ABSTRACT
Prangos ferulacea (Jashir) plant possesses various phytoconstituents such as coumarins, flavonoids, alkaloids, umbelliferon, and monoterpenes and has been used in traditional medicine. The presence of flavonoids in aerial parts of Prangos ferulacea necessities further examination of using them as a source of natural colorants for developing natural and beautiful shades on wool yarns. This will add one more new effective natural dye source and ease the dependency of the present-day textile industry on synthetic colorants. Simple adsorption of aqueous dye extract on the wool followed by evaluation of buildup properties using 28 different binary metal combinations as mordants were studied in this study. The dyeing was performed by exhaust dyeing method and dyed samples were analyzed through reflectance spectroscopy and analyzed in terms of color strength, CIEL*a*b*, and CIEL*c*ho values. Color characteristics were evaluated on a spectrophotometer under D65 illuminant and 10° standard observer. FT-IR and SEM analyses were performed to characterize P. ferulacea dyed wool yarns. The results showed that overall 29 different shades having very good to excellent fastness properties were produced by the use of different metallic salt combinations in natural dyeing of wool yarns using aerial parts of P. ferulacea as a source of natural dye.
摘要
阿魏草含有香豆素类、黄酮类、生物碱、伞形科、单萜类等多种植物成分,在传统医药中有广泛的应用. 阿魏酸普拉戈斯(Prangos ferulacea)的地上部分含有黄酮类化合物,需要进一步研究将其用作天然着色剂的来源,以便在羊毛纱线上形成自然美丽的色调. 这将增加一种新的有效天然染料来源,并缓解当今纺织工业对合成着色剂的依赖. 本研究研究了水性染料萃取物在羊毛上的简单吸附,然后使用28种不同的二元金属组合作为媒染剂评估其堆积性能. 采用排气染色法进行染色,通过反射光谱分析染色样品,并根据颜色强度、CIEL*a*b*和CIEL*c*ho值进行分析. 在D65光源和10°标准观察者下,在分光光度计上评估颜色特性. 采用FT-IR和SEM分析对阿魏酸棕榈酸酯染色羊毛纱线进行了表征. 结果表明,在羊毛纱线的天然染色中,使用不同的金属盐组合,以阿魏酸P.ferulacea的上半部分作为天然染料来源,总共生产了29种不同的色度,具有很好的至优异的牢度性能.
Introduction
Natural products that are not just “eco-friendly” and “organic” but also synthesized or produced “ethically” became more and more common among consumers in the present era of scientific and research developments. The present research is being focused on the strategies to substitute petrochemical products by plant-derived natural materials (Rather et al. Citation2016; Rather, Shahid-Ul-Islam, and Mohammad Citation2015; Zhang, Shahid-Ul-Islam Rather, and Li Citation2022). Natural dyes and pigments have made a comeback in the textile dyeing sector as a valuable alternative to most of the synthetic dyes, which are thought to be harmful to humans (Carcinogenic) and aquatic ecosystems (Ahlström, Eskilsson, and Björklund Citation2005; Javadian, Angaji, and Naushad Citation2014; Rather, Haji, and Shabbir Citation2021). Biodegradable, sustainable, and environmental-friendly natural colorants derived from renewable resources like plants and insects are gaining popularity in the dyeing industrial sectors (Zhang et al. Citation2021; Zhou et al. Citation2022). A variety of chemical compounds present in dye-yielding plants including tannins, anthraquinones, alkaloids, naphthoquinones, flavonoids, and carotenoids are being studied for their potential usage to produce colorful textiles of different hues and tones (Koh and Hong Citation2014; Rather et al. Citation2016, Citation2021; Sadeghi-Kiakhani et al. Citation2021). Because of the inherent biological properties of dye components (tannins, flavonoids, anthraquinones, naphthoquinones, and carotenoids), researchers have employed them for the production of functional textile materials with insect repellent, antimicrobial, antioxidant, UV protection, and fluorescent properties (Rastogi, Jain, and Negi Citation2022; Rather et al. Citation2019; Safapour, Sadeghi-Kiakhani, and Eshaghloo-Galugahi Citation2018; Zhou et al. Citation2020, Citation2022). More recently, researchers are exploring the use of waste by-products of plant origin to reduce the cost of natural dyeing processes in addition to the introduction of new dye sources (Amutha et al. Citation2022; Rather et al. Citation2020; Zhang et al. Citation2021; Zhou et al. Citation2022).
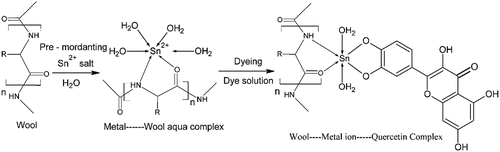
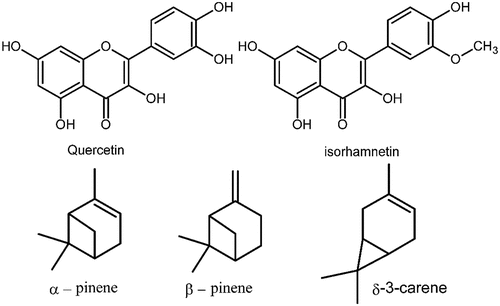
Natural dyeing is a centuries-old tradition in Iran, where textile items such as fibers, yarns, and other clothing’s are soaked in a precise combination of pigments, resulting in the production of beautiful colors. In Achaemenid-era (c. 550–330 BC) famed Pazyryk rug was recovered from the Bolshoy Ulagan dry valley of the Altai Mountains in Kazakhstan in 1949 which bears natural yellow, red, blue, and green colors providing a clue of the long history of natural dye processes in this area (Mouri et al. Citation2014). The development of carpet dyeing was witnessed in the Safavid era (1501 – 1736) with crimson red, blue, green, pale yellow, and orange colors which are similar to the present-day pigments. The dyers also practiced the mixing of two or more colors, providing hues that are different from each other. Mixing violet and red created bright magenta and further addition of yellow pigment turns it into persimmon orange (Aali et al. Citation2012; Pollard et al. Citation2008). Prangos ferulacea (L.) Lindl. plant (Jashir) from the order Umbellales, the family of Umbelliferae (Apiaceae) and subfamily of Smyrneae grows wildly in different parts of eastern and western Iran. P. ferulacea is used as animal fodder and for yogurt seasoning (Bruno et al. Citation2021; Sadraei et al. Citation2013). In Turkey (eastern cities), P. ferulacea is used to provide a characteristic aroma with antimicrobial properties to Otlu cheese. In Persia, P. ferulacea has been explored for use in flok medicine as an antimicrobial, tonic for gastrointestinal and liver disorders, anti-inflammatory, anti-viral, and anti-helminthic agent. It also contains emollient, anti-flatulent, and sedative properties (Kafash-Farkhad, Asadi-Samani, and Rafieian-Kopaei Citation2013). Moreover, it has been reported that P. ferulacea is a rich source of different antioxidants (Delnavazi et al. Citation2017). The protective effects of P. ferulacea are directly related to the presence of phenolic compounds (Coruh, Celep, and Özgökçe Citation2007; Mavi et al. Citation2004). The different classes of compounds found in different parts of P. ferulacea are coumarin, flavonoids, terpenoids, phytosterols, and other miscellaneous compounds. From the aerial parts of P. ferulacea, quercetin and isorhamnetin have been isolated and characterized by 1H-NMR, 13C-NMR, EI-MS, and UV spectral analysis (Delnavazi et al. Citation2017; Mottaghipisheh et al. Citation2020) (). Reverse phase HPLC of P. ferulacea aerial parts extract revealed that active dyeing components present are 3-O-glucuronides of quercetin and isorhamnetin, or rutin (Mouri et al. Citation2014). The nature of the sugar moiety P. ferulacea flavonol glycosides was determined by acid hydrolysis and the products were analyzed by LC-MS (Bigge et al. Citation1995). Further, it was found that the flavonol glycosides in P. ferulacea are identical to those found in Anethum graveolens L. which had previously been identified as quercetin 3-O-β-D-glucuronide and isorhamnetin 3-O-β-D-glucuronide (Teuber and Herrmann Citation1978). Additionally, β-pinene (43.1%), α-pinene (22.1%), and δ-3-carene (16.9%) were the main components of the essential oil component of the aerial parts of this plant (Bruno et al. Citation2021; Delnavazi et al. Citation2017).
Figure 1. Chemical structures of some main phytochemicals present in the aerial parts of P. ferulacea plant.
The results of a recent study (Mouri et al. Citation2014) have shown that the dried plant parts of Prangos species can be directly used for dyeing processes as the dried parts are devoid of any free aglycones. Flavonoid-based natural dyeing of wool fibers presents a novel and cleaner production technology for combating antibiotic resistance with the successful production of the antimicrobial and antioxidant wool substrate (Zhou et al. Citation2022). In light of the conclusion drawn by Mouri et al. (Mouri et al. Citation2014) and Zhou et al. (Zhou et al. Citation2022), P. ferulacea can be used as a source of natural yellow dye for the production of yellow-colored wool yarns. It was recently discovered that P. ferulacea can be used to dye wool fibers and the optimization of dyeing parameters was done using the response surface methodology approach (Barani and Rahimpour Citation2014). However, we herein report for the first time the pre-mordanting of wool with P. ferulacea dye in conjunction with binary metal salt combinations to develop shades of acceptable colorimetric and fastness properties with specific hues and tones. The results have been discussed well in terms of the possible relationship between dye structure, the coordination ability of metal ions, and metal-wool-dye interactions.
Experimental
Materials and chemicals
Commercial scoured 100% wool yarn (20/4 Nm), used as pile yarn for handmade carpet production, was used in this study. Aerial parts of P. ferulacea plant were used as a source of natural dye, purchased from the local market in Yazd, Iran. Different metal salts [aluminum sulfate (Al2(SO4)3.18 H2O), copper sulfate (CuSO4.5 H2O), iron sulfate (FeSO4.5 H2O), tin chloride (SnCl2.2 H2O), potassium dichromate (K2Cr2O7), nickel chloride (NiCl2.6 H2O), cobalt sulfate (CoSO4.7 H2O), and zinc sulfate (ZnSO4.7 H2O)] purchased from Merck Germany (Laboratory-grade), were used as mordants. Nonionic detergent (Nikogen SDN) was used for the scouring of wool. Distilled water was used for the preparation of solutions, mordanting, and dyeing.
Methods
Dyeing with and without mordants
Before mordanting and dyeing, wool yarns were scoured in a solution of 5 g/L Nikogen SDN (nonionic detergent) at 60°C for 30 min with a liquor ratio of 40:1. The air-dried wool yarns were then pre-mordanted using twenty-eight different binary metal mordant combinations with a liquor ratio of 50:1 at 95°C for 60 min (Rather et al. Citation2020). Mordanted samples were thoroughly rinsed with running tap water several times to remove surplus (unused) mordants and dried under shade. The details of the binary mixed metal mordant combinations with respective concentrations are provided in Supplementary data (Table S1). Dyeing experiments were performed with a liquor ratio of 40:1 for 75% (o.w.f.) P. ferulacea aerial parts extract under the acid condition of pH 5 (adjusted by acetic acid) at simmering point (93ºC) for 60 min (Rather et al. Citation2021). Finally, the dyed samples were removed from the dyeing solution and washed thoroughly under running tap water. The samples were dried in shade and stored for the fastness rating assessments.
Color parameter assay
The colorimetric characteristics (L*, a*, b*, c*, ho, and K/S) of wool yarn dyed with 75% (o.w.f.) P. ferulacea natural dye were recorded with a reflectance color-eye XTH spectrometer, X-Rite Inc. in the range of 360–750 nm under the D65 illuminant and 10° standard observer. The color coordinate L* corresponds to the brightness index (100 = white; 0 = black), a* corresponds to the red-green coordinate of color space diagram (+ive = red; -ive = green), b* corresponds to the yellow-blue coordinate (+ive = yellow; -ive = blue), c* gives the color purity or vividness – dullness ratio (100 = vivid; 0 = dull) and h0 corresponds to the hue angle (Shahid-Ul-Islam Rather et al. Citation2017). The color strength (K/S) is related to the adsorption of the dye onto the wool surface and gives the depth of the shade (Rather et al. Citation2017). The data of color parameters are the average of 4 individual measurements.
Colorfastness assay
Wash fastness measurements of all dyed wool yarns were tested as per the ISO 105-C10:2006(E) standard. The samples were washed for a period of 30 min at 60°C and were evaluated for color change and degree of staining on adjacent cotton and wool fabric on gray scale. Rub fastness tests were performed as per ISO 105-X12:1993 (E) standard. The Lightfastness test was done as per ISO 105 B02:1988 (E) standard using a xenon arc lamp and samples were analyzed with a blue scale.
FT-IR spectral analysis of dyed wool yarns
FT-IR spectra of the powdered native, Al/Fe mordanted , and Al/Fe mordanted dyed wool yarn samples was recorded using a Jasco 680-plus FT-IR spectrometer with 4 cm−1 resolution between 4000–500 cm−1 (Rather, Shahid-Ul-Islam, and Mohammad Citation2015).
Surface morphology of dyed wool yarns
Surface morphology of native, Al/Fe mordanted , and Al/Fe mordanted dyed wool yarns was observed using TESCAN MIRA3 FEG Scanning Electron Microscope at 10 kV accelerating voltage. Samples were glued to aluminum stubs with colloidal silver paint for conductivity and sputter coated with gold for 3 min in an argon atmosphere (Rather et al. Citation2016).
Results and discussion
Color strength (K/S) values
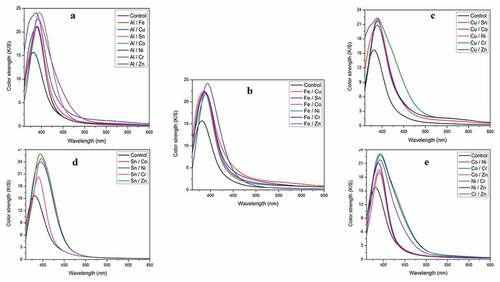
shows the variation of color strength (K/S) dyed wool yarns mordanted with the binary mixed metal combinations. Thus, by varying the combination of metal mordants in conjunction with 75.0% (o.w.f.) P. ferulacea aerial parts extract, we can achieve a range of colors with varying hues and tones. From the results, it is observed that wool yarns dyed P. ferulacea dye extract exhibit significant variations in K/S values due to different mordant combinations. Out of all binary metal combinations, the highest K/S values were observed with Al/Cu, Al/Sn, Fe/Sn, Sn/Co, Sn/Ni, and Sn/Zn with values of 24.78, 24.39, 25.05, 24.65, 24.13, and 26.78 respectively (Supplementary data; Tables S1-S5). This is because metal mordants form chelate complexes with the phenolic group’s of dye molecules and functional groups (amino and carbonyl) of wool fiber (Malacaria et al. Citation2022; Rather et al. Citation2016; Uddin Citation2015) (). Such coordination bonding enhances the interaction between dye molecules and textile substrate, subsequently resulting in higher color depth (higher K/S values) (Ayele et al. Citation2020). In a particular binary metal combination, the retaining effect (bright hues) of a particular metal (e.g. Sn mordant in various Sn combinations) is due to the higher coordination ability (more charge to size ratio) of Sn compared to Al, Fe, Co, Ni and Zn metal ions which is evident from the higher K/S values of corresponding combinations (Rather et al. Citation2018; Rather, Shahid-Ul-Islam, and Mohammad Citation2015). From the photographs of dyed wool yarns (Supplementary data; Figures S1-S5), it is evident that most of the Sn combinations (Sn/Al, Sn/Fe, Sn/Co, Sn/Ni, and Sn/Zn) mordanted wool yarns have retained their characteristic bright hues (Shabbir et al. Citation2019). While in Sn/Cu and Sn/Cr combinations, more contributions come from Cr and Cu compared to Sn. It is clear that varying the amounts and the nature of mordants in a particular combination with constant amount of dye, helps to develop a range of yellow colored shades on wool yarns with different hues and tones.
Color parameters
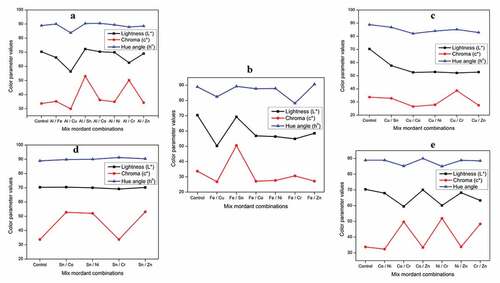
Lightness (L*), chroma (c*), and Hue angle (ho)
From the colorimetric values (CIEL*a*b* and CIEL*c*ho) presented in Tables S1-S5 for various binary mixed metal mordanted wool yarns, it is clear that application of P. ferulacea dye extract produced shades of high lightness (L*) and low color saturation (c*) with hue angle ranging between 82° to 92° (). Among all binary mixed metal combinations, the copper series (Cu/Sn, Cu/Co, Cu/Ni, Cu/Cr, Cu/Zn) showed lower lightness (L*) values compared to the aluminum, iron, and tin series. Among the Cu combinations, Cu/Co and Cu/Cr have the lowest L* values of 52.52 and 52.10, respectively. Similarly, Sn/Zn and Sn/Al mordanted samples show the highest color saturation values (c*) of 53.11 and 53.02, respectively. The current results are on par with the studies of Shabbir et al. for the dyeing of wool fibers with tannin colorants from T. chebula bark extract in conjunction with aluminum, tin, and iron as mordants (Rather et al. Citation2020; Shabbir et al. Citation2019).
Figure 4. Lightness, chroma, and hue angle variation of binary mixed mordanted wool yarns (a) aluminum combinations; (b) Iron combinations; (c) Copper combinations; (d) Tin combinations; and (d) Cobalt and nickel combinations.
a* - b* color coordinates
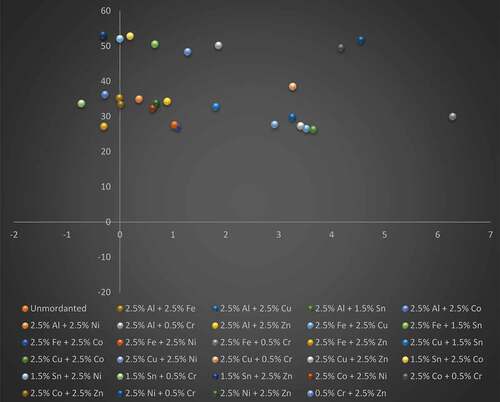
displays the plotted a*–b* graphs of P. ferulacea dyed wool yarns mordanted with different binary metal mordants. As depicted from the a*-b* graph, the data plotted for most of the developed shades for binary mixed mordanted wool yarns lie in the red-yellow zone of the color space diagram. Exceptionally, the a*-b* plots of Al/Fe, Al/Sn, Al/Co, Fe/Zn, Sn/Cr, and Sn/Zn lie in the green-yellow coordinate of the color space diagram with dominant yellow color. The mordanted samples show significant variations in the color of dyeing imparted to wool yarns by the use of different metal combinations. Dyeing with all mordant combinations resulted in color shades with low a* and high b* values, indicating less reddish and more yellowish tincture except Fe/Cr combination which is shifted more toward the red region of the yellow-red coordinate with a* value of 6.28. Darker shades were provided by the iron and copper combination series which possessed comparatively lower b* values and provided the greatest change in color appearance with a shift from yellow to yellowish-brown color. In general, a*–b* values were in good agreement with the visual assessment.
Fastness properties
Light, wash and rub fastness properties of the fastness of P. ferulacea dyed wool yarns were evaluated as per the respective ISO standards. shows the fastness characteristics of control dyed and mordanted dyed samples. Control dyed samples possess a lightfastness rating of 4 (good lightfastness) and a color change rating of 4 (Very good). Application of binary mixed metal mordant combinations has increased the fastness properties concerning the nature and strength of interactions between wool, metal, and dye molecules (Rather et al. Citation2020). Among different mordant series, it was found that tin combinations possess low color change fastness properties with a rating of 4 and lightfastness ratings of 5–6. No or least staining was observed on adjacent cotton and wool fabrics with ratings in the range of 4–5. Similarly, the results for rub fastness for all the shades were assessed using grayscale and in all the cases, dry rub fastness ratings were better than wet rub fastness properties with ratings between 4 and 5.
Table 1. Colorfastness of binary mix metal mordanted wool yarns dyed with 75.0% (o.w.f.) Prangos ferulacea natural dye.
FT-IR spectral analysis of dyed wool yarns
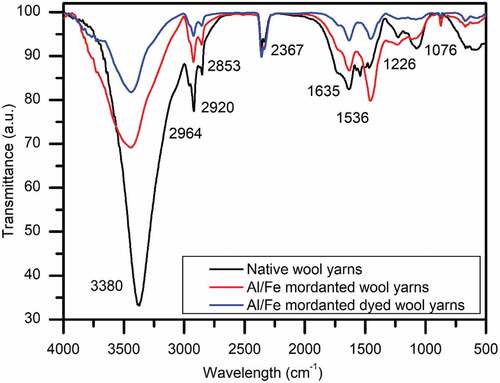
The infrared spectra of native wool yarns indicate the characteristic absorption bands assigned to the peptide bond which is the fundamental structural unit of the polypeptide chain. The different atoms of the peptide bond (IR active) oscillate/vibrate; giving rise to three different IR spectral bands designated as amide I, II, and III bands (Wojciechowska, Włochowicz, and Wesełucha-Birczyńska Citation1999). The amide-I represents the C=O vibrations and falls in the range of 1690–1600 cm−1. Amide-II band is linked with the deformation of N-H and stretching vibration of -C-N bond and it appears in the range of 1580–1480 cm−1. Likewise, amide-III band is linked with the vibrations of C-H and C-N which appears in the region of 1350–1220 cm−1 (Khan et al. Citation2012). The position of amide bands is attributed to the changes in the conformation of keratin molecule.
The IR spectrum of native wool yarns has its own characteristic vibrational bands/FT-IR spectral bands: a high intensity and sharp band at 3380 cm−1 representing N-H and O-H stretching frequency. Sharp band at 2964 cm−1 represents symmetric stretching of methylene group (-CH2) (Rather, Shahid-Ul-Islam, and Mohammad Citation2015). Two more bands at 2920 and 2853 cm−1 represents asymmetric methylene stretching frequencies. Strong peaks at 1635 cm−1 represents amide-I (-C=O) stretching band. Sharp absorption peak around 1536 cm−1 is linked to the secondary amide, N-H bending, and C-N wagging vibrations (Ricci et al. Citation2015). 1226 cm−1 peak represents the virbations linked with amide-III band of peptide bond. Peak at 1076 cm−1 belongs to the S=O vibrations of cysteic monoxide group of polypeptide chain of wool fiber (Belukhina, Milasiene, and Ivanauskas Citation2021; McGregor, Liu, and Wang Citation2018). The wave number shift and intensities of respective bands were found to be affected significantly by mordanting with Al/Fe mordanting and subsequent dyeing with 75.0% (o.w.f.) P. ferulacea aerial parts extract (), which have been briefly summarized in .
Table 2. Characteristics FT-IR spectral bands (cm −1) of native, Al/Fe mordanted, and Al/Fe mordanted dyed wool yarns.
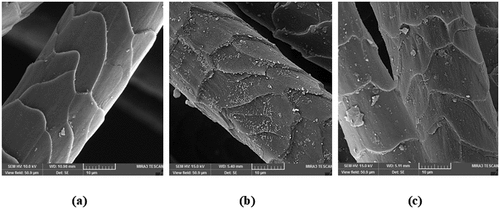
Surface morphology of dyed wool yarns
The surface morphological features of native, mordanted (Al/Fe), and mordanted dyed wool yarns are shown in . SEM images of native wool yarns show normal surface morphology (). It can be clearly seen that mordanting with Al/Fe mordant combinations and subsequent dyeing processes does not alter surface morphology of wool yarns. However, the surface of mordanted sample reveals coarser surfaces due to deposition of well dispersed metal particles with no or little aggregation (). shows that mordanted wool yarns dyed with 75% (o.w.f.) P. ferulacea aerial parts extract showed deposition of dye molecules on its surface. The surface of mordanted dyed yarn sample showed darkening effect due to the deposition of more dye molecules which clearly indicates role of metal ions in increasing color depth (coating) of dyed wool yarn (Rather et al. Citation2016).
Conclusion
The present research was initiated for the development of colored wool yarns with 75.0% (o.w.f.) P. ferulacea aerial parts extract in conjunction with different binary mixed metal salt combinations. The research examinations asserted the use of P. ferulacea natural dye for the development of yellow-colored wool yarns with industrially acceptable colorimetric (CIEL*a*b* and CIEL*c*ho) and fastness (light, wash, and rub) characteristics. The maximum improvement in the dye uptake (K/S) value of 26.78 was recorded with 1.5% (o.w.f.) of stannous chloride +2.5% (o.w.f.) of zinc sulfate combination for 75.0% dye concentration. Each metallic salt-mordant combination produced deep yellow to yellowish-brown shades with significant differences in the hue of the color. The higher values of color depth (K/S) of mordanted samples compared to the control dyed samples were due to the coordination complex-forming ability of metal ions with dye molecules within the fiber structure. The increase in the adsorption of dye molecules and surface characteristics after mordanting and dyeing procedures were verified by FT-IR spectral analysis and SEM images. The surface morphology showed no damage to the cuticle surface of wool yarns under high temperature (93 °C) dyeing. Therefore it can be concluded that different binary metal salt combinations can be successfully utilized for producing beautiful natural yellow shades on wool yarns with P. ferulacea aerial parts extract.
Highlights
Binary mixutres of 8 metal mordants were used for premordanting of wool yarns.
28 Binary mordanted wool yarns were dyed with Prangos ferulacea natural colorants.
Different new and sober color shades with industrially acceptable colorimetric and fastness properties were obtained.
Supplemental Material
Download MS Word (1.3 MB)Acknowledgments
Tabriz Islamic Art University is gratefully acknowledged for all the supports throughout this reaserch study.
Supplemental Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15440478.2022.2134267
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Aali, A., A. Abar, N. Boenke, M. Pollard, F. Rühli, and T. Stöllner. 2012. Ancient salt mining and salt men: The interdisciplinary chehrabad douzlakh project in north-western Iran. Antiquity 86 (333): f1-f5. doi:10.1017/S0003598X0050060X.
- Ahlström, L.-H., C. S. Eskilsson, and E. Björklund. 2005. Determination of banned azo dyes in consumer goods. TrAc Trends in Analytical Chemistry 24 (1):49–13. doi:10.1016/j.trac.2004.09.004.
- Amutha, K., S. G. Annapoorani, P. Sakthivel, and N. Sudhapriya. 2022. Ecofriendly dyeing of textiles with natural dyes extracted from commercial food processing waste materials. Journal of Natural Fibers 1–18. doi:10.1080/15440478.2021.1993506.
- Ayele, M., T. Tesfaye, D. Alemu, M. Limeneh, and B. Sithole. 2020. Natural dyeing of cotton fabric with extracts from mango tree: A step towards sustainable dyeing. Sustainable Chemistry and Pharmacy 17:100293. doi:10.1016/j.scp.2020.100293.
- Barani, H., and S. Rahimpour. 2014. The dyeing procedures evaluation of wool fibers with prangos ferulacea and fastness characteristics. Advances in Materials Science and Engineering. doi:10.1155/2014/497278.
- Belukhina, O., D. Milasiene, and R. Ivanauskas. 2021. Investigation of the possibilities of wool fiber surface modification with copper selenide. Materials 14 (7):1648. doi:10.3390/ma14071648.
- Bigge, J., T. Patel, J. Bruce, P. Goulding, S. Charles, and R. Parekh. 1995. Nonselective and efficient fluorescent labeling of glycans using 2-amino benzamide and anthranilic acid. Analytical Biochemistry 230 (2):229–38. doi:10.1006/abio.1995.1468.
- Bruno, M., V. Ilardi, G. Lupidi, L. Quassinti, M. Bramucci, D. Fiorini, A. Venditti, and F. Maggi. 2021. Composition and biological activities of the essential oil from a sicilian accession of prangos ferulacea (L.) Lindl. Natural Product Research 35 (5):733–43. doi:10.1080/14786419.2019.1598996.
- Coruh, N., A. S. Celep, and F. Özgökçe. 2007. Antioxidant properties of prangos ferulacea (L.) lindl., chaerophyllum macropodum boiss. and heracleum persicum desf. from apiaceae family used as food in eastern anatolia and their inhibitory effects on glutathione-S-transferase. Food Chemistry 100 (3):1237–42. doi:10.1016/j.foodchem.2005.12.006.
- Delnavazi, M.-R., M. Soleimani, A. Hadjiakhoondi, and N. Yass. 2017. Isolation of phenolic derivatives and essential oil analysis of Prangos ferulacea (L.) Lindl. aerial parts. Iranian Journal of Pharmaceutical Research: IJPR 16 (Suppl):207.
- Javadian, H., M. T. Angaji, and M. Naushad. 2014. Synthesis and characterization of polyaniline/γ-alumina nanocomposite: A comparative study for the adsorption of three different anionic dyes. Journal of Industrial and Engineering Chemistry 20 (5):3890–900. doi:10.1016/j.jiec.2013.12.095.
- Kafash-Farkhad, N., M. Asadi-Samani, and M. Rafieian-Kopaei. 2013. A review on phytochemistry and pharmacological effects of prangos ferulacea (L.) lindl. Life Science Journal 10 (SUPPL):360–67.
- Khan, S. A., A. Ahmad, M. I. Khan, M. Yusuf, M. Shahid, N. Manzoor, and F. Mohammad. 2012. Antimicrobial activity of wool yarn dyed with rheum emodi l.(indian rhubarb). Dyes and Pigments 95 (2):206–14. doi:10.1016/j.dyepig.2012.04.010.
- Koh, E., and K. H. Hong. 2014. Gallnut extract-treated wool and cotton for developing green functional textiles. Dyes and Pigments 103:222–27. doi:10.1016/j.dyepig.2013.09.015.
- Malacaria, L., C. La Torre, E. Furia, A. Fazio, M. C. Caroleo, E. Cione, L. Gallelli, T. Marino, and P. Plastina. 2022. Aluminum (III), iron (III) and copper (II) complexes of luteolin: Stability, antioxidant, and anti-inflammatory properties. Journal of Molecular Liquids 345:117895. doi:10.1016/j.molliq.2021.117895.
- Mavi, A., Z. Terzi, U. Özgen, A. Yildirim, and M. Coşkun. 2004. Antioxidant properties of some medicinal plants: prangos ferulacea (apiaceae), sedum sempervivoides (crassulaceae), malva neglecta (malvaceae), cruciata taurica (rubiaceae), rosa pimpinellifolia (rosaceae), galium verum subsp. verum (rubiaceae), urtica dioica (urticaceae). Biological & Pharmaceutical Bulletin 27 (5):702–05. doi:10.1248/bpb.27.702.
- McGregor, B., X. Liu, and X. Wang. 2018. Comparisons of the fourier transform infrared spectra of cashmere, guard hair, wool and other animal fibres. The Journal of the Textile Institute 109 (6):813–22. doi:10.1080/00405000.2017.1372057.
- Mottaghipisheh, J., T. Kiss, B. Tóth, and D. Csupor. 2020. The Prangos genus: A comprehensive review on traditional use, phytochemistry, and pharmacological activities. Phytochemistry Reviews 19 (6):1449–70. doi:10.1007/s11101-020-09688-3.
- Mouri, C., A. Aali, X. Zhang, and R. Laursen. 2014. Analysis of dyes in textiles from the Chehrabad salt mine in Iran. Heritage Science 2 (1):1–8. doi:10.1186/s40494-014-0020-3.
- Mouri, C., V. Mozaffarian, X. Zhang, and R. Laursen. 2014. Characterization of flavonols in plants used for textile dyeing and the significance of flavonol conjugates. Dyes and Pigments 100:135–41. doi:10.1016/j.dyepig.2013.08.025.
- Pollard, A. M., D. R. Brothwell, A. Aali, S. Buckley, H. Fazeli, M. H. Dehkordi, T. Holden, A. Jones, J. Shokouhi, and R. Vatandoust. 2008. Below the salt: A preliminary study of the dating and biology of five salt-preserved bodies from zanjan province, Iran. Iran 46 (1):135–50. doi:10.1080/05786967.2008.11864741.
- Rastogi, D., A. Jain, and A. Negi. 2022. Development of mosquito repellent finish for textiles using neem oil: an eco-friendly approach. In Muthu, Subramanian Senthilkannan (eds), Sustainable Approaches in Textiles and Fashion: Manufacturing Processes and Chemicals, 183–95. Springer, Singapore. doi:10.1007/978-981-19-0538-4_9
- Rather, L. J., S. Akhter, R. A. Padder, Q. P. Hassan, M. Hussain, M. A. Khan, and F. Mohammad. 2017. Colorful and semi durable antioxidant finish of woolen yarn with tannin rich extract of Acacia nilotica natural dye. Dyes and Pigments 139:812–19. doi:10.1016/j.dyepig.2017.01.018.
- Rather, L. J., A. Ali, Q. Zhou, S. A. Ganie, K. Gong, Q. M. R. Haque, and Q. Li. 2020. Instrumental characterization of merino wool fibers dyed with Cinnamomum camphora waste/fallen leaves extract: An efficient waste management alternative. Journal of Cleaner Production 273:123021. doi:10.1016/j.jclepro.2020.123021.
- Rather, L. J., M. Azam, M. Shabbir, M. N. Bukhari, M. Shahid, M. A. Khan, Q. M. R. Haque, and F. Mohammad. 2016. Antimicrobial and fluorescence finishing of woolen yarn with Terminalia arjuna natural dye as an ecofriendly substitute to synthetic antibacterial agents. RSC Advances 6 (45):39080–94. doi:10.1039/C6RA02717B.
- Rather, L. J., Q. F. Dar, Q. Zhou, L. Haofan, and Q. Li. 2021. Binary mix metal mordant dyeing of merino wool fibers using cinnamomum camphora waste/fallen leaves extract: A brief statistical analysis of color parameters. The Journal of the Textile Institute 112 (5):742–51. doi:10.1080/00405000.2020.1779166.
- Rather, L. J., A. Haji, and M. Shabbir. 2021. Innovative and emerging technologies for textile dyeing and finishing. USA: Scrivener-Wiley.
- Rather, L. J., M. Shabbir, M. N. Bukhari, M. Shahid, M. A. Khan, and F. Mohammad. 2016. Ecological dyeing of woolen yarn with Adhatoda vasica natural dye in the presence of biomordants as an alternative copartner to metal mordants. Journal of Environmental Chemical Engineering 4 (3):3041–49. doi:10.1016/j.jece.2016.06.019.
- Rather, L. J., M. Shabbir, Q. Li, and F. Mohammad. 2019. Coloration, UV protective, and antioxidant finishing of wool fabric via natural dye extracts: Cleaner production of bioactive textiles. Environmental Progress & Sustainable Energy 38 (5):13187. doi:10.1002/ep.13187.
- Rather, L. J., M. Shabbir, F. Mohammad, and Q. Li. 2020. Terminalia arjuna dyed woolen yarn-effect of binary and ternary metal salt combinations: A greener route for production of ecofriendly textiles. Journal of Natural Fibers 17 (12):1693–705. doi:10.1080/15440478.2019.1588830.
- Rather, L. J., Shahid-Ul-Islam, and F. Mohammad. 2015. Study on the application of Acacia nilotica natural dye to wool using fluorescence and FT-IR spectroscopy. Fibers and Polymers 16 (7):1497–505. doi:10.1007/s12221-015-4879-8.
- Rather, L. J., Shahid-ul-Islam, M. Shabbir, M. N. Bukhari, F. Mohammad, and M. A. Khan. 2018. Adhatoda vasica in conjunction with binary combinations of metal salts and biomordants as an effective textile dye to produce novel shades on wool. Journal of Natural Fibers 15 (4):611–23. doi:10.1080/15440478.2017.1349712.
- Rather, L. J., Q. Zhou, A. Ali, Q. M. R. Haque, and Q. Li. 2021. Valorization of agro-industrial waste from peanuts for sustainable natural dye production: Focus on adsorption mechanisms, ultraviolet protection, and antimicrobial properties of dyed wool fabric. ACS Food Science & Technology 1 (3):427–42. doi:10.1021/acsfoodscitech.1c00005.
- Ricci, A., K. J. Olejar, G. P. Parpinello, P. A. Kilmartin, and A. Versari. 2015. Application of fourier transform infrared (FTIR) spectroscopy in the characterization of tannins. Applied Spectroscopy Reviews 50 (5):407–42. doi:10.1080/05704928.2014.1000461.
- Sadeghi-Kiakhani, M., A. R. Tehrani-Bagha, S. Safapour, S. Eshaghloo-Galugahi, and S. M. Etezad. 2021. Ultrasound-assisted extraction of natural dyes from Hawthorn fruits for dyeing polyamide fabric and study its fastness, antimicrobial, and antioxidant properties. Environment, Development and Sustainability 23 (6):9163–80. doi:10.1007/s10668-020-01017-0.
- Sadraei, H., Y. Shokoohinia, S. Sajjadi, and M. Mozafari. 2013. Antispasmodic effects of Prangos ferulacea acetone extract and its main component osthole on ileum contraction. Research in Pharmaceutical Sciences 8 (2):137.
- Safapour, S., M. Sadeghi-Kiakhani, and S. Eshaghloo-Galugahi. 2018. Extraction, Dyeing, and antibacterial properties of crataegus elbursensis fruit natural dye on wool yarn. Fibers and Polymers 19 (7):1428–34. doi:10.1007/s12221-018-7643-z.
- Shabbir, M., L. J. Rather, M. N. Bukhari, S. Shahid-Ul-Islam, M. Shahid, M. A. Khan, and F. Mohammad. 2019. Light fastness and shade variability of tannin colorant dyed wool with the effect of mordanting methods. Journal of Natural Fibers 16 (1):100–13. doi:10.1080/15440478.2017.1408521.
- Shahid-ul-Islam, L. J. Rather, M. Shabbir, M. N. Bukhari, M. Shahid, M. Ali Khan, and F. Mohammad. 2017. Bi and tri metal salt combinations plus colorants extracted from timber industry waste as effective dyeing materials to produce novel shades on wool. Journal of Natural Fibers 14 (4):586–96.
- Teuber, H., and K. Herrmann. 1978. Flavonol glycosides of leaves and fruits of dill (Anethum graveolens L.). II. Phenolics of spices (author’s transl). Zeitschrift Fur Lebensmittel-Untersuchung Und-Forschung 167 (2):101–04. doi:10.1007/BF01136136.
- Uddin, M. G. 2015. Extraction of eco-friendly natural dyes from mango leaves and their application on silk fabric. Textiles and Clothing Sustainability 1 (1):1–8. doi:10.1186/s40689-015-0007-9.
- Wojciechowska, E., A. Włochowicz, and A. Wesełucha-Birczyńska. 1999. Application of fourier-transform infrared and raman spectroscopy to study degradation of the wool fiber keratin. Journal of Molecular Structure 511:307–18. doi:10.1016/S0022-2860(99)00173-8.
- Zhang, Y., Shahid-ul-Islam, L. J. Rather, and Q. Li. 2022. Recent advances in the surface modification strategies to improve functional finishing of cotton with natural colourants-A review. Journal of Cleaner Production 335: 130313. doi:10.1016/j.jclepro.2021.130313
- Zhang, Y., Q. Zhou, L. J. Rather, and Q. Li. 2021. Agricultural waste of Eriobotrya japonica L.(Loquat) seeds and flora leaves as source of natural dye and bio-mordant for coloration and bio-functional finishing of wool textile. Industrial Crops and Products 169:113633. doi:10.1016/j.indcrop.2021.113633.
- Zhou, Q., L. J. Rather, A. Ali, W. Wang, Y. Zhang, Q. M. R. Haque, and Q. Li. 2020. Environmental friendly bioactive finishing of wool textiles using the tannin-rich extracts of Chinese tallow (Sapium sebiferum L.) waste/fallen leaves. Dyes and Pigments 176:108230. doi:10.1016/j.dyepig.2020.108230.
- Zhou, Q., L. J. Rather, S. S. Mir, A. Ali, Q. M. R. Haque, and Q. Li. 2022. Bio colourants from the waste leaves of ginkgo biloba l. tree: wool dyeing and antimicrobial functionalization against some antibiotic-resistant bacterial strains. Sustainable Chemistry and Pharmacy 25:100585. doi:10.1016/j.scp.2021.100585.