ABSTRACT
Our objective was to research ways to introduce regenerative agriculture into the fragile landscape of the Southern Altiplano of Bolivia. The quinoa (Chenopodium quinoa) boom (2010–2014) had stimulated farmers to clear large areas of native vegetation: a climax community of shrubs, grasses and cacti. Most fields were soon abandoned, and native plants did not grow back spontaneously. Wind was rapidly eroding the sandy soils. Botanical exploration, informed by local knowledge, discovered species and ecotypes of wild plants, especially shrubs, legumes, grasses and cacti that could be grown as intercrops and as live barriers to control erosion. These plants were evaluated in farmers’ fields, using participatory research. New varieties of quinoa were developed by conventional plant breeding. Researchers learned to grow wild shrubs and grasses in live barriers, to control soil erosion. Wild, native lupines were cultivated for the first time, to use as cover crops. Native cacti were grown in nurseries to encourage farmers to plant them near fields. The new quinoa varieties were better adapted to the local environment. We conclude that this innovative, broad-spectrum research agenda is a kind of plant breeding at the level of the whole landscape. These multiple lines of research are important for developing a diverse, integrated, regenerative agriculture.
Introduction
“We are destroying the productivity of the same soil from which we demand a relentless increase in production.”
Christopher Rhodes (Citation2017)
The earliest experiences with the Green Revolution, beginning with the breeding of high-yielding wheat varieties in Mexico in the 1950s, were focused on a narrow goal: raising yields enough to enable smallholder farmers to feed their families. The model focused on one crop at a time (e.g., wheat, then maize) to make it more responsive to synthetic fertilizers, while encouraging the use of chemical pesticides (Mann Citation2018).
Agrochemicals, combined with tractor tillage, provoked soil degradation. One third of all agricultural soil across the globe is now degraded, e.g., half the topsoil in Iowa has been lost since the 1880s. Plowing releases carbon, and kills some of the living things in the soil. Tillage also breaks up the delicate structures (glomalin) made by fungi that hold the soil together. Mycorrhizal fungi and microbes in the soil extract mineral nutrients from rock fragments and help to break down organic matter so that plants can use it. Microbes trade phosphorous to plants for sugars. Predatory arthropods, nematodes and protozoa eat the microbes and release the nutrients from their bodies back into the soil. These living things make soil more fertile, but they are harmed by the heavy use of chemical fertilizer and soil tillage (Montgomery Citation2017).
In the 1980s, Robert Rodale argued that “regenerative agriculture” was needed to restore the health of the farm environment (Rodale Citation1989; Sherwood et al. Citationin press). Regenerative agriculture (and similar approaches such as agroecology) seek to mimic or harness complex ecological processes, e.g., building healthy soils and conserving water by keeping a permanent ground cover, by diversifying crop species and varieties, and by integrating crops with livestock (Anderson et al. Citation2021), and through integrated “systems” thinking, rather than isolating components (Rhodes Citation2017).
Regenerative agriculture is “soil-centric, rather than seed-centric,” enhancing soil organic matter (SOM) while improving the biogeochemical cycling of carbon, water, nitrogen, phosphorous and other nutrients. Global agriculture is already producing enough food to feed 10 million people, although 30% of it is wasted (Lal Citation2020). It is important to break the vicious cycle of: produce, waste, degrade, pollute and repeat (Lal Citation2020).
The techniques of regenerative agriculture (adapted from Brown Citation2018; Lal Citation2020; Montgomery Citation2017; Sherwood et al. Citationin press) include:
Eliminating and reducing pesticides
Minimizing soil disturbance, e.g., by direct seeding without plowing
Maintaining ground cover, e.g., through cover crops, mulch, trees and other perennials which nourish the soil and conserve moisture
Adding organic fertilizers and sowing plants that improve the soil (e.g., nitrogen-fixing legumes) while avoiding chemical fertilizers
Integrating animals into the system. The grazing, trampling and manuring improves soil health.
Regenerative agriculture is being taken to scale on various commercial family farms. For example, across a period of 20 years, North Dakota farmer Gabe Brown tripled soil carbon, from a low of 1.7%, by using a rotation of diverse cover crops, and by grazing them with cows and other livestock. His wheat yields increased from under 40 bushels per acre to over 60 (i.e., from 2.7 tons/ha to 4 tons/ha). Grasses and other annual plants evolved to be grazed on. This low-input, plant-animal system works because when livestock wound plants, they exude compounds which enrich the soil (Montgomery Citation2017; Brown Citation2018).
In this article, we argue that a diverse, integrated, regenerative agriculture is best supported by multiple strands of complementary research. Few farm environments are more difficult to regenerate than the quinoa (Chenopodium quinoa) system in the Southern Altiplano of Bolivia. Agriculture is barely possible in this high, dry, environment: above 3600 meters in elevation. It’s cold much of the time, with daily minimum temperatures averaging 5.7°C, and annual low temperatures reaching −23.5°C. Annual rainfall varies from just 60 mm to 250 mm. In this harsh environment the climax ecological community is a dwarf forest of shrubs and grasses (). Quinoa is the only crop that will grow on the Southern Altiplano, although llamas (Lama glama) thrive here. Some farmers also grow the semi-domesticated qañawa (Chenopodium pallidicaule) for subsistence (Bonifacio Citation2019a).
Table 1. Dominant shrubs and grasses of the Southern Altiplano.
Before the mid-twentieth century, farmers would grow quinoa in fields sheltered by the gently rolling hills of the Southern Altiplano. Farmers agreed that the open plains were too cold to grow a crop. Yields were low in the hills, about 150 kg per hectare (personal observation), and farmers would take their harvested quinoa (and llama jerky – dried meat) on trains of llamas to distant peasant communities to trade for potatoes (Solanum tuberosum subspecies Andigenum), maize (Zea mays), Andean roots and tubers such as oca (Oxalis tuberosa) and other crops.
About 2010, quinoa became fashionable as a health food in northern countries. In 1998, the world production of quinoa was 49,400 tons, almost half of which (20,921 tons) was from Bolivia. By 2016, world production had tripled to 148,720 tons. The Bolivian quinoa harvest had more than tripled, to 65,548 tons (Montero and Romero Citation2017).
From 2010 to 2014, the quinoa boom largely destroyed native vegetation and pasturelands on the plains of the Southern Altiplano of Bolivia (Risi, Rojas, and Pacheco Citation2015). Fragile, sandy soils were plowed with heavy tractors (Orsag et al. Citation2013). Since 2014, Bolivian quinoa production and exports have fallen, at least partly because of competition from other countries, which have started producing the crop, leaving many recently converted fields in Bolivia exposed to soil erosion by wind (Durán Citation2019).
During the quinoa boom (2010–2014), prices rose from as low as $0.50 per kg to about $6 per kg (McDonell Citation2018). The preferred quinoa varieties to export were the large, white-grained quinua real (“royal quinoa”), once grown in the narrow isthmus between the two salt flats: Uyuni and Coipasa. During the boom, these varieties rapidly expanded to most of the Southern and Central Altiplano (Bonifacio et al. Citation2014a).
On the Southern Altiplano, much of the land on the plains was owned by communities, not by individuals. Farmers began to plow up large areas of land, which had never been farmed before. Planting quinoa on the open plains may have been made possible because climate change had increased temperatures enough to do so, and not just in the surrounding hills (Gandarillas et al. Citation2014). This is not unusual: the ranges of crops are expected to shift in much of the world, e.g., wheat and barley are expected to soon be planted at higher altitudes in Ethiopia (Gebresamuel et al. Citation2022).
The senior author’s personal observations of the area across many years suggest that 70 to 80% of the native climax vegetation was plowed up in the early 2010s. This astounding rate of deforestation was especially wasteful, since only about 30% of the cleared land is still cultivated. The soil is about 70% sand, 30% gravel, with less than 1% organic matter, making it erosion-prone when mechanically plowed (Bonifacio et al. Citation2014a). Little or no vegetation has grown back on the cleared land (personal observation).
High winds in the Southern Altiplano can lift soil particles as high as 50 cm above the ground, leading to annual nitrogen losses of 7.5 kg/ha (Alandia et al. Citation2012). Live barriers (trees and shrubs usually planted along field edges, perpendicular to the direction of the prevailing winds) can be an effective soil conservation method (Udhaya and Sakthinathan Citation2017). Even at the start of the quinoa boom, the lead author and colleagues at Proinpa (Foundation for the Promotion and Investigation of Andean Products) anticipated an ecological disaster, and by 2010 had an integrated research agenda involving perennial plant species, cover crops, and livestock (llamas). The perennial plants could be planted as live barriers to mitigate wind-caused soil erosion ().
Native Andean shrubs
Proinpa began research on native shrubs in 2009–2010. Early studies suggested that live barriers could be made with the native shrub sup’u t’ula (Parastrephia lepidophylla). It was assumed that the shrub could be attractive to farmers because of its various uses, e.g., domesticated llamas and wild vicuñas (Vicugna vicugna) browse on young sup’u t’ula, and local people use it as firewood (Bonifacio et al. Citation2014a).
Basic research on the biology and reproduction of these shrubs produced several theses, conducted in collaboration with Proinpa. They showed that sup’u t’ulu and another dominant shrub, uma t’ula (Parastrephia lucida) were more likely to germinate in nurseries if composted manure was added to the soil (Chillo Citation2015; Huanca Citation2016).
Llamas are part of the quinoa farming system, eating the grasses that cover the fallow land, and enriching the soil with their manure. While llamas refuse quinoa stalks, they will eat the jipi, the chaff left over from threshing quinoa (Mollisaca and Bonifacio Citation2021). This quinoa system also includes wild, native legumes, growing spontaneously in some places. However, with proper management, native lupines could be widely grown as a cover crop in quinoa fields.
Native legumes
With the destruction of native vegetation on the Altiplano, researchers at Proinpa realized that the quinoa crop should include a legume intercrop. However, no known legume crops could survive the local conditions.
Broad beans (or faba beans) are native to the Old World, but have long been grown in the lower, warmer Andean valleys of Bolivia. Dry soils and high temperatures have major negative consequences on the growth of this edible legume (Kibbou Citation2022). Under advice from NGOs, farmers planted broad beans, peas and other legumes, which all died. Researchers hypothesized that a wild, native legume, such as the lupine, might grow as a cover crop, when intercropped with quinoa (Bonifacio Citation2014a).
In the native Aymara language of the Altiplano, q’ila q’ila is a generic name for wild lupines; like the synonym t’uqu t’uqu; the term reflects the popping sound the mature pods make when exploding. Other synonyms in Aymara include salqa (“escape”), sarqawi “in the process of escaping,” salqiri “erratic, deceitful” (Bonifacio, Aroni, and Villca Citation2018a). The q’ila q’ila on the southern Altiplano include various species, e.g., Lupinus chilensis, L. otto-butchinii and L. montanus (Bonifacio et al. Citation2014a), just some of the 83 species known in the Andes (Jacobsen and Mujica (Citation2006) and 85 in South America (Barney Citation2011). The plants tend to grow in wild stands as small as 100 to 200 m2, and as large as 1 km2 (Bonifacio et al. Citation2014a).
Tolerance to drought and frost make the nitrogen-fixing q’ila q’ila a promising candidate for a cover crop. The seedlings of q’ila q’ila emerge with the summer rains of December-January. The plants grow slowly throughout the winter (June-July), flowering in November-December, bearing seeds in December-January. If lupines are planted in December-January as an intercrop, three months after quinoa is planted, these legumes can cover the soil after quinoa is harvested. Q’ila q’ila generates 8 tons of dry matter per hectare, fixing 100 kg of nitrogen, potentially raising quinoa yields by 1 t/ha. Q’ila q’ila can also be planted after quinoa is harvested, as an improved fallow plant (Bonifacio et al. Citation2014a).
By 2008, Proinpa was studying the native lupines for their potential as an intercrop in quinoa (Bonifacio et al. Citation2014a). While llamas reject dry quinoa stalks, the animals readily eat green quinoa plants, potentially placing herders at odds with quinoa growers, whose scattered, unfenced plots are difficult to protect from grazing – another reason for the decline in livestock numbers since the mid-twentieth century. However, if properly managed, more llamas would produce more manure, which could fertilize more quinoa (Bonifacio et al. Citation2014a). Fields on the Bolivian Altiplano with wild legumes had 1.8 times as much carbon and 1.6 times as much nitrogen as other parcels (Sivila de Cary and Hervé Citation2006).
Quinoa and cactus
This paper focusses on shrubs as live barriers, and intercropping with native lupines, but this research was complemented by crop breeding and studies of native cactus. Proinpa bred six new large-grained, white varieties of quinoa to be better adapted to the area (e.g., early-maturing and mildew-resistant) (Bonifacio, Vargas, and Aroni Citation2014b; Bonifacio, Gómez-Pando, and Rojas Citation2014c; Bonifacio Citation2019a). Native cactus had also suffered from land clearing, and restoring them would help to protect the soil, while also providing fruit that local people enjoy eating. The research team studied the reproduction of cactus (e.g., how to harvest and plant the seeds in nurseries). The pasacana cactus (Trichocereus pasacana) is also part of the climax ecological community and will become endangered unless steps are taken to protect it (Bonifacio Citation2019b; Caviña and Bonifacio Citation2020). Together, these four topics (quinoa, cactus, shrubs, lupines) formed an integrated research agenda for regenerative agriculture for the Altiplano.
The present study suggests that several species of native shrubs can be used as live barriers to control soil erosion on the Southern Altiplano. Wild, native lupines can be sown as an intercrop in quinoa to contribute to improved soil fertility in this part of Bolivia.
Native Andean shrubs
Methods and materials: live barriers of native shrubs
In 2017–2018, Proinpa began research trials on live barriers in the farm communities of Sevaruyo, in the department of Oruro, and Chita and Chacala, both in Potosí, on Bolivia’s Southern Altiplano, in collaboration with 25 farmers (including seven women). Sevaruyo is further north of Chita and Chacala, and receives more rain.
The researchers suggested to the farm communities that the live barriers should have three lines of shrubs, to block the wind. However, some of the farmers were reluctant to devote this much land to the live barriers, proposing other designs instead, e.g., using only two rows of native shrubs ().
Table 2. Design of live barriers with four native shrub species.
Proinpa gave training and free seedlings to the collaborating farmers, who provided their land, labor, time and creativity. The barriers were planted as a mix of ñak’a t’ula, sup’u t’ula, uma t’ula and a native grass: sikuya (Stipa ichu) ().
Figure 2. Organized farmer-experimenters plant a live barrier of native plants as a windbreak to control soil erosion.

Farmers observed that another grass, sikuya alto (Anatherostipa orurensis), was fast-growing and might function well in the live barrier. At the suggestion of the farmers, this species was added to the trials.
The trials aimed to determine which species had the highest rate of survival and which ones adapted best to the local environments. In meetings with the researches, community members agreed to collect data on the number of plants that survived, their height, and the diameter of the soil-surface area covered by the shrubs. Each farmer had a notebook to write down data, with support from Proinpa staff.
Because these plants grow slowly, the team collected data only three times: 1) January-May, to determine growth after the brief rains, 2) June-September, following the windy, Bolivian winter, 3) October-December, after the driest part of the year. Later, the results of the trials were analyzed with the farmers. In the community of Chita, nonparticipating farmers also expressed interest in the research, so a meeting was held to analyze the results with all interested local people there.
Results: live barriers
Live barrier experiments in farmers’ fields
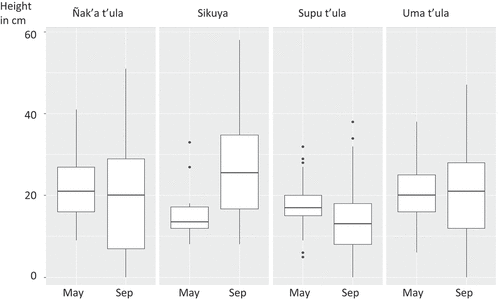
The native species transplanted to the live barriers survived the wettest part of the year (January-May). More plants died in the winter (June-September), due to frost, strong winds and damage from grazing llamas. No plants died during the warmer, spring months of October-December. Of the species, sikuya had the highest rates of survival ().
Table 3. Death of recently transplanted live barrier plants by season and species.
During the data analysis, the farmers observed that only the uma t’ula and sikuya grew during the harsh winter (Jun-Sep), while the other plant species actually became shorter due to the cold and wind, and because of grazing by llamas, sheep, jackrabbits or hares (Lepus sp.), the highland tuco-tuco (a burrowing rodent, Ctenomys opimus) and other animals (). Uma t’ula is not palatable, and animals will not eat it, which explains why it increases in size year-round.
During the winter the young shrub plants perform best in the less sandy soils. Farmers like shrub species that provide fodder to livestock, but browsing pressure may also increase the mortality and decrease the growth of these plants. Uma t’ula and sikuya may be the more promising species for live barriers, because animals do not eat them.
Ñak’a t’ula and sup’u t’ula did not grow between May and September. On average, the plants diminished from 21 cm to 20 cm (ñak’a t’ula) and from 17 cm to 14 cm (sup’u t’ula). At the same time, sikuya grew from 14 cm to 26 cm and uma t’ula went from 20 cm to 21 cm ().
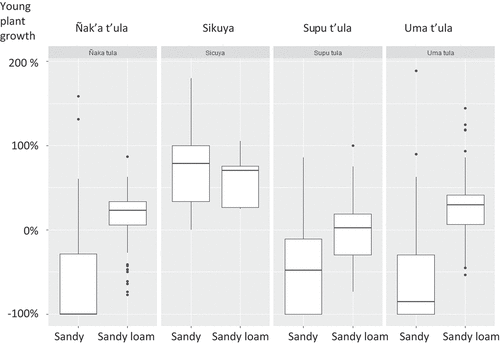
In sandy soils, the winter was especially hard on the plants. From May to September, on sandy soils, the ñak’a t’ula lost all of its above-ground tissue. Sup’u t’ula lost 50% and uma t’ula 80%. Only sikuya grew (80%). In sandy loam soils, all of the shrub species grew, although sup’u t’ula grew only slightly ().
Botanical exploration (shrubs and grasses)
Besides the four shrub species identified above, in the agricultural season of 2018–2019, the lead author and colleagues sought out additional species, looking for tall plants with accessible seed, well-adapted to sandy soils and where llamas were observed to browse. Explorations around the communities of Orinoca and Andamarca in Oruro, and Chacala and Chita in Potosí identified t’it’i t’ula (Lepidophyllun quadrangulare), kaylla (Margiricarpus cristata), siwinqa (Cortadria sp.), pasto aguja (Nassella neesiana), sikuya grande or sikuya alto (Anatherostipa orurensis) and lamphaya (Lampaya castellani). By 2019–2020 the research team began to study the germination of these species in nurseries. Lamphaya and pasto aguja failed to germinate when planted, but the other species could be reproduced in nurseries (See Section 3.1).
The seed was germinated in native soil in trays. The seedlings were transferred to black plastic bags until they were mature enough to transplant to the live barriers (). Proinpa maintained two nurseries, one at the research station of Khipakhipani, near Viacha, La Paz, on the Central Altiplano, and another one run by a community member in Chacala. The collection, processing, planting and transplanting of native shrubs is demonstrated in a farmer-to-farmer learning video, Living windbreaks to protect the soil (Agro-Insight and Proinpa Citation2019).
Transplanting native shrubs allows the roots to be placed deep into the soil. This allows the seedlings to take root, while seeds sown directly in the field will not survive. The seeds die before or soon after they emerge from the soil, from drought and because of wind erosion that uncovers the shallow roots. Transplanting allows the roots to be placed deeper into the soil, where wind will not uncover them.
In the field, the research team identified four new species of native shrubs, and two grasses, as candidates to test in live barriers. Tara t’ula or tara tara (Fabiana densa) is a tall shrub. It thrives in the low hills around the plains, in stony, sandy and sandy-loam soils. The research team observed parasitic wasps visiting the nectaries on the shrub’s flowers. These parasitic Hymenoptera help to control the quinoa caterpillar (Eurisacca quinoa).
Lamphaya (Lampaya castellani) is an evergreen shrub, which produces abundant leaf litter. The leaves and branches spread over the soil, protecting it from erosion. The stems thrive even when covered by windblown sand. The lamphaya’s leathery leaves resist abrasion damage from wind and sand.
Kaylla (Margyricarpus cristatus) is low-growing, evergreen shrub with small thorns. It is favored by browsing llamas. The plant performs so well in poor soil that a single-species stand of kaylla indicates degraded soil.
T’iti t’ula (Lepidophyllun quadrangulare) thrives in sandy soils, even in dunes.
Waylla ichhu (Festuca orthopylla) means “roofing thatch” in Aymara, because its tall, stiff stalks are ideal for keeping out the rain. This grass can reach 1.6 meters in height and grows in sandy soil, even in dunes. Llamas graze on the leaves, flowers and the tender shoots. This tall, hardy plant may be an ideal live barrier species.
Siwinqa (Cortaderia sp.) may be well-suited as a windbreak species, because it can easily reach 2.2 meters in height. It is spontaneously spreading from the hills and foothills onto the plains, perhaps favored by the warming climate.
The research team collected seed samples from these candidate species and found that the shrubs (tara t’ula, t’iti túla, and lamphaya) were easy to collect. They all germinate readily when planted in trays of native soil and watered, except for lamphaya, which has a hard seedcoat and seems to enter into dormancy when the seeds reach maturity.
After the botanical explorations, the team began to experiment with the seed. Of 1,000 seedlings of t’iti t’ula, 837 survived the winter in the Proinpa nursery. Tara t’ula was susceptible to frost, but by February, 2020, 1400 seedlings had been reared in the nursery in Chacala and transplanted in farmers’ fields as live barriers.
Experiments showed that fresh seed, collected from wild stands before the pods had dried, germinated at rates of 95% or higher, even when kept in the refrigerator for 10 months. Dried seed, collected from plants after the pods had burst open, and scarified, germinated at rates of 62% to 86%. This suggests that the easiest way to collect seed may be to gather it when it is mature, but not yet dried, and plant it while it is still fresh (Bonifacio et al. Citation2018b).
Scaling up
Since 2010, Proinpa has planted over 100 live barriers (8200 linear meters) in farmers’ fields in the Southern Altiplano. Recently, people have started to visit the Proinpa nurseries, asking to buy native plants. The idea of planting live barriers may be starting to appeal to local people. Having a great number of well-established barriers and seeing their effects have clearly added motivation.
Native Andean legumes
Farmer experiments (method)
In 2014–2015, Proinpa planted trial fields of wild lupines in the fields of three farmers (two men and one woman) in Chacala and Colchani, both in Potosí, Bolivia. The wild lupines are poorly described botanically. The species observed on the Altiplano could be as many as nine species, although they may be just three (an annual, a biannual species and a perennial). Proinpa has identified nine ecotypes.
The biennial wild lupine has a 16-month cycle, making it ideal to intercrop with quinoa, which has a 6-month cycle. Quinoa is planted in September, taking advantage of the limited residual moisture. In January, 4 months later, wild lupines can be planted as an intercrop, between the rows of quinoa. When the quinoa is harvested the following March, the wild lupines remain as a soil cover. The lupines will still be growing when quinoa is planted the following September, but they will not be in the way. Farmers can plant another field, or plow up the lupines, or hand-plant the quinoa between the rows of the small legume plants.
Farmer experiments (results)
With encouragement from Proinpa, several farmers on the Southern Altiplano have been experimenting with intercrops of wild, native lupines. For example, in 2015, local farmer Valentín Mamani in Chacala planted a field of wild, native lupines (q’ila q’ila), which he intercropped with quinoa for three seasons. In 2019, he decided to plow the lupines into the soil. When the researchers visited his plot, they found that the hardy lupines had spread spontaneously to neighboring fields.
In 2015, local farmer Lidia Ramos in Colchani planted a relay crop of native lupines in a field of quinoa. In 2017, she decided to incorporate the lupines into the soil, and plant quinoa. In January of 2018, researchers observed new, volunteer lupine plants growing in her field. In February 2019, the farmer again plowed the field, incorporating the volunteer lupines. By January 2020, a second generation of volunteer lupines was growing in the field between the rows of quinoa.
In January 2017, local farmer Teodocia Vásquez used a tractor to plant native lupines in her field in Chacala, but germination was poor. The following year, January 2018, she planted lupines again, by hand and with a hand-drawn seed drill. The plants thrived and the farmer plowed them into the soil in February 2019. In October 2019, she again planted quinoa, and the lupines germinated spontaneously with the onset of the rains in January 2020.
These three farmer experiments suggest that the wild, native lupines are highly adapted to the natural environment of the Southern Altiplano. These legumes are so well-adapted to the local sandy soil that they only need to be planted once. After that, they will self-seed and reproduce in an area where they were sown.
Collaborative trials with farmers (method)
In the agricultural season of 2018–2019, a plot of wild lupines was planted on a family farm in Chacala. In 2019–2020, another plot was added in Chita; these are the two driest, southern-most communities.
Collaborative trials with farmers (results)
In February, 2019, two plots of wild, native lupines were planted in the community of Chacala, both in farmer’s fields. On the first plot, on the Cruz family farm, lupines were planted with a hand-pulled seed drill invented by the lead author. Eighty percent of the seed germinated, although the plants grew slowly because of weeds and grazing by livestock.
On the second plot, on the Sánchez family farm, some of the seed (Orinoca ecotype) was planted with a tractor, and some (Habas Cancha and Choclito ecotypes) was planted with the hand-pulled seed drill. The germination rate was higher for the lupines planted with the seed drill (80%) than for those planted with a tractor (60%). Lupines on both the Cruz and Sánchez farms were nearly wiped out by grazing llamas, sheep and wildlife.
In 2020, another parcel of the Orinoca ecotype of lupine was planted in the community of Chita, with a tractor, and 60% of the seed germinated.
From 2010 to 2022, wild lupines were planted in 19 farmers’ fields, in a total of 3.8 hectares. Orinoca is one of the most promising ecotypes of wild lupine. Observations of these fields over several years confirmed that if conditions do not permit this ecotype to bear seed its second year, it lives a third year, to yield seed. This flexibility makes the Orinoca ecotype especially adaptable to the Southern Altiplano.
Wild pheasants (p’isaqa) dig up germinating seeds of lupines to eat the sprouts. Llamas and other animals eat the green lupine plants. Flies (Delia sp.) lay their eggs on the soil where their larvae hatch, and penetrate the root of the germinating plantlet, often killing it. Some noctuid (Lepidoptera) larvae also feed on the leaves.
These collaborative farmer-scientist trials suggest that it may be possible to plant and manage wild lupines, which can be intercropped with quinoa. However, more research is needed on how to improve the survival of planted lupines. For example, the Orinoca ecotype performs well on sandy soils, but problems include the Delia fly, sheep and hares. Future research will include planting this lupine in dry conditions, because the fly favors moister soil. With damage from sheep, it may be possible to coordinate grazing times with livestock owners.
On-station germination trials (method)
Seed was collected at four levels of maturity: 1) green 2) yellow 3) physiologically mature, but not dry, and 4) completely mature and dry. Seed was scarified and planted in trays in the Proinpa nurseries. Data were collected on the germination rates of each level of seed maturity.
On-station germination trials (results)
In 2019 and 2020, the research team collected native lupine seed in the wild and tested it for germination at different stages of maturity. When the seeds are mature and dry, the pods shatter and the seed enters a dormancy of several years (Bonifacio, Aroni, and Villca Citation2018a). However, seed that is not dry, even green seed, germinates readily. After a week, almost all of the seed had germinated, except for the dry grains (). Results are shown for the Orinoca ecotype but were similar for the Qalamarca ecotype (results not shown).
Table 4. Germination rate (%) of wild lupine seed in different stages of maturity in one week.
Botanical exploration (method)
Seeds of wild legumes were collected in Chacala, Chita, Orinoca, Habas Cancha, Chaquilla, Chiutaca and Tica-Tica. Seed was selected from plants that flowered in October and November (just before the onset of the rains). Experiments were conducted with mature fresh seed (that had not dried), and with dried seed that had been scarified (mixed with river sand and then treading or machine mixing it for 60 min.) (Bonifacio Flores et al. Citation2018b).
Botanical exploration (results)
The different ecotypes of lupines are adapted to different natural environments within the Altiplano. Some ecotypes can be eaten by livestock when green. Some lupines have plant toxins, but their amounts decrease as the plants age. Most of the wild lupines on the Altiplano can be eaten by livestock and wildlife when dry (Bonifacio, Aroni, and Villca Citation2018a). There are lupine ecotypes adapted to all of the Altiplano, but each one is restricted to certain environments ().
Table 5. Selected ecotypes of wild lupines of the Bolivian Altiplano.
Cactus
The pasakana (Trichocereus tarapacana pasacana) is a tall (6–8 meter) cactus, native to the Southern Altiplano. Habitat damage and drought have endangered this plant species. The research team studied the pasakana using a mix of local knowledge, direct observation and experimentation. The common name for this cactus in Spanish (“pasacana”) comes from the Aymara term for the fruit, whereas the native term for the plant itself is “qhiwilla” or “qhiwayllu” (Bonifacio Flores Citation2019b).
The pasakana is adapted to volcanic soils. Researchers measured the height of transplanted individuals, and observed healthy regrowth on plants that had lost their thorns in accidental fires. These measurements confirmed that the cactus grows slowly (5.0 to 9.3 cm/year). The plants do not flower until they are 10–12 years old. An exceptional individual that is 10 meters tall may be 180 years old. This cactus is adapted to volcanic soils. Its diurnal flowers are visited by medium-sized, native bees, so the pasakana is probably cross pollinated. Each cactus yields 11–19 sweet, juicy fruits per year, up to one kilo per plant (Bonifacio Flores Citation2019b).
Local people know that (in this land of erratic weather) the flowering of the pasakana signals that it is a good time to plant potatoes (Bonifacio Flores Citation2019b; Choquetopa Citation2021). In the wild, fruits dry on the plant, eventually falling to the ground. Ants gather the seeds and take them to their nests, which is how at least some of these cacti are seeded. The seed coats are hard, and the seed remains dormant for years. In nurseries, researchers germinate the seed by first scarifying them (rubbing them with sand), to break their dormancy (Bonifacio Flores Citation2019b). Seedlings of pasakana and other native plants from the Proinpa nursery in Viacha are now being shared with interested farmers and local governments (Bentley Citation2018).
Discussion
Discussion: live barriers
Various species of wild native shrubs and grasses can potentially be grown as live barriers, to prevent soil erosion by wind. However, when the plants are small, some species suffer from the cold and the effects of grazing. Most of these wild species perform better in sandy loam soil than in the more abundant sandy soil. Four species have been relatively well studied and several more have been added to the research agenda. Some of these species seem especially promising, e.g., kaylla thrives in highly degraded soil (of which there is quite a lot on the Southern Altiplano), and siwinqa, pampas grass, grows to be two meters tall, possibly conserving soil better than the shorter, shrub species.
We hypothesize that without climate change, it may not have been necessary to plant t’ula in nurseries. Before global warming, the shrubs may have self-seeded. It is also possible that removing more than half of the climax plant community has impaired its ability to reproduce itself. Or perhaps reducing the llama population has negatively impacted the plants’ ecology, and their ability to reproduce.
Discussion: native legumes
Farmer experiments across several years suggest that native lupines will survive when planted in farmers’ fields, whereas domesticated legumes will not. The native lupines seed themselves, and even when plowed into the soil will grow back in the quinoa field.
In the collaborative trials some lupine plants suffered from grazing by livestock and wildlife. If the lupines are grown on a larger scale, steps may have to be taken to protect them from animals.
Native lupines planted in farmers’ fields have high rates of germination, especially when planted by hand or with a hand-pulled seed drill. Some ecotypes (e.g., Orinoca) perform especially well in the cold, dry climate of the Southern Altiplano, suggesting that it is possible to cultivate wild ecotypes.
The lupines can be easily planted by selecting seed before the pods are completely dry. Even green seed is viable, which helps to explain why native lupines grow back as volunteers when the plants are plowed under.
These native legumes can be planted from seed, to intercrop them in quinoa fields, providing ground cover to help manage soil erosion, while enriching the soil with nitrogen and carbon.
Conclusion
The increases in quinoa prices in the early 2010s allowed thousands of indigenous Bolivian peasant households to briefly escape poverty, only to be thrust back into it when the bonanza ended. After the Southern Altiplano was largely stripped of vegetation, native plants failed to grow back in the sandy soil. Regenerative agriculture for these conditions required research that was narrowly focused on some topics (e.g., seed germination of wild plants), while also considering the whole farming and natural environment.
New quinoa varieties were bred to meet (national) market demand, while also being adapted to the local environment. Botanical exploration identified shrubs and grasses that could be planted to form windbreaks for soil-erosion control. Plant biology was studied, as was their ability to live as live barriers on the edges of quinoa fields. Cactus biology was also researched, as these plants can be grown between the quinoa fields.
Since no domesticated legumes could withstand local conditions, wild species were identified and studied as quinoa intercrops. Several of the lupine ecotypes performed well in the area. Llamas, which were once common as livestock in the area, are now being encouraged on the landscape, along with their wild relatives, the vicuña. The animals browse on the windbreaks, eat the lupine cover crops and manure the soil. Llamas can be fed on the chafe (jipi) salvaged from quinoa threshing.
Some topics remain to be examined further; e.g., llamas may overgraze some of the live barrier species, especially in their first year, and damage the young plants. But this is a management issue that farmer-experimenters can help to resolve.
This research suggests that an integrated system can be developed, built around a food crop (quinoa), a cover crop (lupines), perennial shrubs and grasses, with livestock (llamas). This approach has also been called “breeding for landscapes,” because the emphasis is on the full farming system and the surrounding natural environment, rather than a reductionist approach to single components (Nicklin Citation2017). All of the species are native, and some are wild. Much remains to be done to promote this regenerative agricultural model among farmers, but developing the key concepts has been a necessary start.
Acknowledgments
This research was generously supported by the Collaborative Crop Research Program (CCRP) of the McKnight Foundation. Thanks to Paul Van Mele and the anonymous reviewer for their valuable comments on a previous draft of this paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Agro-Insight and Proinpa. 2019. “Living Windbreaks to Protect the Soil.” [Video]. www.accessagriculture.org
- Alandia, G., S. Calderón, B. Condori, and S. Jacobsen. 2012. Quantification of Wind Erosion under Four Different Types of Vegetation Cover in Quinoa Fields of the Southern Bolivian Highlands. Wageningen University: Agro Environ (Poster).
- Anderson, C. R., J. Bruil, M. J. Chappell, C. Kiss, and M. P. Pimbert. 2021. Agroecology Now! Transformations Towards More Just and Sustainable Food Systems. Cham, Switzerland: Springer Nature.
- Barney, M. 2011. “Biodiversidad y Ecogeografía del Género Lupinus L. (Leguminosae) en Colombia.” M.Sc. Thesis, Universidad Nacional de Colombia, Palmira, Colombia.
- Bentley, J. 2018. Awakening the Seeds. Agro-Insight blog. https://www.agroinsight.com/blog/?p=2839
- Bonifacio, A. 2019a. “Improvement of Quinoa (Chenopodium Quinoa Willd.) and Qañawa (Chenopodium Pallidicaule Aellen) in the Context of Climate Change in the High Andes.” Ciencia e Investigación Agraria 46 (2): 113–124. doi:10.7764/rcia.v46i2.2146.
- Bonifacio, A., G. Aroni, and M. Villca. 2018a. “Adaptación y Perspectivas de Aprovechamiento del Lupino Silvestre en Sistemas de Producción del Altiplano.” Revista de Agricultura 57: 10–18.
- Bonifacio, A., G. Aroni, M. Villca, P. Ramos, M. Alarcón, and A. Gandarillas. 2014a. “El Rol Actual y Potencial de las Q’ila Q’ila (Lupinus spp.) en Sistemas de Producción Sostenible de Quinoa.” Revista de Agricultura 54: 19–28.
- Bonifacio Flores, A. 2019b. “Características Morfológicas y Reproductivas de la Pasakana (Trichocereus tarapacana subsp. Pasacana) en el Distrito Municipal de Orinoca, Sur Carangas, Oruro.” Apthapi 5 (2): 1564–1573.
- Bonifacio Flores, A., M. Alcon, G. Aroni, and M. Villca. 2018b. “Métodos de Recolección y Tratamiento de Semilla de Salqa o Q’ila-Q’ila (Lupinus spp.).” Revista de Investigación e Innovación Agropecuaria y de Recursos Naturales 5 (2): 81–89.
- Bonifacio, A., L. Gómez-Pando, and W. Rojas. 2014c. “Mejoramiento Genético de Quinua y el Desarrollo de Variedades Modernas.“ In Estado del Arte de la Quinua en el Mundo en 2013, edited by D. Bazile, H. D. Bertero, and C. Nieto, 203–226. Rome: Food and Agriculture Organization of the United Nations (FAO), and Montpellier: Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD).
- Bonifacio, A., A. Vargas, and G. Aroni. 2014b. “Generación de Variedades en un Contexto de Mercado y Cambio Climático.” Revista de Agricultura 54: 29–35.
- Brown, G. 2018. Dirt to Soil: One Family’s Journey into Regenerative Agriculture. White River Junction, Vermont: Chelsea Green Publishing.
- Caviña Fernández, J. G., and A. Bonifacio Flores. 2020. “Características de la Pasacana (Trichocereus pasacana) y Multiplicación Dirigida en la Estación Experimental Kiphakiphani.” Apthapi 6 (1): 1805–1819.
- Chillo Quispe, E. 2015. “Efecto de Abonos Orgánicos y Bioinsumos en el Crecimiento de la Uma T’ula (Parastrephya lucida) en el Centro Experimental Quipaquipani, Viacha.” Thesis, Universidad Mayor de San Andrés, La Paz, Bolivia.
- Choquetopa Rodríguez, B. 2021. Indicadores Naturales para Pronosticar el Tiempo en el Sur de Oruro, Bolivia. Jeffery Bentley (Ed). Cochabamba: Agro-Insight and the McKnight Foundation.
- Durán, T. 2019. “Quinua Export. Producto Milenario, Mercado E Instituciones En el Altiplano Boliviano.” Temas Sociales 45: 10–35.
- Gandarillas, A., W. Rojas, A. Bonifacio, and N. Ojeda. 2014. “La Quinua en Bolivia: Perspectivas de la Fundación Proinpa.” Chapter 5 In Estado del Arte de la Quinoa en el Mundo en 2013, edited by D. Bazile, H. D. Bertero, and C. Nieto, 410–431. Rome: FAO, and Montpellier: CIRAD.
- Gebresamuel, G., H. Abrha, H. Hagos, E. Elias, and M. Haile. 2022. ““Empirical Modeling of the Impact of Climate Change on Altitudinal Shift of Major Cereal Crops in South Tigray, Northern Ethiopia.” Journal of Crop Improvement 36 (2): 169–192. doi:10.1080/15427528.2021.1931608.
- Huanca Tusco, M. L. 2016. “Multiplicación Masiva y Crecimiento Acelerado de Sup’u T’ula (Parastrephia lepidophylla Cabrera) con Fines de Repoblamiento en Zonas Productoras de Quinua.” Thesis, Universidad Mayor de San Andrés, La Paz, Bolivia.
- Jacobsen, S., and A. Mujica. 2006. “El Tarwi (Lupinus mutabilis Sweet.) y sus Parientes Silvestres.” In Botánica Enconómica de los Andes Centrales, edited by R. M. Moraes, B. Øllgaard, L. P. Kvist, F. Borchsenius, and H. Balslefe, 458–462. La Paz, Bolivia: Universidad Mayor de San Andrés.
- Kibbou, F., K. El Bouhmadi, H. Marrou, T. R. Sinclair, and M. E. Ghanem. 2022. “Impact of Drought and Temperature Constraints on Development and Growth of Faba Bean (Vicia Faba L.).” Journal of Crop Improvement 36 (1): 57–72. doi:10.1080/15427528.2021.1906811.
- Lal, R. 2020. “Regenerative Agriculture for Food and Climate.” Journal of Soil and Water Conservation 75 (5): 123A–124A. doi:10.2489/jswc.2020.0620A.
- Mann, C. C. 2018. The Wizard and the Prophet: Two Remarkable Scientists and Their Dueling Visions to Shape Tomorrow’s World. New York: Vintage Books.
- McDonell, E. 2018. “The Quinoa Boom Goes Bust in the Andes.” Nacla https://nacla.org/news/2018/03/12/quinoa-boom-goes-bust-andes
- Mollisaca Mamani, P. E., and A. Bonifacio Flores. 2021. “Rendimiento y Análisis Bromatológico de Subproductos de Trilla de Cuatro Variedades de Quinua (Chenopodium quinoa Willd.) en Kiphakiphani, La Paz – Bolivia.” Revista de Investigación e Innovación Agropecuaria y de Recursos Naturales 8 (3): 59–65. doi:10.53287/vjfz8823in19b.
- Montero, C. V., and C. A. Romero. 2017. Análisis Económico de la Producción Nacional de la Quinua. Lima, Perú: Ministerio de Agricultura y Riego.
- Montgomery, D. R. 2017. Growing a Revolution: Bringing Our Soils Back to Life. New York: Norton.
- Nicklin, C. 2017. Breeding Pipeline: Proinpa Landscapes 2000-2017. McKnight Foundation Collaborative Crop Research Program (CCRP). Poster.
- Orsag, V., L. León, O. Pacosaca, and E. Castro. 2013. “Evaluación de la Fertilidad de los Suelos para la Producción Sostenible de Quinua.” T’inkazos 33: 89–112.
- Rhodes, C. J. 2017. “The Imperative for Regenerative Agriculture.” Science Progress 100 (1): 80–129. doi:10.3184/003685017X14876775256165.
- Risi, J., W. Rojas, and M. Pacheco. 2015. Producción y Mercado de la Quinua en Bolivia, 315. La Paz: Instituto Interamericano de Cooperación para la Agricultura (IICA), Gráfica Real.
- Rodale, R. 1989. Oral History Interview by Jane Gates with Robert Rodale, Alternative Farming Systems Information Center, Oral History Interview Series. Beltsville, Maryland, USA: National Agriculture Library, US Department of Agriculture.
- Sherwood, S., M. Caulfield, M. Paredes, R. M. Borja, and P. Oyarun. inpress. “Response-ability: Establishing Regenerative Soil Management in the Northern Andes,” Chapter 36.” In Biological Approaches to Regenerative and Resilient Soil Systems, edited by N. Uphoff and J. Thies. Boca Raton: CRC Press.
- Sivila de Cary, R., and D. Hervé. 2006. “Efecto de Leguminosas Nativas en Terrenos en Descanso sobre la Microbiota del Suelo durante un Cultivo de Papa (Altiplano Central Boliviano.” Ecología en Bolivia 41 (3): 154–166.
- Udhaya, D., and B. Sakthinathan. 2017. “Windbreaks and Shelterbelts for Soil Conservation: A Review.” International Journal of Chemistry Studies 1 (2): 1–4.