?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This work describes an extraction method for giant reed fibers from stems and leaves based on chemical soaking and crushing through a rolling mill. Obtained fibers, together with the shredded plant (stems + leaves), are characterized in terms of chemical composition, thermal stability, morphology, and crystallinity. Mechanical properties of fibers have also been assessed (single fiber tensile tests). The results show that the proposed method allows obtaining fibers with higher cellulose content (near 70%), good thermal stability (10% weight loss over 270°C), higher density, and better mechanical properties than other Arundo fibers previously reported in the literature. Fibers from leaves are thinner and show higher crystallinity than those from stems (72 μm vs. 157 μm, 73% vs. 67% crystallinity, respectively), although mechanical properties are similar for both (around 900 MPa for tensile strength and over 45 GPa for elastic modulus). Analysis of the microstructure shows that fibers consist of microfiber bundles, and the removal of a thin layer of non-cellulosic nature is clear; fibers provide a rougher, cleaner surface than shredded raw material.
摘要
本工作描述了一种从茎和叶中提取巨型芦苇纤维的方法,该方法基于化学浸泡和通过轧机破碎. 获得的纤维以及切碎的植物(茎+叶)具有化学成分、热稳定性、形态和结晶度的特征. 还评估了纤维的机械性能(单纤维拉伸试验). 结果表明,与文献中先前报道的其他Arundo纤维相比,所提出的方法可以获得纤维素含量更高(接近70%)、热稳定性好(270°C以上失重10%)、密度更高和机械性能更好的纤维. 叶纤维比茎纤维更薄,结晶度更高(分别为72 μm和157 μm,73%和67%结晶度),但两者的机械性能相似(拉伸强度约为900 MPa,弹性模量超过45 GPa). 微观结构分析表明,纤维由超细纤维束组成,并清除了一层薄薄的非纤维素性质; 纤维提供了比粉碎原料更粗糙、更清洁的表面.
Introduction
Arundo donax L. is a perennial grass from the Gramineae family, with an uncertain origin due to its small size and the high number of chromosomes found in this species, although many authors place it in East Asia (Jensen et al. Citation2018). Although giant reed produces flowers, seeds are not usually viable, and reproduction takes place through the rhizome or shoot fragmentations. Stems are hollow and have diameters around 2–3 cm, reaching up to 6 m in height, with stem-clasping leaves along the entire stem (Csurhes Citation2016). Giant reed is generally recognized as an interesting source of biomass due to its high productivity and low requirements (Jensen et al. Citation2018), as it can be grown in almost any type of soil and with a minimal amount of water (Ahmed Citation2016), with high thermal and pathogen resistance (Accardi et al. Citation2015).
This species is mainly studied as a feedstock for biorefineries, especially for ethanol and bioenergy products (Accardi et al. Citation2015; Jensen et al. Citation2018). Reed has also gained attention for obtaining other biomolecules, such as furfural or levulinic acid, among others (Antonetti et al. Citation2015; Di Fidio et al. Citation2020; Raspolli Galletti et al. Citation2013). Other uses of Arundo have been investigated, such as paper and pulp (Shatalov and Pereira Citation2013), biochar production (Ahmed Citation2016), as filler in concrete (Ismail and Jaeel Citation2014), or for oil spill recovery (Piperopoulos et al. Citation2021). Its cultivation is also proposed as a strategy for soil bioremediation (Fernando et al. Citation2016; Tarek et al. Citation2015).
Compared to the extensive research performed on biofuels and biomolecules, only a little attention has been paid to the obtaining of reed composites, mainly related to particle board production (Barreca et al. 2019; García-Ortuño et al. Citation2011; C. E. Ferrández-García et al. Citation2012; Andreu-Rodriguez et al. 2013; M. T. Ferrández-García et al. Citation2020; Ferrandez-Garcia et al. Citation2019; Ferrández Villena et al. Citation2020). Those studies on composite production use shredded vegetal material (Bessa et al. Citation2020, Citation2021; Fiore et al. Citation2014; Fiore, Scalici, and Valenza Citation2014) or bast fibers obtained by combining with a teeth brush (Fiore, Scalici, and Valenza Citation2014; Scalici, Fiore, and Valenza Citation2016) or rudimentary mechanical procedures (Ortega et al. Citation2021; Suárez et al. Citation2021). From the limited papers found in the literature in this field, these mainly focus on thermoset resins (Bessa et al. Citation2021; Chikouche et al. Citation2015; Fiore, Scalici, and Valenza Citation2014), being polylactic acid (PLA) (Fiore et al. Citation2014) and polyethylene (PE) used as thermoplastic matrixes (Ortega et al. Citation2021; Suárez et al. Citation2021). This paper focuses on the characterization of Arundo fibers, obtained from the stems and the leaves, using a combination of chemical and mechanical procedures that provide finer fibers.
Arundo is still considered one of the worst invasive plants in the world (Jiménez-Ruiz et al. Citation2021), and several actions take place periodically to try to control their spread in many parts of the world. This invasive character is due to its rapid and easy propagation but also to the difficulties in removing its rhizomatous root system. To achieve successful eradication, the continuous application of herbicides, such as glyphosate (Jiménez Auzmendi Citation2014; Martín, Carolina, and Barroso Citation2019), or biological/mechanical treatments (Jiménez-Ruiz et al. Citation2021) are needed, not even arriving to a complete eradication of the plant (Jensen et al. Citation2018). For example, control campaigns have been performed in Murcia (Aymerich et al. Citation2012) or Canary Islands (Jiménez Auzmendi Citation2014) in Spain, Oregon in USA (Martín, Carolina, and Barroso Citation2019), and Queensland in Australia (Csurhes Citation2016).
Consequently, a large amount of residual biomass is available in different areas with potential interest as reinforcement of composites, among other possible uses, to advance toward more efficient use of resources and a zero waste strategy.
Materials and methods
Materials preparation
Specimens of Arundo donax L. plants have been collected in Gran Canaria (28.081344−15.473501) and show 2–3 cm diameter and around 4–5 m height. The rhizomes were removed, and three different materials were prepared: leaves, stems, and raw material. Leaves and stems were used separately for fiber obtaining. For characterization purposes, all samples were milled in a Retsch ZM 200 mill to a particle size lower than 0.5 mm. All reagents were purchased in analytical grade from Sigma Aldrich and were used without further modification.
Fiber obtaining
Fibers from the stems were obtained after 7–10 days of soaking them in a 1 N NaOH solution and subsequent crushing of the resulting material in a lab-made mechanical device, as described in Suárez et al. (Citation2021). Once obtained, fibers were washed in water until neutral pH and dried under room conditions for 3–4 days. Fibers were then cut to 3–5 mm length, washed again to remove fine dust, dried at 60°C in a forced air dryer device TG 200 from Retsch, and then sieved in an AS200 Control device from Retsch, using 75 and 800 μm sieves to remove fines and clustered particles.
Fibers from the leaves were also obtained after immersing the green leaves in a 1 N NaOH solution for 3 weeks, and then just by shaking them in water. Once washed, they were cut, washed again, dried, and sieved, as explained for fibers obtained from stems.
Finally, the raw material was chopped, dried at room conditions, and then processed twice in an SM 300 cutting mill from Retsch, using a 10 mm screen. The last step to prepare the shredded material was washing with water, drying with forced air and sieving.
For stems, a fiber extraction yield of around 34% is obtained (on a dry basis), while it is quite lower for leaves (8%). shows the aspect of the obtained materials; fibers are thinner than those obtained in other published works (Fiore, Scalici, and Valenza Citation2014; Ortega et al. Citation2021; Suárez et al. Citation2021).
Chemical characterization
Humidity, ash content, extractives, cellulose, hemicellulose, and lignin were determined. The lignin content was determined using the Klason method according to the ANSI/ASTM 1997a standard (American National Standard Institute Citation1977), which consists of hydrolysis with H2SO4. Acid-soluble lignin (ASL) was determined from the resulting liquor, measuring absorbance at 205 nm, using an extinction coefficient of 110 l/g·cm (Proietti et al. Citation2017) in a Cary 60 UV–VIS spectrophotometer from Agilent Technologies. Holocellulose content was also determined following a gravimetric method (Browning Citation1967), in which acetic acid and sodium chlorite were used for the sample delignification. Once this sample was obtained, the total cellulose content was obtained following ANSI/ASTM 1977b standard (American National Standard Institute Citation1977). Hemicellulose is calculated as holocellulose minus cellulose contents.
Extractive content was determined following NREL/TP-510-42619 (Sluiter et al. Citation2008), consisting of two subsequent extraction processes, with water and ethanol, respectively.
Three replicas from three different batches were used for all these tests. The results are expressed as average values ± standard deviations.
Density determination
The real density of the samples was measured in an Ultrapyc 5000 Foam gas pycnometer from Anton Paar, using nitrogen as flow gas, at a target pressure of 68.9 kPa (10.0 psi), with temperature control targeted at 20.0°C. A small cell (10 cm3) was used, running 15 scans per sample under the flow mode and setting the apparatus for fine powder.
Fourier Transformed Infrared (FTIR) spectroscopy
FTIR spectra were obtained in a Perkin Elmer spectrum Two spectrophotometer from Perkin Elmer, under the attenuated total reflectance (ATR) mode, from 4000 to 500 cm−1, at a resolution of 4 cm−1, obtaining each spectrum as the average of 64 scans. Intensities in absorbance of peaks at 2900 cm−1, 1420 cm−1, 1375 cm−1, and 893 cm−1 have been calculated in Perkin Elmer software to calculate the crystallinity of the samples.
Thermal characterization
Thermogravimetric tests (TGA) were conducted to determine the thermal stability of the samples. TGA was performed in a Netzsch TG 2091F1 Libra apparatus, from 20°C to 900°C at a heating rate of 10°C/min under a nitrogen atmosphere (20 ml/min), using alumina crucibles. The first derivative (DTG) curve was calculated, and values of temperature for 5%, 10%, 20%, and 50% mass loss were determined.
Morphology
Sample dimensions were obtained from optical microscopy observations performed in an Olympus B×51 microscope at 5x and reflected light. A minimum of 50 particles were measured for each sample, with at least 3 measurements per particle.
The sample’s morphology was assessed using a Hitachi TM3030 Scanning Electron Microscopy (SEM). Samples were placed in copper tape and coated via electrical glow discharge of Au/Pd target in an SC7620 Mini Sputter Coater from Quorum Technologies. “Fibers” surface and section were observed at different magnifications, at 15 kV.
X-ray diffraction (XRD)
The crystallinity of the samples was obtained from diffraction patterns with Cu Kα radiation (l = 1.5406 Å) in a Seifert URD6 apparatus, using 30 kV and 40 mA. Diffractograms were recorded from 2θ = 10° to 2θ = 40° in 0.05 steps. The presented data were smoothed using the Savitzky–Golay function (polynomial order 2, points of window 15). Crystallinity was determined according to the following equation (Wang et al. Citation2016):
where I002 is the diffraction intensity of the cellulose I crystalline region (maximum intensity in the 2θ angle range between 20° and 23°), and Iam is the diffraction intensity of the amorphous cellulose region (minimum intensity in the 2θ angle range between 18° and 19°).
Mechanical properties
Single fiber tests were performed on leaves and stem fibers to determine their tensile strength and elastic modulus. Thirty fibers were tested per sample in a Dongguan Liyi Test Equipment LY-1065 tester at 1 mm/min rate and a gauge length of 30 mm, following ASTM D3379–75. “Fibers” diameter was determined as the average value of 8 measurements, performed in a micrometer, considering a circular section of the fibers.
Results and discussion
Chemical characterization
shows the average values for the obtained fibers and shredded material composition. The composition of the shredded material is similar to Arundo stems found in other studies. In particular, ashes have been found in higher content in leaves than in stems (Jensen et al. Citation2018; Martínez-Sanz et al. Citation2018), as also found for fibers obtained in this study, being the average content of ashes in stems between 1.9% and 5.5% in most studies (Chikouche et al. Citation2015; Davide et al. Citation2016; Shatalov and Pereira Citation2005). As expected, lignin content for raw shredded material is much higher than for processed fibers, accounting for around 35%; other authors have reported similar values for total lignin, approximately 30% (Davide et al. Citation2016) and Klason lignin, about 20% for the stems without leaves (Bessa et al. Citation2021; Suárez et al. Citation2021).
Table 1. Composition (in %) and density of shredded material and fiber samples (average values ± standard deviation).
Total extractives for these materials are below 3.0% due to the fiber extraction or the washing of the shredded material (data not shown). As expected, the process followed for fiber obtaining removes hemicellulose in the plant and leads to a fiber with relatively high cellulose content, in the range of jute or sisal fibers, and, in any case, higher than for fibers obtained in previous works (around 45% (Fiore, Scalici, and Valenza Citation2014; Suárez et al. Citation2021)). Lignin content is higher in fibers from stems than in fibers from leaves and within the ranges found in the literature. As stems show a woody rigid structure and contain a higher amount of lignin than leaves, fibers from stems also show higher lignin content than fibers from the leaves. No significant differences in cellulose and hemicellulose content are found for both types of fibers.
Density determination
shows the density values for the three materials studied; fibers from leaves show higher density than fibers from stems, which is also higher than from shredded material. For the same species, density measurements can be correlated to the plant’s chemical composition (Charca, Tenazoa, and Junior Citation2021); the material with higher density has higher cellulose content and lower lignin amount. It then appears that Arundo fibers obtained in this research show higher density than shredded material and higher cellulose content, as seen in , due to the procedure followed for fiber extraction. Removing hemicellulose and lignin allows for a higher proportion of cellulose, resulting in a higher density of the fibers. Fiore, Scalici, and Valenza (Citation2014) determined a density for Arundo fibers obtained by the mechanical procedure of 1.168 g/cm3 and cellulose content of 43.59%; fibers obtained here show around 50% more cellulose and densities around 40% higher, while shredded material shows similar composition and density than fibers obtained in that research.
FTIR spectroscopy
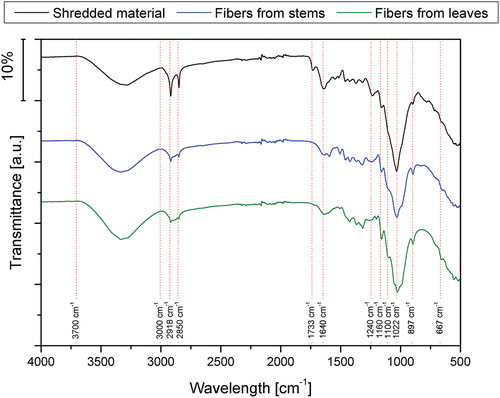
shows the average recorded spectra for the samples of shredded material, fibers from leaves, and fibers from stems. Typical bands for lignocellulosic materials can be observed, namely:
Broad band at 3000–3700 cm−1, assigned to O-H stretching.
The double peak at 2918 and 2850 cm−1, attributed mainly C-H aliphatic groups in hemicellulose (Chikouche et al. Citation2015), which decreases in intensity due to the fiber extraction process, is also confirmed in chemical composition assays.
The sharp peak at 1733 cm−1, related to C=O stretching in lignin and hemicellulose (Bessa et al. Citation2020; Scalici, Fiore, and Valenza Citation2016), is found for shredded material but not for fibers. This is correlated with the intensity reduction at 1240 cm−1, also found for fibers, attributed to C-O stretching in lignin (Fiore, Scalici, and Valenza Citation2014).
The peak at 1640 cm−1 is reduced for fibers, as the removal of hemicellulose and lignin leads to less absorbed water (Martínez-Sanz et al. Citation2018).
High-intensity peaks at 1022 cm−1 (C=O stretching ring vibration in cellulose) are observed for the three samples, although the relative intensity is higher for fibers than for raw material. This may suggest higher cellulose content for fibers, as observed in the quantification analysis. C-O-C asymmetric stretching at 1160 cm−1 also appears clearly for fibers, while it is only a light shoulder for shredded material. The precise definition of a peak at 1100 cm−1 may also indicate the higher presence of cellulose I crystals (Martínez-Sanz et al. Citation2018).
Therefore, in summary, it appears that the procedure proposed for Arundo fiber extraction allows obtaining a fiber of good quality to be used in composites, according to the literature, where several treatments (soda, plasma, silanization, etc.) are applied to materials from Arundo to improve their surface, in terms of chemical bonding. Fibers obtained only by crushing or peeling show the peak at 1740 cm−1, which can be removed by performing an alkaline treatment (Suárez et al. Citation2021); this is also in agreement with results obtained from chemical composition tests, which show lower lignin and hemicellulose contents in samples obtained with the procedure described in this paper than for those obtained using only mechanical means.
The Total Crystallinity Index (TCI) and the Lateral Order Index (LOI) can be calculated from FTIR spectra after baseline correction and normalizing (El Oudiani, Msahli, and Sakli Citation2017), thus giving an approximate idea of the crystallinity of samples. The first one, TCI, is obtained by dividing the absorbance of peaks at 1375 cm−1 and 2900 cm−1, being the value obtained proportional to the degree of crystallinity of the cellulose. The second one, LOI, is obtained by dividing absorbance at 1420 cm−1 by that at 893 cm−1, considering this last an empirical crystallinity index and also attributed to the proportion of cellulose I (Sang Youn et al. Citation2005). As observed in , TCI and LOI values are higher for fiber samples than for shredded materials, thus indicating that fibers show higher crystallinity, a fact that was otherwise expected, as seen in the chemical composition of the three materials. Apparently, fibers from leaves have a higher amount of crystalline cellulose, while fibers from stems have a higher-order degree in the entire structure. Similar conclusions arise if areas of the bands at 1640, 897, and 667 cm−1 are considered (Abidi and Manike Citation2018). A higher area at 1640 cm−1 is related to higher water absorption, directly related to higher amorphous content, while more significant areas in the other two peaks are linked to higher cellulose crystallinity.
Table 2. Approximation of crystallinity of samples by FTIR spectrum.
Thermal characterization
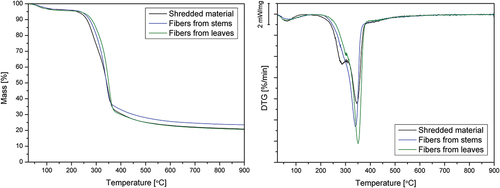
TGA curves for all considered materials show a similar course (). However, leaf fibers seem to have higher degradation temperatures than fibers from stems, which are also more stable than shredded material. Residual biomass also has similar values for the three materials.
The most important peak found in the derivative curves is related to cellulose degradation, arising between 338°C and 350°C for these samples (pure cellulose has a maximum pyrolysis rate at 355°C (Yang et al. Citation2007)); fibers show a higher degradation rate at this peak than shredded material (). Besides, DTG curves also show some differences in their shape. Only for shredded material samples, the main peak has a shoulder at around 290.8°C due to the hemicellulose degradation (hemicellulose pyrolysis takes place before cellulose degradation, starting at about 220°C (Bessa et al. Citation2020)) (). When considering hemicellulose and cellulose pyrolysis ranges (210–315°C and 315–400°C, respectively), shredded material shows a weight loss of 27.9% in the first step and 37.1% in the second one, being the first decrease considerably lower for fiber samples (23.1% and 16.8% for stems and leaves fibers, respectively), while the α-cellulose-related step shows higher values (39.6% and 48.5%). These values are in the range of those found by other authors for Arundo-treated fibers, although with lower peak temperatures (up to 340°C as the maximum value) (Bessa et al. Citation2020). Scalici and collaborators also obtained the first shoulder on the DTG curve at 285°C and maximum weight loss (30%) at around 340 ºC for fibers obtained from leaves (Scalici, Fiore, and Valenza Citation2016).
Table 3. Temperatures for 5%, 10%, 20%, and 50% weight loss, residual mass DTG peak values.
Arundo materials obtained and characterized in this work show similar or even higher temperature stability than other fibers from the same species (due to the proposed extraction process) or other vegetal fibers. The onset degradation temperature is found to be around 300ºC, thus confirming that it can be considered an excellent candidate to be used in composite production; weight loss starts over 270°C, in the range (or even higher) than other commonly used vegetal fibers, such as flax or jute (Fiore, Scalici, and Valenza Citation2014). Considering the analyzed fibers as potential reinforcement of thermoplastic composites, it is essential to define the temperature range that will allow their melt processing with polymer without degradation of the lignocellulosic structure. According to the methodology described by Bledzki et al. (Bledzki, Mamun, and Volk Citation2010), the starting decomposition temperature (Td) of natural fibers is assumed to be the temperature value associated with a 1% mass loss above 150°C. This approach is due to completely removing free and bonded moisture contents in lignocellulosic fibers below the selected temperature. Td values are additionally presented in . The reported test results agree with the spectroscopic analysis results, namely the estimated water absorption determined as the increase in absorbance intensity at 1620 cm−1.
Morphology
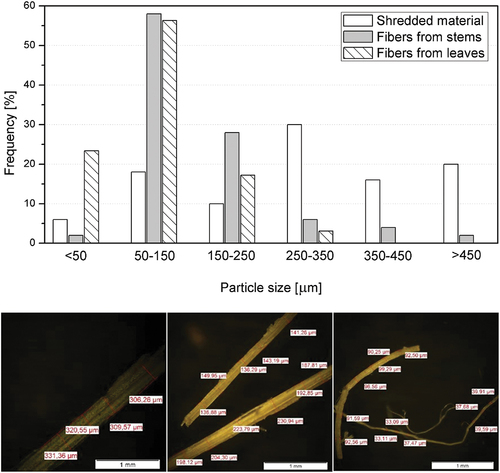
Optical microscopy has been used to determine diameters’ distribution for the three different samples; fibers obtained from the leaves are thinner (71.6 ± 35.1 μm) than those obtained from the stems (156.5 ± 73.5 μm). Shredded particles show the biggest size (370.3 ± 133.0 μm). As observed in , the shredded sample shows some thin fibers (lower than 50 μm in diameter), which were released during the mechanical shredding procedure, although these are merely testimonials. Most fibers have diameters between 50 and 150 microns, while the leaf fibers show an important percentage of fibers under 50 microns and stem fibers between 150 and 250 microns. Shredded material has a uniform distribution at bigger particle sizes (250–350 μm).
Figure 4. Particle size distribution (frequency vs. particle size, in microns) (above). Pictures from optical microscope and measurements for (a) shredded material, (b) stem fiber, and (c) leaves fiber (below).
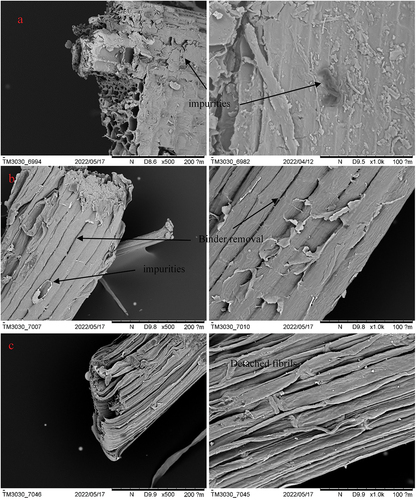
On the other hand, SEM micrographs () show clear differences between shredded and fiber samples. Fiber bundles of aligned microfibrils can be observed for all samples, usually happening for cellulosic fibers. These bundles are covered with a layer probably made of lignin or hemicellulose for shredded material (Khan et al. Citation2020). In contrast, this layer is reduced and appears broken for fiber samples (both from leaves and stems), giving a rougher aspect. This is in agreement with assays performed, which show lower hemicellulose content for fibers than for shredded material. Thinner fibers can be observed for fibers from leaves, as also determined in optical microscopy; this may be due to the higher lignin content, which bonds fibers together, as observed in SEM pictures. Fiber cell wall (Figure S1.c) has the same geometrical structure and different sizes of wall and lumen as already mentioned (Fiore, Scalici, and Valenza Citation2014). Cellulose is arranged in a spiral shape through the cellular wall; in some samples, elongated vascular cells and pit membranes (Lian et al. Citation2019) for water and nutrient exchange can also be observed (see Figure S1).
X-ray diffraction (XRD)
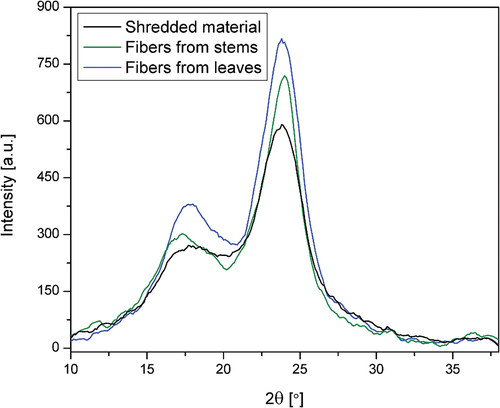
The XRD plots presenting X-ray diffraction patterns made for three types of fibers are shown in . For all curves, a dominant peak at 2θ in the range of 22–23° can be noted, referred to as the crystallographic plane (002) characteristic for crystalline forms of cellulose I (Wang et al. Citation2016). The curves differ in peak intensities, taking the higher values for fibers and the lowest for shredded material. Additionally, the peak in the (101) crystallographic plane, also attributed to the crystalline cellulose phase (Barreto et al. Citation2010), is more distinct for fibers from stems. In the result of the calculations made for X-ray data according to EquationEquation (1)(1)
(1) , the samples of natural fibers showed degrees of crystallinity at the level of 59.1%, 66.7%, and 73.1% for shredded material, fibers from stems, and leaves, respectively. Despite the changeable values concerning TCI resulting from a different methodology (Liu et al. Citation2012), the trend is comparable; the highest crystallinity characterizes fibers obtained from leaves.
Generally, the higher the cellulose content, the higher the CI. Some authors have even reported a linear correlation between these two parameters (Abidi and Manike Citation2018). The cellulose in the fibers obtained in this work account for over 65% of the total biomass, and CI is over 60%, while other authors report cellulose contents of 35% and 43% have revealed CI of 50% and 66%, respectively (Bessa et al. Citation2020; Chikouche et al. Citation2015). Alkali-treated fibers show higher crystallinity values, increasing up to 58.2% and 75.4%.
Mechanical properties
Single fiber tests have allowed obtaining tensile strength and elastic modulus for the two types of fibers obtained in this work; fibers from stems show an average strength of around 905 MPa, varying from 600 to 1200 MPa, and an elastic modulus close to 42 GPa (31–56 GPa). Fiore and collaborators (Fiore, Scalici, and Valenza Citation2014) reported 248 MPa and 9.4 GPa for fibers obtained from stems, which are quite lower than those shown here and those reported for other natural fibers, such as abaca (400–980 MPa; 8–20 GPa), jute (393–773 MPa; 27 GPa), flax (345-1035MPa; 28 GPa) or bamboo (140–1000 MPa, 11–89 GPa) (Odesanya et al. Citation2021).
Fibers from leaves provide similar values, around 1000 MPa for tensile strength and 39 GPa for elastic modulus, although with higher dispersion of results, especially for modules. Tensile strength varied from 400 to 1500 MPa (a standard deviation of 300 MPa), while elastic modulus values from 12 to nearly 74 GPa were obtained (19 GPa deviation). This behavior for leaf fibers was also reported in (Scalici, Fiore, and Valenza Citation2016), although they also received lower values (173.90 ± 146.09 MPa for strength and 15.96 ± 12.17 GPa for modulus). The higher dispersion of results may be related to the increased distribution of diameters found for this material. On the other hand, as observed in SEM pictures, leaf fibers show detached microfibrils, which could explain the variability of results.
Fiber’s tensile strength and elastic modulus have been analyzed following a two-parameter Weibull distribution, as found in other works in the literature (Andersons et al. Citation2005; Fiore, Scalici, and Valenza Citation2014; Fiore, Valenza, and Di Bella Citation2011). The probability plots for these two properties can be found in the supplementary material (Figure S2). These graphs show that the data obtained fit a Weibull distribution quite well, as demonstrated by the correlation parameter in . summarizes the statistical parameters obtained in such analysis. For a Weibull distribution, the shape parameter is related to the data variability, while the scale parameter corresponds to the 63.2%ile of the data. In this case, the results obtained are pretty close to normality, as the shape factor is in, or close to, the 2–4 range (Fiore, Valenza, and Di Bella Citation2011).
Table 4. Statistical parameters for tensile strength (in MPa) and modulus (in GPa) for Arundo fibers.
Conclusions
This study confirms that Arundo donax can be used as a source for high cellulose fibers with interesting mechanical properties and good thermal stability, suitable for thermoplastic composite production. It has been determined that fibers from leaves are thinner (diameter around 70 μm) and show higher crystallinity values (from FTIR and XRD analysis) than fibers from stems, which are thicker (around 150 μm). Thermal stability is similar for both, with an onset temperature of over 300°C. SEM pictures show the different morphology between shredded material and fibers and not much difference between fibers from stems and leaves, apart from fibrillation and lower fiber diameters. Fibers are formed by microfiber bundles and provide a rough surface due to the removal of binding agents, which may lead to a higher surface and, thus, better attachment to the polymeric matrix.
Highlights
Arundo donax can be used as a source for high cellulose fibers with good mechanical properties
Leaves fibers are thinner and more crystalline than stem fibers
Arundo fibers show good thermal stability, with an onset temperature of over 300 °C
The proposed method allows for the obtaining of fibers with good mechanical properties
Fibers are formed by microfiber bundles and provide a rough surface
Supplemental Material
Download MS Word (33.2 KB)Supplemental Material
Download MS Word (1.2 MB)Acknowledgments
This research was funded by European Funding for Regional Development (FEDER), INTERREG MAC 2014-2020 program, providing funds for MAC Inv2Mac project (Grant number MAC2/4.6d/229). Luis Suárez also acknowledges the funding through the PhD grant program cofinanced by the Canarian Agency for Research, Innovation and Information Society of the Canary Islands Regional Council for Employment, Industry, Commerce and Knowledge (ACIISI) and by the European Social Fund (ESF) (Grant number TESIS2021010008). The authors would also like to thank Dr Katarzyna SKóczewska and MSc Sławomir Michałowski for their invaluable help and technical support in the field of XRD analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15440478.2022.2131687
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Abidi, N., and M. Manike. 2018. X-ray diffraction and FTIR investigations of cellulose deposition during cotton fiber development. Textile Research Journal 88 (7):719–14. doi:10.1177/0040517516688634.
- Accardi, D. S., P. Russo, R. Lauri, B. Pietrangeli, and L. Di Palma. 2015. From soil remediation to biofuel: Process simulation of bioethanol production from Arundo donax. Chemical Engineering Transactions 43:2167–72. doi:10.3303/CET1543362.
- Ahmed, M. J. 2016. Potential of Arundo donax L. stems as renewable precursors for activated carbons and utilization for wastewater treatments: Review. Journal of the Taiwan Institute of Chemical Engineers 63 (June):336–43. doi:10.1016/j.jtice.2016.03.030.
- American National Standard Institute. 1977. “ANSI/ASTM, 1977b. standard test methods for alpha-cellulose in wood D 1103-60.” Washington DC, USA.
- Andersons, J., E. Sparniņš, R. Joffe, and L. Wallström. 2005. Strength distribution of elementary flax fibres. Composites Science and Technology 65 (3–4):693–702. doi:10.1016/J.COMPSCITECH.2004.10.001.
- Antonetti, C., E. Bonari, D. Licursi, N. Nassio Di Nasso, and A. M. Raspolli Galletti. 2015. Hydrothermal conversion of giant reed to furfural and levulinic acid: Optimization of the process under microwave irradiation and investigation of distinctive agronomic parameters. Molecules 20 (12):21232–353. doi:10.3390/molecules201219760.
- Aymerich, F. R., M. A. E. Selma, I. H. García, V. M. Z. Pérez, P. F. Celdrán, R. V. López, J. M. Fernández, and J. M. Ródenas. 2012. “Seguimiento y Análisis de Las Actuaciones Para El Control Experimental de La Caña Común (Arundo Donax) En La Región de Murcia.” Murcia.
- Barreto, A. C. H., M. A. Esmeraldo, D. S. Rosa, P. B. A. Fechine, and S. E. Mazzetto. 2010. Cardanol biocomposites reinforced with jute fiber: Microstructure, biodegradability, and mechanical properties. Polymer Composites 31 (11):1928–37. doi:10.1002/PC.20990.
- Bessa, W., D. Trache, M. Derradji, H. Ambar, M. Benziane, and B. Guedouar. 2021, July. Effect of different chemical treatments and loadings of Arundo donax L. fibers on the dynamic mechanical, thermal, and morphological properties of bisphenol a aniline based polybenzoxazine composites. ( pc.26215) Polymer Composites 42 (10):5199–208. doi: https://doi.org/10.1002/pc.26215.
- Bessa, W., D. Trache, M. Derradji, H. Ambar, A. F. Tarchoun, M. Benziane, and B. Guedouar. 2020. Characterization of raw and treated Arundo donax L. cellulosic fibers and their effect on the curing kinetics of bisphenol a-based benzoxazine. International Journal of Biological Macromolecules 164 (December):2931–43. doi:10.1016/j.ijbiomac.2020.08.179.
- Bledzki, A. K., A. A. Mamun, and J. Volk. 2010. Barley husk and coconut shell reinforced polypropylene composites: The effect of fibre physical, chemical and surface properties. Composites Science and Technology 70 (5):840–46. doi:10.1016/J.COMPSCITECH.2010.01.022.
- Browning, B. L. 1967. Methods of wood chemistry. volumes I & II.methods of wood chemistry. volumes I & II. New York: Interscience Publishers. Vol. 102. http://www.biblio.com/book/methods-wood-chemistry-volume-1-browning/d/710364055.
- Charca, S., C. Tenazoa, and H. S. Junior. 2021. Chemical composition of natural fibers using the measured true density. Case study: Ichu fibers. Journal of Natural Fibers 1–6. doi:10.1080/15440478.2021.1952143.
- Chikouche, M. D. L., A. Merrouche, A. Azizi, M. Rokbi, and S. Walter. 2015. Influence of alkali treatment on the mechanical properties of new cane fibre/polyester composites. Journal of Reinforced Plastics and Composites 34 (16):1329–39. doi:10.1177/0731684415591093.
- Csurhes, S. 2016. “Invasive weed risk assessment: Giant reed Arundo Donax.” https://www.daf.qld.gov.au/__data/assets/pdf_file/0006/59973/IPA-Giant-Reed-Risk-Assessment.pdf.
- Davide, B., A. Salanti, M. Orlandi, D. S. Ali, and L. Zoia. 2016. Biorefinery process for the simultaneous recovery of lignin, hemicelluloses, cellulose nanocrystals and silica from rice husk and Arundo donax. Industrial Crops and Products 86 (August):31–39. doi:10.1016/j.indcrop.2016.03.029.
- Di Fidio, N., A. M. Raspolli Galletti, S. Fulignati, D. Licursi, F. Liuzzi, I. De Bari, and C. Antonetti. 2020. Multi-step exploitation of raw Arundo donax L. for the selective synthesis of second-generation sugars by chemical and biological route. Catalysts 10 (1):79. doi:10.3390/catal10010079.
- Fernando, A. L., B. Barbosa, J. Costa, and E. G. Papazoglou. 2016 January. Giant reed (Arundo donax L.). Bioremediation and Bioeconomy 77–95. doi:10.1016/B978-0-12-802830-8.00004-6.
- Ferrández-García, C. E., J. Andreu-Rodríguez, M. T. Ferrández-García, M. Ferrández-Villena, and T. García-Ortuño. 2012. Panels made from giant reed bonded with non-modified starches. BioResources 7 (4):5904–16. doi:10.15376/biores.7.4.5904-5916.
- Ferrandez-Garcia, M. T., C. Eugenia Ferrandez-Garcia, T. Garcia-Ortuño, A. Ferrandez-Garcia, and M. Ferrandez-Villena. 2019. Experimental evaluation of a new giant reed (Arundo donax L.) composite using citric acid as a natural binder. Agronomy 9 (12):882. doi:10.3390/agronomy9120882.
- Ferrández-García, M. T., A. Ferrández-García, T. García-Ortuño, C. E. Ferrández-García, and M. Ferrández-Villena. 2020. Assessment of the physical, mechanical and acoustic properties of Arundo donax L. biomass in low pressure and temperature particleboards. Polymers 12 (6):1361. doi:10.3390/POLYM12061361.
- Ferrández Villena, M., C. E. F. Garcia, T. García Ortuño, A. Ferrández García, and M. T. Ferrández García. 2020. The influence of processing and particle size on binderless particleboards made from Arundo donax L. rhizome. Polymers 12 (3):696. doi:10.3390/polym12030696.
- Fiore, V., L. Botta, R. Scaffaro, A. Valenza, and A. Pirrotta. 2014. PLA based biocomposites reinforced with Arundo donax fillers. Composites Science and Technology 105 (December):110–17. doi:10.1016/j.compscitech.2014.10.005.
- Fiore, V., T. Scalici, and A. Valenza. 2014. Characterization of a new natural fiber from Arundo donax L. as potential reinforcement of polymer composites. Carbohydrate Polymers 106 (1):77–83. doi:10.1016/j.carbpol.2014.02.016.
- Fiore, V., A. Valenza, and G. Di Bella. 2011. Artichoke (Cynara cardunculus L.) fibres as potential reinforcement of composite structures. Composites Science and Technology 71 (8):1138–44. doi:10.1016/J.COMPSCITECH.2011.04.003.
- Francesco, B., A. M. Gabarron, J. A. F. Yepes, and J. J. P. Pérez. 2019. Innovative use of giant reed and cork residues for panels of buildings in Mediterranean area. Resources, Conservation and Recycling 140 (January):259–66. doi:10.1016/j.resconrec.2018.10.005.
- Galletti, A. M. R., C. Antonetti, E. Ribechini, M. P. Colombini, N. N. O Di Nasso, and E. Bonari. 2013. From giant reed to levulinic acid and gamma-valerolactone: A high yield catalytic route to valeric biofuels. Applied Energy 102 (February):157–62. doi:10.1016/j.apenergy.2012.05.061.
- García-Ortuño, T., J. Andréu-Rodríguez, M. T. Ferrández-García, M. Ferrández-Villena, and C. E. Ferrández-García. 2011. Evaluation of the physical and mechanical properties of particleboard made from giant reed (Arundo donax L.). BioResources 6 (1):477–86. doi:10.15376/biores.6.1.477-486.
- Ismail, Z. Z., and A. J. Jaeel. 2014. A novel use of undesirable wild giant reed biomass to replace aggregate in concrete. Construction and Building Materials 67 (September):68–73. doi:10.1016/j.conbuildmat.2013.11.064.
- Javier, A.-R., E. Medina, M. T. Ferrandez-Garcia, M. Ferrandez-Villena, C. E. Ferrandez-Garcia, C. Paredes, M. A. Bustamante, and J. Moreno-Caselles. 2013. Agricultural and industrial valorization of Arundo donax L. Communications in Soil Science and Plant Analysis 44 (1–4):598–609. doi:10.1080/00103624.2013.745363.
- Jensen, E. F., M. D. Casler, K. Farrar, J. M. Finnan, R. Lord, C. Palmborg, J. Valentine, and I. S. Donnison. 2018. Giant reed: From production to end use. In Perennial Grasses for Bioenergy and Bioproducts, ed. E. Alexopoulou, 107–50. London: Academic Press Inc. Elsevier.
- Jiménez Auzmendi, E. 2014. Plantas Invasoras, ¿ Batalla Perdida? ¿ O No ? Investigación, Gestión Y Técnica Forestal, En La Región de La Macaronesia 135–55. Universidad de La Laguna. Editor: Juan Carlos Santamarta Cerezal. 2014. 978-84-617-3391-0. Madrid: Colegio de Ingenieros de Montes. Accessed at: https://mdc.ulpgc.es/utils/getfile/collection/MDC/id/177834/filename/218410.pdf
- Jiménez-Ruiz, J., L. Hardion, J. P. Del Monte, B. Vila, and M. I. Santín-Montanyá. 2021. Monographs on invasive plants in Europe N° 4: Arundo donax L. Botany Letters 168 (1):131–51. doi:10.1080/23818107.2020.1864470.
- Khan, A., R. Vijay, D. L. Singaravelu, M. R. Sanjay, S. Siengchin, F. Verpoort, K. A. Alamry, and A. M. Asiri. 2020. Characterization of natural fibers from Cortaderia selloana grass (pampas) as reinforcement material for the production of the composites. Journal of Natural Fibers. doi:10.1080/15440478.2019.1709110.
- Lian, C., R. Liu, C. Xiufang, S. Zhang, J. Luo, S. Yang, X. Liu, and B. Fei. 2019. Characterization of the pits in parenchyma cells of the moso bamboo [Phyllostachys edulis (Carr.) J. Houz.] Culm. Holzforschung 73 (7):629–36. doi:10.1515/HF-2018-0236.
- Liu, Y., D. Thibodeaux, G. Gamble, P. Bauer, and D. VanDerveer. 2012. Comparative investigation of Fourier transform infrared (FT-IR) spectroscopy and X-ray diffraction (XRD) in the determination of cotton fiber crystallinity. Applied Spectroscopy 66 (8):983–86. doi:10.1366/12-06611.
- Martín, S., J. A. G. Carolina, and J. Barroso. 2019. Control of volunteer giant reed (Arundo donax). Invasive Plant Science and Management 12 (1):43–50. doi:10.1017/inp.2018.36.
- Martínez-Sanz, M., E. Erboz, C. Fontes, and A. López-Rubio. 2018. Valorization of Arundo donax for the production of high performance lignocellulosic films. Carbohydrate Polymers 199 (November):276–85. doi:10.1016/j.carbpol.2018.07.029.
- Odesanya, K. O., R. Ahmad, M. Jawaid, S. Bingol, G. O. Adebayo, and Y. H. Wong. 2021. Natural fibre-reinforced composite for ballistic applications: A review. Journal of Polymers and the Environment 29 (12):3795–812. doi:10.1007/s10924-021-02169-4.
- Ortega, Z., F. Romero, R. Paz, L. Suárez, A. N. Benítez, and M. D. Marrero. 2021. Valorization of invasive plants from macaronesia as filler materials in the production of natural fiber composites by rotational molding. Polymers 13 (13):2220. doi:10.3390/polym13132220.
- Oudiani, A. E., S. Msahli, and F. Sakli. 2017. In-depth study of agave fiber structure using Fourier transform infrared spectroscopy. Carbohydrate Polymers 164 (May):242–48. doi:10.1016/J.CARBPOL.2017.01.091.
- Piperopoulos, E., A. Khaskhoussi, V. Fiore, and L. Calabrese. 2021. Surface modified Arundo donax natural fibers for oil spill recovery. Journal of Natural Fibers 1–16. doi:https://doi.org/10.1080/15440478.2021.1961343.
- Proietti, S., S. Moscatello, M. Fagnano, N. Fiorentino, A. Impagliazzo, and A. Battistelli. 2017. Chemical composition and yield of rhizome biomass of Arundo donax L. grown for biorefinery in the Mediterranean environment. Biomass & Bioenergy 107 (December):191–97. doi:10.1016/j.biombioe.2017.10.003.
- Sang Youn, O., D. I. Yoo, Y. Shin, H. C. Kim, H. Y. Kim, Y. S. Chung, W. H. Park, and J. H. Youk. 2005. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydrate Research 340 (15):2376–91. doi:10.1016/J.CARRES.2005.08.007.
- Scalici, T., V. Fiore, and A. Valenza. 2016. Effect of plasma treatment on the properties of Arundo donax L. leaf fibres and its bio-based epoxy composites: A preliminary study. Composites Part B: Engineering 94 (June):167–75. doi:10.1016/j.compositesb.2016.03.053.
- Shatalov, A. A., and H. Pereira. 2005. Kinetics of organosolv delignification of fibre crop Arundo donax L. Industrial Crops and Products 21 (2):203–10. doi:10.1016/j.indcrop.2004.04.010.
- Shatalov, A. A., and H. Pereira. 2013. High-grade sulfur-free cellulose fibers by pre-hydrolysis and ethanol-alkali delignification of giant reed (Arundo donax L.) stems. Industrial Crops and Products 43 (1):623–30. doi:10.1016/j.indcrop.2012.08.003.
- Sluiter, A., R. Ruiz, C. Scarlata, J. Sluiter, and D. And Templeton NREL/TP-510-42619 - Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP). Technical Report NREL/TP-510-42619. January 2008. Accessible on: https://www.nrel.gov/docs/gen/fy08/42619.pdf
- Suárez, L., J. Castellano, F. Romero, M. D. Marrero, A. N. Benítez, and Z. Ortega. 2021. Environmental hazards of giant reed (Arundo donax l.) in the Macaronesia region and its characterisation as a potential source for the production of natural fibre composites. Polymers 13 (13):2101. doi:10.3390/polym13132101.
- Tarek, A., N. Elhawat, É. Domokos-Szabolcsy, J. Kátai, L. Márton, M. Czakó, H. El-Ramady, and M. G. Fári. 2015. Giant reed (Arundo donax L.): A green technology for clean environment. Phytoremediation: Management of Environmental Contaminants 1:1–20. doi:10.1007/978-3-319-10395-2_1.
- Wang, C., S. Bai, X. Yue, B. Long, and L. P. Choo-Smith. 2016. Relationship between chemical composition, crystallinity, orientation and tensile strength of kenaf fiber. Fibers and Polymers 2016 (11):1757–64. doi:10.1007/S12221-016-6703-5.
- Yang, H., R. Yan, H. Chen, D. H. Lee, and C. Zheng. 2007. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86 (12–13):1781–88. doi:10.1016/j.fuel.2006.12.013.