distinct chemical and nanostructure properties have been investigated. Nanoparticles have garnered more interest due to their increased surface area for enzyme attachment.[Citation99] Owing to entrainment or adsorption, the depletion of the enzyme-substrate complex can be avoided by using functionalized microporous substrates like membranes.[Citation99] The immobilization of nanoparticles, including zinc oxide, titanium dioxide, copper oxide, and iron oxide, onto the polymeric membrane have been investigated for the purpose of eliminating environmental pollutants and conducting anti-biofouling studies.[Citation100–103] presents the numerous studies on industrial effluents that have demonstrated the efficiency of laccase in eliminating difficult-to-remove contaminants.[Citation137–141]
Table 2. Application of laccase as a biocatalyst on different industrial effluents.
Applications of Free Biocatalysts
The languid process of applying plants and microorganisms in bioremediation encourages the use of isolated microbial enzymes from entire cells to expedite the process.[Citation104] There are two types of free enzymatic systems that can remove pollutants: crude enzymes and purified enzymes. There are three crude enzymes derived from white-rot fungal cultures, namely laccase, lignin peroxidase and manganese peroxidase. The most widely used bioremediation enzymes are laccase and horseradish peroxidase, which perform best at a pH 7 and temperature 25–40°C. This is because they oxidize a variety of contaminants by taking recourse to their low specificity for their substrates.[Citation105] While both systems are capable of efficiently transforming dangerous substances, there are benefits and drawbacks to each of their strategies. Using unpurified enzymes instead of refined enzymes and skipping the purification step is less expensive. The extract of crude oxidoreductase enzymes may also include mediators, which raise the enzyme redox potential and broaden the range of environmental pollutants that can be transformed.[Citation106] The biodegradation rate of laccase is enhanced up to 40% when mediators such syringaldehyde, 1-hydroxybenzotriazole, or 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid are present in comparison to the isolated, purified enzyme.[Citation105] In contrast, the use of purified enzymes has several benefits, such as more effective and focused pollution remediation, a decreased risk of inhibition, and high activity throughout a wide variety of procedure parameters.[Citation107] Purified enzymes are more accustomed to the ideal conditions for enzyme reactions, so they have fewer side effects, and are typically used to improve the control of the process.[Citation108] Finding a balance that considers enzyme price, purity, activity, and capacity to convert a wide spectrum of dangerous chemicals is therefore necessary for economic wastewater treatment.
Several studies have shown that ligninolytic enzymes, both crude and refined, can eliminate contaminants in both batch and continuous processes. shows a summary of the removal of pollutants from industrial wastewater using free enzymes.[Citation142–151] Using P. chrysosporium crude extract, Li et al.[Citation109] removed almost 90% of naproxen (starting concentration 10 mg/L) in 2 days. This was shown to be superior to whole-cell treatment, which only removed 68% after 2 days. The outcomes showed that the extracellular enzymes of P. chrysosporium had a significant impact on naproxen elimination. An investigation involving laccase-assisted biodegradation revealed that laccase generated by Trametes versicolor also contributed to the elimination of naproxen, resulting in nearly total breakdown in 5 h.[Citation110] The quantitative degradation efficiency of laccase isolated from Trametes versicolor and Streptomyces cyaneus microbes for mefenamic acid and diclofenac was investigated by Margot et al..[Citation111] Fungal laccase was found to be more effective than bacterial laccase under typical municipal wastewater settings (10–25°C and neutral pH), resulting in the rapid breakdown of both pharmaceutical contaminants. Mefenamic acid and diclofenac were completely eliminated by fungal laccase after a 12-day period of incubation, however bacterial laccase was able to destroy roughly half of both pollutants in the same 12-day time. Laccase removed 78% of the tetracycline after 18 h, whereas esterase removed 50% of the erythromycin after 16 h.[Citation112]
Table 3. Bioremediation of pollutants by free biocatalysts.
However, difficulties with bioremediation when utilizing free enzymes may arise in certain situations. An enzyme can become more potent than the problematic contaminant, making it a bigger environmental risk. The purification and extraction method for solving this issue is costly and time-consuming because enzymes are potentially unstable and quickly disintegrate in a hostile environment devoid of organisms. Enzyme waste also happens throughout the bioremediation procedure.[Citation58] One common solution to these problems is enzyme immobilization.
Application of Immobilized Biocatalysts
When free enzymes are employed in bioremediation processes, they demonstrate high activity. However, because enzymatic recovery post bioremediation is impossible, large-scale application of free enzymes is constrained by their low stability and high production costs.[Citation113] To solve the cost concerns for large-scale effluent treatment, several strategies have been developed.[Citation114] These include applying ultra-filtration membranes to prevent enzyme loss with effluent, immobilizing biocatalysts onto various matrices for simple enzyme extraction from the treated wastewater samples and producing enzymes during treatment by growing microorganisms on cost-effective feedstocks.[Citation115] Enzyme immobilization on solid supports has been shown in multiple studies to increase stability and activity.[Citation3,Citation116] Biocatalyst immobilization is the process of transforming a soluble biocatalyst to an insoluble state by binding it to an insoluble substrate or encasing it in a synthetic or natural matrix. It limits the mobility of an enzyme or microbial cell without affecting its ability to perform catalytic tasks.[Citation117] Immobilization generally improves enzymatic stability, resistance, reusability, activity, selectivity, improves separation and purification and overall efficiency.[Citation3] Catalytic properties of immobilized enzymes can be maintained for extended time periods. The protective and supportive structure of immobilizer materials aids preservation of enzyme properties across broader pH and temperature ranges.[Citation118] Immobilized enzymes demonstrate resilience to inhibitory effects from various components in wastewater. Immobilized enzymes also improve the process efficiency and the purity of the finished product.[Citation119] However, because of structural conformational changes and heterogeneity on the immobilization support, immobilized biocatalysts may become less active than their free form in some cases.[Citation120] The immobilization procedure affects the properties of the enzyme; hence the choice of the immobilization methodology controls the cost of the enzyme, biocatalytic functionality, and inactivation dynamics.[Citation115,Citation121,Citation122] Recent studies describing the biodegradation of different pollutants by immobilized biocatalytic systems have been summarized in .[Citation152–158]
Table 4. Bioremediation of pollutants by immobilized horseradish or laccase biocatalysts.
Drawbacks of immobilizing enzymes include a possible lower activity levels than native enzymes. The stability of an enzyme may be decreased by improper immobilization. Also, the matrix may interact with the enzyme and impede the reaction. Reactants may be prevented from accessing the reactive enzyme sites. In cases where a substrate is insoluble, immobilization may not be the optimal solution.[Citation58] One significant observation is the sharp decline in the rapid activity of native enzymes, like proteases or amylases, following immobilization due to diffusion restrictions. This decreases the immobilized enzyme appeal from an economic standpoint.[Citation123]
Immobilization methods include entrapment, encapsulation, covalent bonding, adsorption, and crosslinking as presented in .[Citation124] A comparison of the benefits and challenges of these immobilization methods is presented in .[Citation58,Citation159,Citation160] The process of immobilization, which involves a chemical reaction with the enzyme molecule, results in minimal enzyme deactivation and stable attachment. Because of covalent bonding, the native enzyme structure of the enzyme is altered, and this may sometimes affect its biocatalytic activity.[Citation125] Entrapment and adsorption techniques generally preserve the structure of the enzyme, but they provide less stability during catalytic activity.[Citation115] The best materials to support environmental wastewater treatment applications are inorganic and hybrid.[Citation126] Selecting the right support, a highly porous material that has a large surface area, can also help to remove pollutants because of simultaneous adsorption and biocatalytic conversion.[Citation113]
Table 5. Benefits and challenges of various immobilization techniques.
Haq and Kalamdhad[Citation127] employed Pseudomonas putida strain immobilized by entrapment on a mixture of polyvinyl alcohol with sodium alginate (PVA-SA) and agar-agar (AA) for effectively treating paper industry wastewater. After 6 days of degradation, the bacterial culture immobilized in PVA-SA polymer effectively eliminated BOD5, COD, TOC and total Kjeldahl nitrogen (TKN), as well as phenol, lignin, and color. The percentages were 69.6%, 78.6%, 81.7%, and 88.0%, 98.4%, 65.6%, and 85.7%, respectively, with 24.8 IU/mL of Lac activity. Pollutant levels decreased by 61.0%, BOD5, 75.9%, COD, 76.5%, TKN, 86.0%, TOC, 97–9.9%, phenol, 62.3%, lignin, and 89.8%, respectively, with 18.5 IU/mL Lac activity when PVA-AA immobilized bacteria were employed. Overall, the results indicated that the bacterial immobilization was highly effective in degrading the pollutants of the paper industry effluent. The reason for this is that PVA-SA may have securely attached bacterial cells more than PVA-AA due to its unstable structures. The strength of the biocarrier matrix was increased by adding PVA to the SA.[Citation127] Enzyme immobilization has been very successful in the treatment of other industrial wastes apart from the paper industry effluents. Homaei[Citation128] reported that Penaeus merguiensis alkaline phosphatase immobilized on gold nanorods for heavy metal detection exhibited outstanding adsorption through both hydrophobic and ionic interactions. The process of immobilization involved combining a suspension of gold nanorods at room temperature with a relative concentrate solution of the enzyme, continuously agitated at 200 rpm for 4 h. The significance of gold nanorods as an immobilization support for alkaline phosphatase is highlighted by the combined effects of a wider temperature and pH profile, increased thermal stability as well as stability at critical pHs, and improved storage qualities.
The immobilized enzymes showed similar biocatalytic behavior when compared to the kinetic parameters of free enzymes, as seen by their similar Km, Kcat, and Vmax values. This suggests that immobilization served to preserve the active structure of the enzyme from disruption and improved the thermal stability of alkaline phosphatase. Naghdi et al.[Citation129] degraded carbamazepine in spiked water and secondary effluent by immobilizing 25 mg of laccase on carboxyl functionalized nanobiochars. The immobilized laccase performed better than free laccase in terms of thermostability by retaining 35–42% its initial activity between 50 and 70°C; the free laccase was unable to retain < 11% of its initial activity. In terms of pH stability, immobilized laccase maintained roughly 36% of its activity at pH > 8 whereas free laccase lost nearly all its activity at same pH level. With the contributions of biodegradation exceeding 45% and adsorption below 35%, approximately 85% of the pollutant degradation was attained. Cross-linked enzyme particles with or without support may be utilized to mitigate costs and diffusional limitations.
In a study by Kumar and Cabana,[Citation130] the suitability of a laccase-based bioprocess for treating a combination of 13 selected pharmaceuticals was examined. Amine functionalized magnetic NPs were used to immobilize laccase as cross-linked enzyme aggregates (MAC-CLEAs) using chitosan/1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride as the cross-linking mechanism. In ideal circumstances, the activity recovery of laccase was 61.4% for the creation of MAC-CLEAs. Almost complete removal efficiency was seen for acetaminophen (97%), atenolol (90%), epoxy-carbamazepine (93%), and diclofenac (95%), at a contact time of 12 h. In addition, the pharmacological transformation efficiency of ketoprofen (48%), trimethoprim (60%), and diazepam (68%), was significant. The presence of an aromatic amine in the chemical structures of mefenamic acid, acetaminophen, and diclofenac may have contributed to their high removal rates by lowering their redox potential and making them more susceptible to oxidation by laccase. Another study by Primožič et al.[Citation131] showed that 93% of the diclofenac was eliminated from wastewater composed of synthetic materials using two forms of immobilization: CLEAs and magnetic CLEAs. The mCLEAs laccase showed superior thermal stability and remained more stable over time, while having a lower diclofenac removal capability than CLEA laccase. However, this indicates that both immobilized laccase forms have the potential to be employed in cleaner industrial practices for the elimination of environmental pollutants. These studies further demonstrate the practical potential of enzyme systems.
Using immobilized enzymes effectively and sustainably in wastewater treatment involves establishing robust enzyme-support bonds and minimizing enzyme elution. Accordingly, covalent immobilization is most frequently used with a variety of organic and inorganic supports, typically modified by carbonyl, amine, or hydroxyl groups.[Citation126] Trametes hirsuta laccase was covalently immobilized onto a specially designed PVDF membrane by Masjoudi et al.,[Citation32] which allowed pharmaceuticals such as carbamazepine and diclofenac to be removed. The immobilized nanobiocatalyst exhibited a notable improvement in thermal and operational stability compared to the soluble enzyme, as well as excellent catalytic efficiency and recuperated activity. In a mini-membrane reactor, immobilized laccase eliminated 27% of carbamazepine and 95% of diclofenac in 2 days. Yanto et al.[Citation132] observed that adding 1 g of Trametes hirsuta laccase immobilized on light enlarged clay aggregates to 100 mL of wastewater resulted in complete decolorization efficiency of the batik wastewater in 1 h. At the end of four consecutive cycles, over 95% of the decolorization efficiency was retained. It is important to emphasize that the enzyme stable, covalent binding to the support was the reason for the limited enzyme discharge from the matrix during storage and repeated use in these investigations.
Enzyme Reusability
The reusability of enzymes is a crucial component for economic feasibility and ultimately broad adoption of its use in industrial wastewater applications.[Citation17] For long-term reusability, choosing enzymes with inbuilt stability within wastewater conditions is essential. Wastewater treatment applications can benefit from the enhanced stability that extremophiles exhibit against fluctuations in salinity, pH and temperature. Maximizing reusability requires streamlining wastewater treatment procedures to reduce enzyme inactivation. The efficacy of immobilized laccases in removing different pollutants and their potential for reuse under varying operating conditions are summarized in .[Citation99,Citation161–164] Enzyme lifespan can be greatly increased by elements like pH regulation, inhibitor removal, and a suitable reactor design. It is occasionally possible to reverse the inactivation of enzymes caused by inhibitor exposure or adverse environments.[Citation133] Enzyme treatments or specific reagent washings are examples of regeneration techniques that can restore enzyme activity and enable further use.[Citation58]
Table 6. Different immobilized laccase efficiency for pollutants removals and their reusability potential.
CURRENT CHALLENGES AND FUTURE OPPORTUNITIES
Compared to traditional techniques, enzymes have a number of benefits, such as high specificity, mild operating conditions, and environmental friendliness. The widespread use of biocatalysts in the treatment of industrial wastewater is still hampered by various challenges despite their potential. These challenges include:
In harsh environments, such as high temperatures, low pH levels, and the presence of inhibitors, enzymes can become inactivated. Their applicability in actual wastewater treatment processes is limited by this instability.
Enzymes can be expensive to produce and purify, which makes it difficult to economically employ them for large-scale industrial wastewater treatment. For biocatalysis to be economically viable, efficient techniques for enzyme recovery and reuse are essential. Nevertheless, creating recycling plans that are both scalable and successful continues to be difficult.
Enzymes have a high specificity, but not all pollutants found in various industrial wastewaters will be broken down by them. Enzyme customization to particular wastewater compositions necessitates continuous research and development.
It is frequently necessary to adjust and modify operational procedures and infrastructure in order to integrate biocatalytic processes into current wastewater treatment plants. This integration may be expensive and time-consuming.
Several studies have demonstrated the significant potential of enzymatic processes in wastewater treatment, as previously noted. Further research is therefore required to investigate how effectively enzymes perform with different types of industrial wastewater. These future opportunities include:
Enzyme engineering: The development of more stable, active, and economically viable enzymes for wastewater treatment appears to be possible thanks to advancements in enzyme engineering techniques like directed evolution and protein design.[Citation134]
Immobilization techniques: Enzymes can be made more stable, reusable, and inhibitor-resistant by immobilizing them on solid supports. Innovative immobilization techniques are being created to meet the unique requirements of treating industrial wastewater.[Citation88]
Enzyme discovery and metagenomics: Metagenomics enables the identification of new enzymes from various environmental sources, which may result in the discovery of enzymes with improved wastewater treatment characteristics.[Citation135]
Reactor design and process optimization: The overall effectiveness and financial viability of biocatalytic wastewater treatment systems can be raised by creating effective reactor designs and streamlining process parameters.[Citation136]
Financial incentives and regulatory support: By offering monetary incentives and regulatory assistance to promote research and development in this field, governments and industries can contribute to the adoption of biocatalysts.
Despite these challenges, biocatalysts have a promising future in the treatment of paper industrial wastewaters. More resilient, affordable, and adaptable enzymes should be created as research and development advances on, increasing their appeal for use in large-scale industrial wastewater treatment applications.
CONCLUSIONS
This review emphasizes the potential of free and immobilized biocatalysts, especially laccase enzymes, for the treatment of industrial wastewater, notably that from paper mills. Enzyme immobilization enhances cost-effectiveness, reusability, and stability for large-scale applications. To maximize the application of immobilized enzymes and evaluate their efficacy in removing priority pollutants, further research is required. Crucial steps include optimizing reactor designs, formulating biocatalysts, and doing techno-economic analyses. By addressing these issues, biocatalysis may become a viable and sustainable technique for treating wastewater, encouraging ethical industrial practices and a cleaner environment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Palani, G.; Arputhalatha, A.; Kannan, K.; Lakkaboyana, S. K.; Hanafiah, M. M.; Kumar, V.; Marella, R. K. Current Trends in the Application of Nanomaterials for the Removal of Pollutants from Industrial Wastewater Treatment—A Review. Molecules 2021, 26(9), 2799. DOI: 10.3390/molecules26092799.
- Kumar, M.; Ngasepam, J.; Dhangar, K.; Mahlknecht, J.; Manna, S. Critical Review on Negative Emerging Contaminant Removal Efficiency of Wastewater Treatment Systems: Concept, Consistency and Consequences. Bioresour. Technol 2022, 352, 127054. DOI: 10.1016/j.biortech.2022.127054.
- Sa, H.; Michelin, M.; Tavares, T.; Silva, B. Current Challenges for Biological Treatment of Pharmaceutical-Based Contaminants with Oxidoreductase Enzymes: Immobilization Processes, Real Aqueous Matrices and Hybrid Techniques. Biomolecules 2022, 12(10), 1489. DOI: 10.3390/biom12101489.
- Osode, A. N.; Okoh, A. I. Impact of Discharged Wastewater Final Effluent on the Physicochemical Qualities of a Receiving Watershed in a Suburban Community of the Eastern Cape Province. CLEAN Soil Air Water. 2009, 37(12), 938–944. DOI: 10.1002/clen.200900098.
- Edokpayi, J. N.; Odiyo, J. O.; Durowoju, O. S. Impact of Wastewater on Surface Water Quality in Developing Countries: A Case Study of South Africa. In Water Quality, Tutu, H., and Grover, P. B., Eds.; 2017; pp. 401–416. Croatia: IntechOpen.
- Munir, H. S.; Feroze, N.; Ikhlaq, A.; Kazmi, M.; Javed, F.; Mukhtar, H. Removal of Colour and Cod from Paper and Pulp Industry Wastewater by Ozone and Combined Ozone/UV Process. Desalin. Water. Treat 2019, 137, 154–161. DOI: 10.5004/dwt.2019.23186.
- Sai Preethi, P.; Hariharan, N. M.; Vickram, S.; Rameshpathy, M.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Yadav, V.; Ravindran, B.; Chang, S. W., et al. Advances in Bioremediation of Emerging Contaminants from Industrial Wastewater by Oxidoreductase Enzymes. Bioresour. Technol 2022, 359, 127444. DOI: 10.1016/j.biortech.2022.127444.
- Kumar, V.; Bansal, V.; Madhavan, A.; Kumar, M.; Sindhu, R.; Awasthi, M. K.; Binod, P.; Saran, S. Active Pharmaceutical Ingredient (Api) Chemicals: A Critical Review of Current Biotechnological Approaches. Bioengineered 2022, 13(2), 4309–4327. DOI: 10.1080/21655979.2022.2031412.
- Pindihama, G.; Gumbo, J.; Oberholster, P. J. Evaluation of a Low Cost Technology to Manage Algal Toxins in Rural Water Supplies. Afr. J. Biotechnol 2011, 10(86), 19883–19889. https://academicjournals.org/journal/AJB/article-full-text-pdf/EE244A536858/.
- Edokpayi, J.; Odiyo, J.; Popoola, O.; Msagati, T. Assessment of Trace Metals Contamination of Surface Water and Sediment: A Case Study of Mvudi River, South Africa. Sustainability 2016, 8(2), 135. DOI: 10.3390/su8020135.
- Edokpayi, J. N.; Nkhumeleni, M.; Enitan-Folami, A. M.; Olaniyi, F. C. Water Quality Assessment and Potential Ecological Risk of Trace Metals in Sediments of Some Selected Rivers in Vhembe District, South Africa. Phys. Chem. Earth, Parts A/B/C 2022, 126, 103111. DOI: 10.1016/j.pce.2022.103111.
- Chen, X.; He, B.; Feng, M.; Zhao, D.; Sun, J. Immobilized Laccase on Magnetic Nanoparticles for Enhanced Lignin Model Compounds Degradation. Chin. J. Chem. Eng 2020, 28(8), 2152–2159. DOI: 10.1016/j.cjche.2020.02.028.
- Rajmohan, K. S.; Chandrasekaran, R.; Varjani, S. A Review on Occurrence of Pesticides in Environment and Current Technologies for Their Remediation and Management. Indian J. Microbiol 2020, 60(2), 125–138. DOI: 10.1007/s12088-019-00841-x.
- Mishra, B.; Varjani, S.; Agrawal, D. C.; Mandal, S. K.; Ngo, H. H.; Taherzadeh, M. J.; Chang, J.-S.; You, S.; Guo, W. Engineering Biocatalytic Material for the Remediation of Pollutants: A Comprehensive Review. Environ. Technol. Innov 2020, 20, 101063. DOI: 10.1016/j.eti.2020.101063.
- Sharma, D.; Chaudhary, R.; Kaur, J.; Arya, S. K. Greener Approach for Pulp and Paper Industry by Xylanase and Laccase. Biocatal. Agric. Biotechnol 2020, 25(25), 101604. D OI: d oi:1 0.1016/j.bcab.2020.101604.
- Chaturvedi, P.; Giri, B. S.; Shukla, P.; Gupta, P. Recent Advancement in Remediation of Synthetic Organic Antibiotics from Environmental Matrices: Challenges and Perspective. Bioresour. Technol 2021, 319, 124161. DOI: 10.1016/j.biortech.2020.124161.
- Zhou, W.; Zhang, W.; Cai, Y. Laccase Immobilization for Water Purification: A Comprehensive Review. Chem. Eng. J 2021, 403, 126272. DOI: 10.1016/j.cej.2020.126272.
- Pekgenc, E.; Yavuzturk Gul, B.; Vatanpour, V.; Koyuncu, I. Biocatalytic Membranes in Anti-Fouling and Emerging Pollutant Degradation Applications: Current State and Perspectives. Sep. Purif. Technol 2022, 282, 120098. DOI: 10.1016/j.seppur.2021.120098.
- Covinich, L. G.; Bengoechea, D. I.; Fenoglio, R. J.; Area, M. C. Advanced Oxidation Processes for Wastewater Treatment in the Pulp and Paper Industry: A Review. Am. J. Environ. Eng 2014, 4(3), 56–70. DOI: 10.5923/j.ajee.20140403.03.
- Branco, R.; Serafim, L.; Xavier, A. Second Generation Bioethanol Production: On the Use of Pulp and Paper Industry Wastes as Feedstock. Fermentation 2018, 5(1), 4–9. DOI: 10.3390/fermentation5010004.
- Bajpai, P. Management of Pulp and Paper Mill Waste, 1st ed.; Springer: Switzerland, 2015; Vol. 431, p. 1.
- Majeke, B. M.; Collard, F. X.; Tyhoda, L.; Gorgens, J. F. The Synergistic Application of Quinone Reductase and Lignin Peroxidase for the Deconstruction of Industrial (Technical) Lignins and Analysis of the Degraded Lignin Products. Bioresour. Technol 2021, 319, 124152. DOI: 10.1016/j.biortech.2020.124152.
- Kumar, V.; Thakur, I. S.; Shah, M. P. Bioremediation Approaches for Treatment of Pulp and Paper Industry Wastewater: Recent Advances and Challenges. Microb. Biorem. Biodegrad. 2020, 1–48. DOI: 10.1007/978-981-15-1812-6_1.
- Abdelaziz, O. Y.; Brink, D. P.; Prothmann, J.; Ravi, K.; Sun, M.; García-Hidalgo, J.; Sandahl, M.; Hulteberg, C. P.; Turner, C.; Lidén, G., et al. Biological Valorization of Low Molecular Weight Lignin. Biotechnol. Adv 2016, 34(8), 1318–1346. DOI: 10.1016/j.biotechadv.2016.10.001.
- Patil, S. V.; Argyropoulos, D. S. Stable Organic Radicals in Lignin: A Review. ChemSuschem 2017, 10(17), 3284–3303. DOI: 10.1002/cssc.201700869.
- Rajput, H.; Changotra, R.; Kumar Sangal, V.; Dhir, A. Photoelectrocatalytic Treatment of Recalcitrant Compounds and Bleach Stage Pulp and Paper Mill Effluent Using Au-Tio2 Nanotube Electrode. Chem. Eng. J 2021, 408, 127287. DOI: 10.1016/j.cej.2020.127287.
- Gavrilescu, D.; Puiȋel, A. Zero Discharge: Technological Progress Towards Eliminating Pulp Mill Liquid Effluent. Environ. Eng. Manage. J 2007, 6(5), 431–439. DOI: 10.30638/eemj.2007.053.
- Gadekar, M. R.; Ahammed, M. M. Use of Water Treatment Residuals for Colour Removal from Real Textile Dye Wastewater. Appl. Water. Sci 2020, 10(7), 160. DOI: 10.1007/s13201-020-01245-9.
- Kumar, A.; Srivastava, N. K.; Gera, P. Removal of Color from Pulp and Paper Mill Wastewater- Methods and Techniques- a Review. J. Environ. Manage 2021, 298, 113527. DOI: 10.1016/j.jenvman.2021.113527.
- Jun, L. Y.; Yon, L. S.; Mubarak, N. M.; Bing, C. H.; Pan, S.; Danquah, M. K.; Abdullah, E. C.; Khalid, M. An Overview of Immobilized Enzyme Technologies for Dye and Phenolic Removal from Wastewater. J. Environ. Chem. Eng 2019, 7(2), 102961. DOI: 10.1016/j.jece.2019.102961.
- Sharma, P.; Bano, A.; Yadav, S.; Singh, S. P. Biocatalytic Degradation of Emerging Micropollutants. Top. Catal 2023, 66(9–12), 676–690. DOI: 10.1007/s11244-023-01790-y.
- Masjoudi, M.; Golgoli, M.; Ghobadi Nejad, Z.; Sadeghzadeh, S.; Borghei, S. M. Pharmaceuticals Removal by Immobilized Laccase on Polyvinylidene Fluoride Nanocomposite with Multi-Walled Carbon Nanotubes. Chemosphere. 2021, 263, 128043. DOI: 10.1016/j.chemosphere.2020.128043.
- Chaturvedi, P.; Shukla, P.; Giri, B. S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and Hazardous Impact of Pharmaceutical and Personal Care Products and Antibiotics in Environment: A Review on Emerging Contaminants. Environ. Res 2021, 194, 110664. DOI: 10.1016/j.envres.2020.110664.
- Vieira, W. T.; de Farias, M. B.; Spaolonzi, M. P.; da Silva, M. G. C.; Vieira, M. G. A. Removal of Endocrine Disruptors in Waters by Adsorption, Membrane Filtration and Biodegradation. a Review. Environ. Chem. Lett 2020, 18(4), 1113–1143. DOI: 10.1007/s10311-020-01000-1.
- Grötzner, M.; Melchiors, E.; Schroeder, L. H.; dos Santos, A. R.; Moscon, K. G.; de Andrade, M. A.; Martinelli, S. H. S.; Xavier, C. R. Pulp and Paper Mill Effluent Treated by Combining Coagulation-Flocculation-Sedimentation and Fenton Processes. Water. Air Soil Pollut 2018, 229(11), 364. DOI: 10.1007/s11270-018-4017-5.
- Motamedi, E.; Kavousi, K.; Sadeghian Motahar, S. F.; Reza Ghaffari, M.; Sheykh Abdollahzadeh Mamaghani, A.; Hosseini Salekdeh, G.; Ariaeenejad, S. Efficient Removal of Various Textile Dyes from Wastewater by Novel Thermo-Halotolerant Laccase. Bioresour. Technol 2021, 337, 125468. DOI: 10.1016/j.biortech.2021.125468.
- Evans, A. E. V.; Mateo-Sagasta, J.; Qadir, M.; Boelee, E.; Ippolito, A. Agricultural Water Pollution: Key Knowledge Gaps and Research Needs. Curr. Opin. Environ. Sustain 2019, 36, 20–27. DOI: 10.1016/j.cosust.2018.10.003.
- Yin, H.; Ma, J.; Li, Z.; Li, Y.; Meng, T.; Tang, Z. Polybrominated Diphenyl Ethers and Heavy Metals in a Regulated E-Waste Recycling Site, Eastern China: Implications for Risk Management. Molecules 2021, 26(8), 2169. DOI: 10.3390/molecules26082169.
- Wu, W.; Wu, P.; Yang, F.; Sun, D. L.; Zhang, D. X.; Zhou, Y. K. Assessment of Heavy Metal Pollution and Human Health Risks in Urban Soils Around an Electronics Manufacturing Facility. Sci. Total Environ 2018, 630, 53–61. DOI: 10.1016/j.scitotenv.2018.02.183.
- Ateş, S.; Ateş, E.; Yazici Guvenc, S.; Can-Güven, E.; Aydın, S.; Varank, G. Removal of Cod, Phenol, and Colour from Olive Mill Wastewater by Iron-Activated Persulphate Process: Multivariate Optimisation Approach. J. Environ. Anal. Chem. 2022, 2022, 1–23. DOI: 10.1080/03067319.2022.2078202.
- Fayiga, A. O.; Ipinmoroti, M. O.; Chirenje, T. Environmental Pollution in Africa. Environ. Dev. Sustain 2017, 20(1), 41–73. DOI: 10.1007/s10668-016-9894-4.
- Gita, S.; Hussan, A.; Choudhury, T. Impact of Textile Dyes Waste on Aquatic Environments and Its Treatment. Environ. Ecol 2017, 35, 2349–2353.
- Kasonga, T. K.; Coetzee, M. A. A.; Kamika, I.; Ngole-Jeme, V. M.; Benteke Momba, M. N. Endocrine-Disruptive Chemicals as Contaminants of Emerging Concern in Wastewater and Surface Water: A Review. J. Environ. Manage 2021, 277, 111485. DOI: 10.1016/j.jenvman.2020.111485.
- Singh, A. K.; Kumar, A.; Chandra, R. Environmental Pollutants of Paper Industry Wastewater and Their Toxic Effects on Human Health and Ecosystem. Bioresour. Technol. Rep 2022, 20, 101250. DOI: 10.1016/j.biteb.2022.101250.
- Kishor, R.; Purchase, D.; Saratale, G. D.; Saratale, R. G.; Ferreira, L. F. R.; Bilal, M.; Chandra, R.; Bharagava, R. N. Ecotoxicological and Health Concerns of Persistent Coloring Pollutants of Textile Industry Wastewater and Treatment Approaches for Environmental Safety. J. Environ. Chem. Eng 2021, 9(2), 105012. DOI: 10.1016/j.jece.2020.105012.
- Feng, S.; Hao Ngo, H.; Guo, W.; Woong Chang, S.; Duc Nguyen, D.; Cheng, D.; Varjani, S.; Lei, Z.; Liu, Y. Roles and Applications of Enzymes for Resistant Pollutants Removal in Wastewater Treatment. Bioresour. Technol 2021, 335, 125278. DOI: 10.1016/j.biortech.2021.125278.
- Zajda, M.; Aleksander-Kwaterczak, U. Wastewater Treatment Methods for Effluents from the Confectionery Industry–An Overview. J. Ecol. Eng. 2019, 20(9), 293–304. DOI: 10.12911/22998993/112557.
- Bhargava, D. A. Physico-Chemical Waste Water Treatment Technologies: An Overview. Int. J. Sci. Res. Edu 2016, 4, 5308–5319. DOI: 10.18535/ijsre/v4i05.05.
- Alshabib, M.; Onaizi, S. A. A Review on Phenolic Wastewater Remediation Using Homogeneous and Heterogeneous Enzymatic Processes: Current Status and Potential Challenges. Sep. Purif. Technol 2019, 219, 186–207. DOI: 10.1016/j.seppur.2019.03.028.
- Khan, S. I.; Zarin, A.; Ahmed, S.; Hasan, F.; Belduz, A. O.; Canakci, S.; Khan, S.; Badshah, M.; Farman, M.; Shah, A. A. Degradation of Lignin by Bacillus Altitudinis Sl7 Isolated from Pulp and Paper Mill Effluent. Water. Sci. Technol 2022, 85(1), 420–432. DOI: 10.2166/wst.2021.610.
- Zdarta, J.; Jesionowski, T.; Meyer, A. S.; Pinelo, M. Removal of Tetracycline in Enzymatic Membrane Reactor: Enzymatic Conversion as the Predominant Mechanism Over Adsorption and Membrane Rejection. J. Environ. Chem. Eng 2022, 10(1), 106973. DOI: 10.1016/j.jece.2021.106973.
- Chandra, R.; Raj, A.; Yadav, S.; Patel, D. K. Reduction of Pollutants in Pulp Paper Mill Effluent Treated by Pcp-Degrading Bacterial Strains. Environ. Monit. Assess 2009, 155(1–4), 1–11. DOI: 10.1007/s10661-008-0413-4.
- Domínguez de María, P. Biocatalysis, Sustainability, and Industrial Applications: Show Me the Metrics. Curr. Opin. Green Sustain. Chem. 2021, 31, 100514. DOI: 10.1016/j.cogsc.2021.100514.
- Erdem, E.; Woodley, J. M. Industrially Useful Enzymology: Translating Biocatalysis from Laboratory to Process. Chem. Catalysis 2022, 2(10), 2499–2505. DOI: 10.1016/j.checat.2022.09.037.
- van Schie, M.; Sporing, J. D.; Bocola, M.; Dominguez de Maria, P.; Rother, D. Applied Biocatalysis Beyond Just Buffers – from Aqueous to Unconventional Media. Options and Guidelines. Green Chem. 2021, 23(9), 3191–3206. DOI: 10.1039/d1gc00561h.
- Zdarta, J.; Jesionowski, T.; Pinelo, M.; Meyer, A. S.; Iqbal, H. M. N.; Bilal, M.; Nguyen, L. N.; Nghiem, L. D. Free and Immobilized Biocatalysts for Removing Micropollutants from Water and Wastewater: Recent Progress and Challenges. Bioresour. Technol 2022, 344, 126201. DOI: 10.1016/j.biortech.2021.126201.
- Shakerian, F.; Zhao, J.; Li, S. P. Recent Development in the Application of Immobilized Oxidative Enzymes for Bioremediation of Hazardous Micropollutants – a Review. Chemosphere 2020, 239, 124716. DOI: 10.1016/j.chemosphere.2019.124716.
- Ekeoma, B. C.; Ekeoma, L. N.; Yusuf, M.; Haruna, A.; Ikeogu, C. K.; Merican, Z. M. A.; Kamyab, H.; Pham, C. Q.; Vo, D. N.; Chelliapan, S. Recent Advances in the Biocatalytic Mitigation of Emerging Pollutants: A Comprehensive Review. J. Biotech 2023, 369, 14–34. DOI: 10.1016/j.jbiotec.2023.05.003.
- Haruna, A.; Chong, F. K.; Ho, Y. C.; Merican, Z. M. A. Preparation and Modification Methods of Defective Titanium Dioxide-Based Nanoparticles for Photocatalytic Wastewater Treatment—A Comprehensive Review. Environ. Sci. Pollut. Res. Int 2022, 29(47), 70706–70745. DOI: 10.1007/s11356-022-22749-8.
- Mohanan, N.; Montazer, Z.; Sharma, P. K.; Levin, D. B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. DOI: 10.3389/fmicb.2020.580709.
- Mousavi, S. M.; Hashemi, S. A.; Iman Moezzi, S. M.; Ravan, N.; Gholami, A.; Lai, C. W.; Chiang, W. H.; Omidifar, N.; Yousefi, K.; Behbudi, G. Recent Advances in Enzymes for the Bioremediation of Pollutants. Biochem. Res. Int 2021, 2021, 5599204. DOI: 10.1155/2021/5599204.
- Show, P. L.; Chew, K. W.; Vo, D. N. Biotechnology and Sustainable Environmental Health Management. Chemosphere 2022, 291, 132798. DOI: 10.1016/j.chemosphere.2021.132798.
- Zhu, B.; Chen, Y.; Wei, N. Engineering Biocatalytic and Biosorptive Materials for Environmental Applications. Trends Biotechnol. 2019, 37, 661–676. DOI: 10.1016/j.tibtech.2018.11.005.
- Noreen, S.; Perveen, S.; Shafiq, N.; Aslam, S.; Iqbal, H. M. N.; Ashraf, S. S.; Bilal, M. Laccase-Loaded Functionalized Graphene Oxide Assemblies with Improved Biocatalytic Properties and Decolorization Performance. Environ. Technol. Innov 2021, 24, 101884. DOI: 10.1016/j.eti.2021.101884.
- Daronch, N. A.; Kelbert, M.; Pereira, C. S.; de Araújo, P. H. H.; de Oliveira, D. Elucidating the Choice for a Precise Matrix for Laccase Immobilization: A Review. Chem. Eng. J 2020, 397, 125506. DOI: 10.1016/j.cej.2020.125506.
- Edri, E.; Armon, N.; Greenberg, E.; Moshe-Tsurel, S.; Lubotzky, D.; Salzillo, T.; Perelshtein, I.; Tkachev, M.; Girshevitz, O.; Shpaisman, H. Laser Printing of Multilayered Alternately Conducting and Insulating Microstructures. ACS Appl. Mater. Interfaces 2021, 13(30), 36416–36425. DOI: 10.1021/acsami.1c06204.
- Sheldon, R. A.; Woodley, J. M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev 2018, 118(2), 801–838. DOI: 10.1021/acs.chemrev.7b00203.
- López, C.; Moreira, M. T.; Feijoo, G.; Lema, J. M. Economic Comparison of Enzymatic Reactors and Advanced Oxidation Processes Applied to the Degradation of Phenol as a Model Compound. Biocatal. Biotransform. 2011, 29(6), 344–353. DOI: 10.3109/10242422.2011.638056.
- Abejón, R.; Belleville, M. P.; Sanchez-Marcano, J. Design, Economic Evaluation and Optimization of Enzymatic Membrane Reactors for Antibiotics Degradation in Wastewaters. Sep. Purif. Technol 2015, 156, 183–199. DOI: 10.1016/j.seppur.2015.09.072.
- “Enzymes Global Market Report. The Business Research Company. https://www.reportlinker.com/p06241900/Enzymes-Global-Market-Report.html?utm_source=GNW. (accessed Aug 23, 2023).
- “Enzymes Market. Precedence Research Pvt. Ltd. https://www.precedenceresearch.com/enzymes-market. (accessed Feb 27, 2024).
- Bhandari, S.; Poudel, D. K.; Marahatha, R.; Dawadi, S.; Khadayat, K.; Phuyal, S.; Shrestha, S.; Gaire, S.; Basnet, K.; Khadka, U., et al. Microbial Enzymes Used in Bioremediation. J. Chem. 2021, 2021, 1–17. DOI: 10.1155/2021/8849512.
- Li, Q.; Liu, J.; Gadd, G. M. Fungal Bioremediation of Soil Co-Contaminated with Petroleum Hydrocarbons and Toxic Metals. Appl. Microbiol. Biotechnol 2020, 104(21), 8999–9008. DOI: 10.1007/s00253-020-10854-y.
- Bentil, J. A.; Thygesen, A.; Mensah, M.; Lange, L.; Meyer, A. S. Cellulase Production by White-Rot Basidiomycetous Fungi: Solid-State versus Submerged Cultivation. Appl. Microbiol. Biotechnol 2018, 102(14), 5827–5839. DOI: 10.1007/s00253-018-9072-8.
- Dhakar, K.; Jain, R.; Tamta, S.; Pandey, A. Prolonged Laccase Production by a Cold and Ph Tolerant Strain of Penicillium Pinophilum (Mcc 1049) Isolated from a Low Temperature Environment. Enzym. Res 2014, 2014, 120708. DOI: 10.1155/2014/120708.
- Cruz, I. D. A.; Cruz-Magalhaes, V.; Loguercio, L. L.; Dos Santos, L.; Uetanabaro, A. P. T.; Costa, A. M. D. A Systematic Study on the Characteristics and Applications of Laccases Produced by Fungi: Insights on Their Potential for Biotechnologies. Prep. Biochem. Biotechnol 2024, 2024, 1–14. DOI: 10.1080/10826068.2023.2297697.
- Cheute, V. M. S.; Uber, T. M.; dos Santos, L. F. O.; Backes, E.; Dantas, M. P.; Contato, A. G.; Castoldi, R.; de Souza, C. G. M.; Corrêa, R. C. G.; Bracht, A., et al. Biotransformation of Pollutants by Pycnoporus Spp. in Submerged and Solid-State Fermentation: Mechanisms, Achievements, and Perspectives. Biomass 2024, 4(2), 313–328. DOI: 10.3390/biomass4020015.
- Caroca, E.; Elorrieta, M.; Palma, C.; Navia, D.; Lebrero, R.; Carvajal, A. Lignocellulosic Residue Valorization in a Sequential Process of Solid‐State Fermentation and Solid Substrate Anaerobic Digestion. J. Chem. Tech & Biotech 2021, 97(6), 1575–1584. DOI: 10.1002/jctb.6967.
- Sharma, D.; Sud, A.; Bansal, S.; Mahajan, R.; Sharma, B. M.; Chauhan, R. S.; Goel, G. Endocellulase Production by Cotylidia Pannosa and Its Application in Saccharification of Wheat Bran to Bioethanol. Bioenergy Res 2017, 11(1), 219–227. DOI: 10.1007/s12155-017-9890-z.
- Debnath, R.; Saha, T. An Insight into the Production Strategies and Applications of the Ligninolytic Enzyme Laccase from Bacteria and Fungi. Biocatal. Agric. Biotechnol 2020, 26, 101645. DOI: 10.1016/j.bcab.2020.101645.
- Bera, S.; Mohanty, K. Areca nut (areca catechu) husks and luffa (luffa cylindrica) sponge as microbial immobilization matrices for efficient phenol degradation. J. Water. Process Eng 2020, 33, 100999. DOI: 10.1016/j.jwpe.2019.100999.
- Giovanella, P.; Vieira, G. A. L.; Ramos Otero, I. V.; Pais Pellizzer, E.; de Jesus Fontes, B.; Sette, L. D. Metal and Organic Pollutants Bioremediation by Extremophile Microorganisms. J. Hazard. Mater 2020, 382, 121024. DOI: 10.1016/j.jhazmat.2019.121024.
- Wentzel, L. C. P.; Inforsato, F. J.; Montoya, Q. V.; Rossin, B. G.; Nascimento, N. R.; Rodrigues, A.; Sette, L. D. Fungi from Admiralty Bay (King George Island, Antarctica) Soils and Marine Sediments. Microb. Ecol 2019, 77(1), 12–24. DOI: 10.1007/s00248-018-1217-x.
- Gundinger, T.; Spadiut, O. A Comparative Approach to Recombinantly Produce the Plant Enzyme Horseradish Peroxidase in Escherichia coli. J. Biotech 2017, 248, 15–24. DOI: 10.1016/j.jbiotec.2017.03.003.
- Rana, R. S.; Singh, P.; Kandari, V.; Singh, R.; Dobhal, R.; Gupta, S. A Review on Characterization and Bioremediation of Pharmaceutical industries’ Wastewater: An Indian Perspective. Appl. Water. Sci 2014, 7(1), 1–12. DOI: 10.1007/s13201-014-0225-3.
- Zdarta, J.; Meyer, A. S.; Jesionowski, T.; Pinelo, M. Developments in Support Materials for Immobilization of Oxidoreductases: A Comprehensive Review. Adv. Colloid. Interface Sci 2018, 258(258), 1–20. DOI: 10.1016/j.cis.2018.07.004.
- Zdarta, J.; Nguyen, L. N.; Jankowska, K.; Jesionowski, T.; Nghiem, L. D. A Contemporary Review of Enzymatic Applications in the Remediation of Emerging Estrogenic Compounds. Crit. Rev. Environ. Sci. Technol 2022, 52(15), 2661–2690. DOI: 10.1080/10643389.2021.1889283.
- Yaashikaa, P. R.; Devi, M. K.; Kumar, P. S. Advances in the Application of Immobilized Enzyme for the Remediation of Hazardous Pollutant: A Review. Chemosphere. 2022, 299, 134390. DOI: 10.1016/j.chemosphere.2022.134390.
- Pirahanchi, Y.; Anoruo, M.; Sharma, S. Biochemistry, Lipoprotein Lipase. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK537040/. (accessed Sep 13, 2023).
- Umar, A.; Ahmed, S. Optimization, Purification and Characterization of Laccase from Ganoderma Leucocontextum Along with Its Phylogenetic Relationship. Sci. Rep 2022, 12(1), 2416. DOI: 10.1038/s41598-022-06111-z.
- Rostami, A.; Abdelrasoul, A.; Shokri, Z.; Shirvandi, Z. Applications and Mechanisms of Free and Immobilized Laccase in Detoxification of Phenolic Compounds — a Review. Korean J. Chem. Eng. 2022, 39, 821–832. DOI: 10.1007/s11814-021-0984-0.
- Su, J.; Fu, J.; Wang, Q.; Silva, C.; Cavaco-Paulo, A. Laccase: A Green Catalyst for the Biosynthesis of Poly-Phenols. Crit. Rev. Biotechnol 2018, 38(2), 294–307. DOI: 10.1080/07388551.2017.1354353.
- Mate, D. M.; Alcalde, M. Laccase: A Multi-Purpose Biocatalyst at the Forefront of Biotechnology. Microb. Biotechnol 2017, 10(6), 1457–1467. DOI: 10.1111/1751-7915.12422.
- Guan, Z. B.; Luo, Q.; Wang, H. R.; Chen, Y.; Liao, X. R. Bacterial Laccases: Promising Biological Green Tools for Industrial Applications. Cell. Mol. Life Sci 2018, 75(19), 3569–3592. DOI: 10.1007/s00018-018-2883-z.
- Yang, X.; Wu, Y.; Zhang, Y.; Yang, E.; Qu, Y.; Xu, H.; Chen, Y.; Irbis, C.; Yan, J. A Thermo-Active Laccase Isoenzyme from Trametes Trogii and Its Potential for Dye Decolorization at High Temperature. Front. Microbiol. 2020, 11, 241. DOI: 10.3389/fmicb.2020.00241.
- Karigar, C. S.; Rao, S. S. Role of Microbial Enzymes in the Bioremediation of Pollutants: A Review. Enzym. Res 2011, 2011, 805187. DOI: 10.4061/2011/805187.
- Zimbardi, A. L.; Camargo, P. F.; Carli, S.; Aquino Neto, S.; Meleiro, L. P.; Rosa, J. C.; De Andrade, A. R.; Jorge, J. A.; Furriel, R. P. A High Redox Potential Laccase from Pycnoporus Sanguineus rp15: Potential Application for Dye Decolorization. Int. J. Mol. Sci 2016, 17(5), 672. DOI: 10.3390/ijms17050672.
- Apriceno, A.; Astolfi, M. L.; Girelli, A. M.; Scuto, F. R. A New Laccase-Mediator System Facing the Biodegradation Challenge: Insight into the NSAIDs Removal. Chemosphere. 2019, 215, 535–542. DOI: 10.1016/j.chemosphere.2018.10.086.
- George, J.; Alanazi, A. K.; Senthil Kumar, P.; Venkataraman, S.; Rajendran, D. S.; Athilakshmi, J. K.; Singh, I.; Singh, I.; Sen, P.; Purushothaman, M., et al. Laccase-Immobilized on Superparamagnetic Iron Oxide Nanoparticles Incorporated Polymeric Ultrafiltration Membrane for the Removal of Toxic Pentachlorophenol. Chemosphere. 2023, 331, 138734. DOI: 10.1016/j.chemosphere.2023.138734.
- Iriarte-Mesa, C.; Diaz-Castanon, S.; Abradelo, D. G. Facile Immobilization of Trametes Versicolor Laccase on Highly Monodisperse Superparamagnetic Iron Oxide Nanoparticles. Colloids Surf. B. 2019, 181, 470–479. DOI: 10.1016/j.colsurfb.2019.05.012.
- Waheed, A.; Baig, U.; Ansari, M. A. Fabrication of Cuo Nanoparticles Immobilized Nanofiltration Composite Membrane for Dye/Salt Fractionation: Performance and Antibiofouling. J. Environ. Chem. Eng 2022, 10(1), 106960. DOI: 10.1016/j.jece.2021.106960.
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Laccase Immobilization on Titania Nanoparticles and Titania-Functionalized Membranes. J. Membr. Sci 2014, 452, 229–240. DOI: 10.1016/j.memsci.2013.10.019.
- Munnawar, I.; Iqbal, S. S.; Anwar, M. N.; Batool, M.; Tariq, S.; Faitma, N.; Khan, A. L.; Khan, A. U.; Nazar, U.; Jamil, T., et al. Synergistic Effect of Chitosan-Zinc Oxide Hybrid Nanoparticles on Antibiofouling and Water Disinfection of Mixed Matrix Polyethersulfone Nanocomposite Membranes. Carbohydr. Polym 2017, 175, 661–670. DOI: 10.1016/j.carbpol.2017.08.036.
- Piotrowska-Długosz, A. The Use of Enzymes in Bioremediation of Soil Xenobiotics. In Xenobiotics in the Soil Environment, Hashmi, M. Z., Kumar, V. Varma, A., Eds.; Springer: Switzerland, 2017; Vol. 49, pp. 243–265.
- Morsi, R.; Bilal, M.; Iqbal, H. M. N.; Ashraf, S. S. Laccases and Peroxidases: The Smart, Greener and Futuristic Biocatalytic Tools to Mitigate Recalcitrant Emerging Pollutants. Sci. Total Environ 2020, 714, 136572. DOI: 10.1016/j.scitotenv.2020.136572.
- Nguyen, L. N.; van de Merwe, J. P.; Hai, F. I.; Leusch, F. D.; Kang, J.; Price, W. E.; Roddick, F.; Magram, S. F.; Nghiem, L. D. Laccase-Syringaldehyde-Mediated Degradation of Trace Organic Contaminants in an Enzymatic Membrane Reactor: Removal Efficiency and Effluent Toxicity. Bioresour. Technol 2016, 200, 477–484. DOI: 10.1016/j.biortech.2015.10.054.
- Robinson, P. K. Enzymes: Principles and Biotechnological Applications. Essays Biochem. 2015, 59, 1–41. DOI: 10.1042/bse0590001.
- Stadlmair, L. F.; Letzel, T.; Drewes, J. E.; Grassmann, J. Enzymes in Removal of Pharmaceuticals from Wastewater: A Critical Review of Challenges, Applications and Screening Methods for Their Selection. Chemosphere. 2018, 205, 649–661. DOI: 10.1016/j.chemosphere.2018.04.142.
- Li, X.; de Toledo, R. A.; Wang, S.; Shim, H. Removal of Carbamazepine and Naproxen by Immobilized Phanerochaete Chrysosporium Under Non-Sterile Condition. N. Biotechnol 2015, 32(2), 282–289. DOI: 10.1016/j.nbt.2015.01.003.
- Marco-Urrea, E.; Perez-Trujillo, M.; Blanquez, P.; Vicent, T.; Caminal, G. Biodegradation of the Analgesic Naproxen by Trametes Versicolor and Identification of Intermediates Using Hplc-Dad-Ms and Nmr. Bioresour. Technol 2010, 101(7), 2159–2166. DOI: 10.1016/j.biortech.2009.11.019.
- Margot, J.; Bennati-Granier, C.; Maillard, J.; Blánquez, P.; Barry, D. A.; Holliger, C. Bacterial versus Fungal Laccase: Potential for Micropollutant Degradation. AMB Express 2013, 3(1), 1–14. DOI: 10.1186/2191-0855-3-63.
- Llorca, M.; Rodriguez-Mozaz, S.; Couillerot, O.; Panigoni, K.; de Gunzburg, J.; Bayer, S.; Czaja, R.; Barcelo, D. Identification of New Transformation Products During Enzymatic Treatment of Tetracycline and Erythromycin Antibiotics at Laboratory Scale by an On-Line Turbulent Flow Liquid-Chromatography Coupled to a High Resolution Mass Spectrometer Ltq-Orbitrap. Chemosphere. 2015, 119, 90–98. DOI: 10.1016/j.chemosphere.2014.05.072.
- Zdarta, J.; Jankowska, K.; Wyszowska, M.; Kijenska-Gawronska, E.; Zgola-Grzeskowiak, A.; Pinelo, M.; Meyer, A. S.; Moszynski, D.; Jesionowski, T. Robust Biodegradation of Naproxen and Diclofenac by Laccase Immobilized Using Electrospun Nanofibers with Enhanced Stability and Reusability. Mater. Sci. Eng. C 2019, 103, 109789. DOI: 10.1016/j.msec.2019.109789.
- Ren, S.; Li, C.; Jiao, X.; Jia, S.; Jiang, Y.; Bilal, M.; Cui, J. Recent Progress in Multienzymes Co-Immobilization and Multienzyme System Applications. Chem. Eng. J 2019, 373, 1254–1278. DOI: 10.1016/j.cej.2019.05.141.
- Bilal, M.; Lam, S. S.; Iqbal, H. M. N. Biocatalytic Remediation of Pharmaceutically Active Micropollutants for Environmental Sustainability. Environ. Pollut 2022, 293, 118582. DOI: 10.1016/j.envpol.2021.118582.
- Sheldon, R. A.; Basso, A.; Brady, D. New Frontiers in Enzyme Immobilisation: Robust Biocatalysts for a Circular Bio-Based Economy. Chem. Soc. Rev 2021, 50(10), 5850–5862. DOI: 10.1039/d1cs00015b.
- Hassan, M. E.; Yang, Q.; Xiao, Z.; Liu, L.; Wang, N.; Cui, X.; Yang, L. Impact of Immobilization Technology in Industrial and Pharmaceutical Applications. 3 Biotech. 3 Biotech. 2019, 9(12), 440. DOI: 10.1007/s13205-019-1969-0.
- Olcucu, G.; Klaus, O.; Jaeger, K.-E.; Drepper, T.; Krauss, U. Emerging Solutions for in vivo Biocatalyst Immobilization: Tailor-Made Catalysts for Industrial Biocatalysis. ACS Sustain. Chem. Eng 2021, 9(27), 8919–8945. DOI: 10.1021/acssuschemeng.1c02045.
- Lassouane, F.; Ait-Amar, H.; Amrani, S.; Rodriguez-Couto, S. A Promising Laccase Immobilization Approach for Bisphenol a Removal from Aqueous Solutions. Bioresour. Technol 2019, 271, 360–367. DOI: 10.1016/j.biortech.2018.09.129.
- Rehman, S.; Wang, P.; Bhatti, H. N.; Bilal, M.; Asgher, M. Improved Catalytic Properties of Penicillium Notatum Lipase Immobilized in Nanoscale Silicone Polymeric Films. Int. J. Biol. Macromol 2017, 97, 279–286. DOI: 10.1016/j.ijbiomac.2017.01.038.
- Asgher, M.; Noreen, S.; Bilal, M. Enhancing Catalytic Functionality of Trametes Versicolor Ibl-04 Laccase by Immobilization on Chitosan Microspheres. Chem. Eng. Res. Des 2017, 119, 1–11. DOI: 10.1016/j.cherd.2016.12.011.
- Adeel, M.; Bilal, M.; Rasheed, T.; Sharma, A.; Iqbal, H. M. N. Graphene and Graphene Oxide: Functionalization and Nano-Bio-Catalytic System for Enzyme Immobilization and Biotechnological Perspective. Int. J. Biol. Macromol 2018, 120, 1430–1440. DOI: 10.1016/j.ijbiomac.2018.09.144.
- Wang, Z.; Liu, Y.; Li, J.; Meng, G.; Zhu, D.; Cui, J.; Jia, S. Efficient Immobilization of Enzymes on Amino Functionalized Mil-125-Nh2 Metal Organic Framework. Biotechnol. Bioprocess Eng 2022, 27(1), 135–144. DOI: 10.1007/s12257-020-0393-y.
- Bayat, Z.; Hassanshahian, M.; Cappello, S. Immobilization of Microbes for Bioremediation of Crude Oil Polluted Environments: A Mini Review. Open Microbiol. J. 2015, 9, 48–54. DOI: 10.2174/1874285801509010048.
- Sheldon, R. A. Enzyme Immobilization: The Quest for Optimum Performance. Adv. Synth. Catal 2007, 349(8–9), 1289–1307. DOI: 10.1002/adsc.200700082.
- Zdarta, J.; Meyer, A.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8(2), 92. DOI: 10.3390/catal8020092.
- Haq, I.; Kalamdhad, A. S. Enhanced Biodegradation of Toxic Pollutants from Paper Industry Wastewater Using Pseudomonas sp. Immobilized in Composite Biocarriers and Its Toxicity Evaluation. Bioresour. Technol. Rep 2023, 24, 101674. DOI: 10.1016/j.biteb.2023.101674.
- Homaei, A. Immobilization of Penaeus Merguiensis Alkaline Phosphatase on Gold Nanorods for Heavy Metal Detection. Ecotoxicol. Environ. Saf. 2017, 136, 1–7. DOI: 10.1016/j.ecoenv.2016.10.023.
- Naghdi, M.; Taheran, M.; Brar, S. K.; Kermanshahi-Pour, A.; Verma, M.; Surampalli, R. Y. Immobilized Laccase on Oxygen Functionalized Nanobiochars Through Mineral Acids Treatment for Removal of Carbamazepine. Sci. Total Environ 2017, 584-585, 393–401. DOI: 10.1016/j.scitotenv.2017.01.021.
- Kumar, V. V.; Cabana, H. Towards High Potential Magnetic Biocatalysts for On-Demand Elimination of Pharmaceuticals. Bioresour. Technol 2016, 200, 81–89. DOI: 10.1016/j.biortech.2015.09.100.
- Primožič, M.; Kravanja, G.; Knez, Ž.; Crnjac, A.; Leitgeb, M. Immobilized Laccase in the Form of (Magnetic) Cross-Linked Enzyme Aggregates for Sustainable Diclofenac (Bio)degradation. J. Clean. Prod 2020, 275, 124121. DOI: 10.1016/j.jclepro.2020.124121.
- Yanto, D. H. Y.; Guntoro, M. A.; Nurhayat, O. D.; Anita, S. H.; Oktaviani, M.; Ramadhan, K. P.; Pradipta, M. F.; Watanabe, T. Biodegradation and Biodetoxification of Batik Dye Wastewater by Laccase from Trametes Hirsuta Edn 082 Immobilised on Light Expanded Clay Aggregate. 3 Biotech. 2021, 11(5), 247. DOI: 10.1007/s13205-021-02806-8.
- Ji, C.; Nguyen, L. N.; Hou, J.; Hai, F. I.; Chen, V. Direct Immobilization of Laccase on Titania Nanoparticles from Crude Enzyme Extracts of P. Ostreatus Culture for Micro-Pollutant Degradation. Sep. Purif. Technol 2017, 178, 215–223. DOI: 10.1016/j.seppur.2017.01.043.
- Al-Maqdi, K. A.; Elmerhi, N.; Athamneh, K.; Bilal, M.; Alzamly, A.; Ashraf, S. S.; Shah, I. Challenges and Recent Advances in Enzyme-Mediated Wastewater Remediation—A Review. Nanomaterials 2021, 11(11), 3124. DOI: 10.3390/nano11113124.
- Chen, G.; Bai, R.; Zhang, Y.; Zhao, B.; Xiao, Y. Application of Metagenomics to Biological Wastewater Treatment. Sci. Total Environ 2022, 807, 150737. DOI: 10.1016/j.scitotenv.2021.150737.
- Mei, J.; Gao, X.; Zou, J.; Pang, F. Research on Photocatalytic Wastewater Treatment Reactors: Design, Optimization, and Evaluation Criteria. Catalysts 2023, 13(6), 974. DOI: 10.3390/catal13060974.
- Kumar, R.; Singh, A.; Maurya, A.; Yadav, P.; Yadav, A.; Chowdhary, P.; Raj, A. Effective Bioremediation of Pulp and Paper Mill Wastewater Using bacillus cereus as a Possible Kraft Lignin-Degrading Bacterium. Bioresour. Technol 2022, 352, 127076. DOI: 10.1016/j.biortech.2022.127076.
- Daâssi, D.; Rodríguez-Couto, S.; Nasri, M.; Mechichi, T. Biodegradation of Textile Dyes by Immobilized Laccase from Coriolopsis Gallica into Ca-Alginate Beads. Int. Biodeter. Biodegrad 2014, 90, 71–78. DOI: 10.1016/j.ibiod.2014.02.006.
- Guardado, A. L. P.; Druon-Bocquet, S.; Belleville, M. P.; Sanchez-Marcano, J. A Novel Process for the Covalent Immobilization of Laccases on Silica Gel and Its Application for the Elimination of Pharmaceutical Micropollutants. Environ. Sci. Pollut. Res. Int 2021, 28(20), 25579–25593. DOI: 10.1007/s11356-021-12394-y.
- Zerva, A.; Pentari, C.; Topakas, E. Crosslinked Enzyme Aggregates (Cleas) of Laccases from Pleurotus Citrinopileatus Induced in Olive Oil Mill Wastewater (Oomw). Molecules 2020, 25(9), 25: 2221. DOI: 10.3390/molecules25092221.
- Zhou, H.; Huang, X.; Jiang, L.; Shen, Q.; Sun, H.; Yi, M.; Wang, X.; Yao, X.; Wu, Y.; Zhang, C., et al. Improved Degradation of Petroleum Contaminants in Hydraulic Fracturing Flowback and Produced Water Using Laccase Immobilized on Functionalized Biochar. Environ. Technol. Innov 2023, 32, 103280. DOI: 10.1016/j.eti.2023.103280.
- Sun, K.; Kang, F.; Waigi, M. G.; Gao, Y.; Huang, Q. Laccase-Mediated Transformation of Triclosan in Aqueous Solution with Metal Cations and Humic Acid. Environ. Pollut 2017, 220, 105–111. DOI: 10.1016/j.envpol.2016.09.028.
- Yang, P.; Zhang, T.; Lu, J. Coupling of Natural Organic Matter–Metal Binding and Laccase-Catalyzed Oxidation of Tetrabromobisphenol a. Environ. Sci. Pollut. Res. Int 2020, 27(24), 30199–30209. DOI: 10.1007/s11356-020-09352-5.
- Ariste, A. F.; Haroune, L.; Saibi, S.; Cabana, H. Enzyme Polymer Engineered Structure Strategy to Enhance Cross-Linked Enzyme Aggregate Stability: A Step Forward in Laccase Exploitation for Cannabidiol Removal from Wastewater. Environ. Sci. Pollut. Res. Int 2021, 28(32), 44051–44063. DOI: 10.1007/s11356-021-13746-4.
- Liu, Y.; Huang, L.; Guo, W.; Jia, L.; Fu, Y.; Gui, S.; Lu, F. Cloning, Expression, and Characterization of a Thermostable and Ph-Stable Laccase from Klebsiella pneumoniae and Its Application to Dye Decolorization. Process Biochem. 2017, 53, 125–134. DOI: 10.1016/j.procbio.2016.11.015.
- Ingole, P. M.; Rathod, V. K. Ultrasound-Assisted Enzymatic Degradation of Naproxen. J. Indian Chem. Soc 2023, 100(8), 101040. DOI: 10.1016/j.jics.2023.101040.
- Garcia-Morales, R.; Rodriguez-Delgado, M.; Gomez-Mariscal, K.; Orona-Navar, C.; Hernandez-Luna, C.; Torres, E.; Parra, R.; Cardenas-Chavez, D.; Mahlknecht, J.; Ornelas-Soto, N. Biotransformation of Endocrine-Disrupting Compounds in Groundwater: Bisphenol A, Nonylphenol, Ethynylestradiol and Triclosan by a Laccase Cocktail from Pycnoporus Sanguineus cs43. Water. Air Soil Pollut 2015, 226(8), 251. DOI: 10.1007/s11270-015-2514-3.
- Wagner, M.; Nicell, J. A. Impact of Dissolved Wastewater Constituents on Peroxidase‐Catalyzed Treatment of Phenol. J. Chem. Tech & Biotech 2002, 77(4), 419–428. DOI: 10.1002/jctb.571.
- Yang, Y.; Li, J.; Shi, H.; Zhai, L.; Wang, X.; Gao, S. Influence of Natural Organic Matter on Horseradish Peroxidase-Mediated Removal of 17 alpha-Ethinylestradiol: Role of Molecular Weight. J. Hazard. Mater 2018, 356, 9–16. DOI: 10.1016/j.jhazmat.2018.05.032.
- Jiang, W.; Li, W.; Xiao, F.; Wang, D.; Wang, Z. Influence of Nom and Ss on the Bpa Removal via Peroxidase Catalyzed Reactions: Kinetics and Pathways. Sep. Purif. Technol 2017, 173, 244–249. DOI: 10.1016/j.seppur.2016.09.029.
- Xu, H.; Guo, M. Y.; Gao, Y. H.; Bai, X. H.; Zhou, X. W. Expression and Characteristics of Manganese Peroxidase from Ganoderma Lucidum in Pichia Pastoris and Its Application in the Degradation of Four Dyes and Phenol. BMC Biotechnol. 2017, 17(1), 19. DOI: 10.1186/s12896-017-0338-5.
- Bilal, M.; Iqbal, H. M.; Hu, H.; Wang, W.; Zhang, X. Development of Horseradish Peroxidase-Based Cross-Linked Enzyme Aggregates and Their Environmental Exploitation for Bioremediation Purposes. J. Environ. Manage 2017, 188, 137–143. DOI: 10.1016/j.jenvman.2016.12.015.
- Abdulaal, W. H.; Almulaiky, Y. Q.; El-Shishtawy, R. M. Encapsulation of Hrp Enzyme Onto a Magnetic fe3o4 Np–Pmma Film via Casting with Sustainable Biocatalytic Activity. Catalysts 2020, 10(2), 181. DOI: 10.3390/catal10020181.
- Pylypchuk, I. V.; Daniel, G.; Kessler, V. G.; Seisenbaeva, G. A. Removal of Diclofenac, Paracetamol, and Carbamazepine from Model Aqueous Solutions by Magnetic Sol–Gel Encapsulated Horseradish Peroxidase and Lignin Peroxidase Composites. Nanomater. 2020, 10(2), 282. DOI: 10.3390/nano10020282.
- Chang, Q.; Huang, J.; Ding, Y.; Tang, H. Catalytic Oxidation of Phenol and 2,4-Dichlorophenol by Using Horseradish Peroxidase Immobilized on Graphene Oxide/Fe(3)o(4). Molecules 2016, 21(8), 1044. DOI: 10.3390/molecules21081044.
- Ahari, S. M.; Tabatabaei, Z. G. Preparation of Chitosan-Carbon Nanotubes Decorated with Zno@ Laccase: Application in the Removal of Bisphenol a from Aqueous Solution. J. Environ. Eng 2022, 149. DOI: 10.1061/JOEEDU.EEENG-6922.
- Mahmoodi, N. M.; Saffar-Dastgerdi, M. H.; Hayati, B. Environmentally Friendly Novel Covalently Immobilized Enzyme Bionanocomposite: From Synthesis to the Destruction of Pollutant. Compos. B: Eng 2020, 184, 107666. DOI: 10.1016/j.compositesb.2019.107666.
- Sharma, K.; Tewatia, P.; Kaur, M.; Pathania, D.; Banat, F.; Rattan, G.; Singhal, S.; Kaushik, A. Bioremediation of Multifarious Pollutants Using Laccase Immobilized on Magnetized and Carbonyldiimidazole-Functionalized Cellulose Nanofibers. Sci. Total Environ 2023, 864, 161137. DOI: 10.1016/j.scitotenv.2022.161137.
- Reis, C.; Sousa, E.; Serpa, J.; Oliveira, R.; Oliveira, R.; Santos, J. Design of Immobilized Enzyme Biocatalysts: Drawbacks and Opportunities. Química Nova 2019, 42, 768–783. DOI: 10.21577/0100-4042.20170381.
- Voběrková, S.; Solčány, V.; Vršanská, M.; Adam, V. Immobilization of Ligninolytic Enzymes from White-Rot Fungi in Cross-Linked Aggregates. Chemosphere. 2018, 202, 694–707. DOI: 10.1016/j.chemosphere.2018.03.088.
- Zhu, Y.; Qiu, F.; Rong, J.; Zhang, T.; Mao, K.; Yang, D. Covalent Laccase Immobilization on the Surface of Poly(vinylidene Fluoride) Polymer Membrane for Enhanced Biocatalytic Removal of Dyes Pollutants from Aqueous Environment. Colloids Surf. B. 2020, 191, 111025. DOI: 10.1016/j.colsurfb.2020.111025.
- Aricov, L.; Leonties, A. R.; Gifu, I. C.; Preda, D.; Raducan, A.; Anghel, D. F. Enhancement of Laccase Immobilization Onto Wet Chitosan Microspheres Using an Iterative Protocol and Its Potential to Remove Micropollutants. J. Environ. Manage 2020, 276, 111326. DOI: 10.1016/j.jenvman.2020.111326.
- Rouhani, S.; Azizi, S.; Maaza, M.; Mamba, B.; Msagati, T. A. M. Covalent Immobilization of Laccase on fe3o4–Graphene Oxide Nanocomposite for Biodegradation of Phenolic Compounds. Environ. Prot. Eng 2021, 47(1), 85–99. DOI: 10.37190/epe210107.
- Yaohua, G.; Ping, X.; Feng, J.; Keren, S. Co-Immobilization of Laccase and Abts Onto Novel Dual-Functionalized Cellulose Beads for Highly Improved Biodegradation of Indole. J. Hazard. Mater 2019, 365, 118–124. DOI: 10.1016/j.jhazmat.2018.10.076.

![Figure 2. Schematic representation of the papermaking process [adapted from 27]. Dashed arrows: fresh water consumption; bold arrows: wastewater generation.](/cms/asset/2968fd00-5ba4-48a8-a821-484fcc96543a/lspr_a_2382313_f0002_b.gif)
![Figure 3. The wastewater treatment processes [adapted from 40].](/cms/asset/cef1075b-d737-4b7e-bab9-907b6a0bd14c/lspr_a_2382313_f0003_oc.jpg)
![Figure 4. Biocatalyst groups used in wastewater treatment [adapted from 50].](/cms/asset/11d16476-cf11-466f-9bac-fe5040d9a770/lspr_a_2382313_f0004_oc.jpg)
![Figure 6. Enzyme immobilization techniques [adapted from 116].](/cms/asset/297ba5ba-4548-4f68-b987-d991f0c35f53/lspr_a_2382313_f0006_oc.jpg)