?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In recent years, many different properties of natural dyestuffs that do not harm the environment and human have been discovered as a result of the search for dyestuffs. Buckthorn based dyes can be considered in this respect, and so they were tested in this study. The berries and leaves of the buckthorn bush trees were used. While dyeing the fabric, along with the dyeing process, other properties that can be added have been investigated. Wool fabrics with no metal salts and with five different metal salts were dyed with natural dye sources at 100°C for 1 hour. After dyeing, all samples were dried at room temperature. Then, the dyed wool samples’ CIE L*a*b* and color efficiency values (K/S) were determined from the obtained colors to show the usability of buckthorn in dyeing. Color fastness to washing, rubbing, perspiration, and light fastness of the samples were also tested, and results were determined in the range of 3–5 points. The T% values between 0.08 and 0.35 for UVA and between 0.09 and 0.18 for UVB were observed in all trials. It has been determined that fabrics treated with buckthorn berries/leaves can provide a protective feature against the harmful rays of the sun.
摘要
近年来,由于对染料的研究,人们发现了许多对环境和人类无害的天然染料的不同性质. 在这方面可以考虑使用沙棘染料,因此在本研究中对其进行了测试. 使用了沙棘树的浆果和叶子. 在对织物进行染色的同时,还研究了染色过程中可以添加的其他性能. 不含金属盐和五种不同金属盐的羊毛织物用天然染料源在100°C下染色1小时. 染色后,所有样品在室温下干燥. 然后,从获得的颜色中确定染色羊毛样品的CIE L*a*b*和颜色效率值(K/S),以显示沙棘在染色中的可用性. 还测试了样品的耐洗牢度、耐摩擦牢度、耐汗牢度和耐光牢度,结果在3-5分范围内测定. 在所有试验中,UVA的T%值在0.08和0.35之间,UVB的T%为0.09和0.18之间. 已经确定,用沙棘浆果/树叶处理过的织物可以提供一种抵御有害阳光的保护功能.
Introduction
The use of natural stuffs in textile finishing has started to be a promising topic in the textile industry. Due to the harm caused by petroleum-based dyestuffs to the environment and human health, research and development in the textile dye industry are particularly focused on renewing producing natural dyestuffs for a sustainable future (Thakker and Sun Citation2021). By 2024, it is expected that the global market share of natural dyes would increase by $5 billion or more. The compound annual growth rate would be 11% from 2018 to 2025 (Natural Dyes Market). Natural dyestuffs are widely used as colorants in the textile, food, pharmaceutical, cosmetic, leather, and lacquer industries today. There are a wide variety of plant-based dyestuffs and many different parts of plants, such as roots, stems, branches, leaves, bark, flowers, seeds, and fruit, can be used as the colorants (Samanta and Konar Citation2011). Phenolic compounds are the most commonly found structures in plants (Khoddami, Wilkes, and Roberts Citation0000), and plants produce flavonoids to protect themselves against external factors such as frost, drought, fungal/microbial activities, and UV radiation (Giusti and Wallace Citation2009). Therefore, in addition to the ability of plant-based natural dye sources to dye textile materials Davulcu et al. (Citation2014); Benli and Bahtiyari (Citation2015)(a); Benli and Bahtiyari Citation2016; Benli (Citation2017); Mia et al. (Citation2022) in recent years, many properties such as antimicrobial (Han and Yang Citation2005; Singh et al. (Citation2005); Gawish et al. (Citation2017); Yılmaz et al. (Citation2020(a)); Yılmaz et al. (Citation2020(b)); Jiang et al. (Citation2022), antioxidant (Andreeva et al. Citation2004), anti-viral (Rachel and Hussain Citation2019) and UV protection (Grifoni et al. Citation2009; Hou et al. Citation2013; Karabulut and Atav Citation2020; Benli and Bahtiyari Citation2021; Mia et al. Citation2021) have been discovered. The sun is the main source of energy on the globe. Only a small portion of UV light is made up by solar energy, and the atmosphere absorbs it depending on its wavelength. Ozone, water vapor, and CO2 in the stratosphere absorb the bulk of high-energy UV-C and around 90% of UV-B radiation. Thus, 94% of the radiation that comes to Earth is UV-A, which penetrates the skin deeply, while only 6% of UV-B comes to surface (Sankaran et al. Citation2021). Exposure to the sun’s harmful UV rays causes a variety of health problems and plays a causal and determining role in acute and chronic skin damage as well as cancer (Afaq and Mukhtar Citation2001). Therefore, the skin should be protected from the harmful rays of the sun. Today, clothes can gain protective properties by a number of chemical substances (TiO2, ZnO) (Khan et al. Citation2018; Mavrić, Tomšič, and Simončič Citation2018; Sivakumar et al. Citation2013). From an ecological point of view, the toxicity of chemicals such as ZnO to environmental bacterial species is of great importance (Dağlıoğlu et al. Citation2016; Londono et al. Citation2017; Ma, Williams, and Diamond Citation2013). Hence, providing protection with environmentally friendly methods is also very important in terms of sustainability and the environment. In this context, the importance of detecting and applying natural materials and structures that have protective properties against the harmful UV rays of the sun has emerged. Researchers are currently exploring natural dyestuffs to ensure the UV protection of garments as ultraviolet radiation (UVR) begins to reach the Earth due to damage to the ozone layer. UV radiation can harm human beings and that is why the UV protection property of garments has become the main concern of garment manufacturers. As is known, natural fibers are widely used in summer garments because of their excellent comfort properties, but these fibers have very poor UV protection (Kociç et al. Citation2019). Natural resources such as dyer’s woad, madder, logwood, brasilwood, lavender, and weld have been reported as dyestuff providing UV protection properties to the textile materials (Grifoni et al. Citation2009, Citation2011, Citation2014). In this study, the dyeing and UV protection properties of buckthorn (Rhamnus petiolaris Boiss) (), a previously unexplored herbal resource from this point were investigated. Buckthorn has been known for centuries. Buckthorn berry dye plant, an old Turkish dyestuff, has a very important place in natural dyestuff sources (Böhmer et al. Citation2002; Cardon Citation2007). The main dyestuff present in the aqueous extract from the dried buckthorn is reported to be a glycoside derivative of rhamnetin (Romani, Zuccaccia, and Clementi Citation2006), and the barks have a lot of flavonoids such as chrysophanol, physcion, emodin (Tanker and Ertan Citation1971), and quercetin glycosides (Deveoglu Citation2012). During the 15thand 17thcenturies, these plants were used to produce yellow colors on a variety of carpets (Deveoglu Citation2019) too. Apart from its usage as a dyestuff, little research has been done on buckthorn’s other properties.
Comlekcioglu et al. reported that R. petiolaris was found to be the most efficient antimicrobial agent on wool yarn against certain microorganisms (Comlekcioglu et al., Citation2017). Davulcu et al. (Citation2014) shown that polypropylene could be dyed via buckthorn through the incorporation of hyper-branched polyester amide into the polymer prior to fiber spinning via buckthorn (Davulcu et al. Citation2014). Rocchetti et al. (Citation2019) determined the antioxidant activities of methanol and aqueous unmature fruit extracts of buckthorn using radical scavenging activities (Rocchetti et al., Citation2019). In this study, not only the dyeing ability but also the UV protection properties of buckthorn plant-based natural dyestuffs were investigated.
Materials and methods
Materials
In this study, the berries and leaves of dried Anatolian Buckthorn (), obtained from the local market, were used as a natural dyestuff source. All natural sources were separately ground into a powder before being used for the finishing of the fabrics. In the study a wool fabric at a weight of 200 g/m2 was used. It was a 2/1 twill fabric and it was in pretreated form so ready for the dyeing. The study employed analytical grades of FeSO4.7 H2O, CuSO4.5 H2O, SnCl2.2 H2O, K2Cr2O7, and KAl(SO4)2.12 H2O (Sigma-Aldrich).
Mordanting-dyeing
Since the mordants are generally substantive against both the colorant and the fiber and make bonds with the dye on the fiber to form an insoluble precipitate, it is used to improve dye adsorption and fastness. Different mordanting techniques such as pre-, post-, or simultaneous mordanting, have been used for centuries. By utilizing different metal salts, which are frequently polyvalent metallic ions that form a complex with the fiber and the pigment, the same dyestuff sources could produce distinct hues or even different colors (Zarkogianni et al. Citation2011). Simultaneous-mordanting methods (dye source and metal salt are applied simultaneously to textile material) (Erdem and Bahtiyari Citation2018) were used for the finishing of wool in this study. This method was performed at a material to liquor ratio of 1:40 for one hour at 100°C. After the herbal sources were prepared for dyeing, they were added to the bath with the fabrics and mordants if necessary. The used herbal source amount was arranged to be equal to the fabric to be dyed. Like the study by Yılmaz (Citation2020), the following metal salts are used as mordants, in the following concentrations based on the fabric weight: FeSO4.7 H2O (3%), CuSO4.5 H2O (3%), SnCl2.2 H2O (3%), K2Cr2O7 (3%) and KAl(SO4)2.12 H2O (20%).
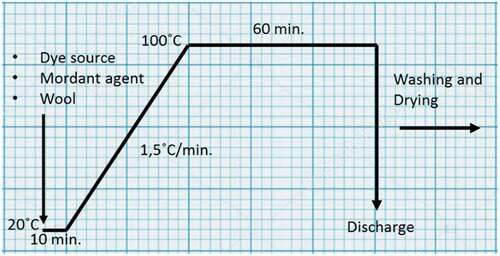
In the dyeing process (), natural dye sources (without a previous extraction) and mordants (if necessary) were added directly to the dye bath (water at pH:7) and the dyeing procedure started. After that, dyed fabrics were rinsed, followed by a hot wash with 1 g/L nonionic surfactant for 10 minutes. Subsequently, warm and cold rinses were managed too. And finally, dyed wool fabrics were dried at room temperature.
Analytical procedures
Color analysis
The dyed wool fabrics were evaluated in terms of color efficiencies (K/S) and CIE L*a*b* color space values by using a Konica Minolta 3600d spectrophotometer (D65/10°). L* describes lightness (100 = white, 0 = black); a* measures redness or greenness; b* measures yellowness or blueness in the CIE system. The K/S value was calculated by Kubelka – Munk Equationequation 1(1)
(1) (McDonald Citation1997).
Where K is the absorption coefficient, S is the scattering coefficient, and R is the fabric’s reflectance at peak wavelength.
Color fastness properties
Testing for washing fastness (with CitationISO 105-C10 standard in test condition of Test A (1)), rubbing fastness (with CitationISO 105-X12 standard), light fastness (with CitationISO 105-B02 standard), and perspiration fastness (with CitationISO 105-E04 standard) tests were applied to dyed fabrics.
UV–Vis absorption
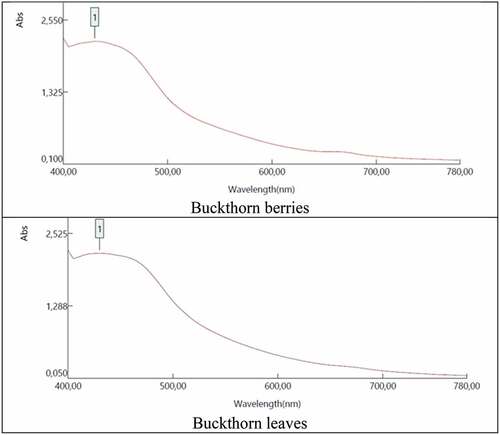
A UV/VIS spectrophotometer (PG Instrument T70) was used to obtain the absorbance curves of the tested natural dye sources. Berries and leaves of buckthorn were tested separately. Water was used as the solvent. Extracts were obtained by taking the amount of fruit and leaves required for dyeing and boiling them at 100°C for one hour. The absorbance curve of these extracts was determined ().
Measurement of UV-protective properties
As in our previous study (Benli and Bahtiyari Citation2021), the UV-protection properties of the samples were measured with the help of the CitationEN 13758–1.2001. standard. The UV-transmittance rates of the undyed and dyed samples were determined. The UV-protection factor (UPF) and ultraviolet transmittance rates (TUVA and TUVB) were calculated (Hou et al. Citation2013).
SEM analysis
The surface changes of the selected samples were determined by scanning electron microscopy (SEM) (Zeiss LS-10).
Results and discussion
In the present study, buckthorn berries and leaves were used as a natural dye source. Generally, there is a complex relationship between the dye molecule, metal salt, and fiber. This complex structure was shown in different studies (Haji, Mehrizi, and Sharifzadeh Citation2016; Uddin Citation2014, Benli Citation2022). The aim of this study was to investigate the protective properties of the dyed wool fabrics with natural dyestuffs against harmful UV rays emitted by the sun.
UV-protective properties
Every textile material serves a different purpose for defense against many elements, including heat, mechanical strain, bad weather, physical, chemical, and biological dangers, electric and electronic dangers, explosions, etc. Since all kinds of protection cannot be provided by a single textile, the development of various protective clothing has gained importance. UPF values of textile materials are affected by chemical composition and fiber structure (Sankaran et al. Citation2021). According to a study by Schuicrer (Citation1997), natural fibers like cotton, wool, and silk have lower UV absorption rates than synthetic fibers like PET (Schuicrer Citation1997). It has been reported that dyed cotton fabrics show higher UPF compared to undyed and bleached cotton (Sankaran et al. Citation2021). Srinivasan and Gatewood (Citation2000) stated that depending on the concentration of dyes in the fabric and the absorptivity of dyes in the UV area, the application of dyes can significantly affect the UV protection offered by a fabric (Srinivasan and Gatewood Citation2000). In general, it is understood that the UV protection property of the fabric improves with the dyeing of the textile surface. It is reported that the UV-blocking capacity of natural dye sources depends on the functional groups’ absorption properties, such as those of flavonoids, anthraquinones, terpenoids, aromatic conjugated systems, etc., that are present in plants (Ammayappan and Jose Citation2015). In , UV protection properties of selected dyed samples were shown. The results for the undyed wool fabrics were almost identical to those obtained in our previous study (Benli and Bahtiyari Citation2021). In this study, the UV protection values of undyed wool fabrics are 8.24% (T(UVA)) and 0.5% (T(UVB)), respectively. The T% values between 0.08 and 0.35% for UVA and between 0.09 and 0.18 for UVB were determined in all trials. In addition, the UPF rating values were determined as 50 + .By dyeing with tested natural sources, a significant increase in UV-Protection features has been gained even in mordant-free dyeings too. But beyond this, it was also found that the transmittance level of the samples changed with the metal ion used in dyeing, and hence, the dyed samples showed significantly higher UV protective properties. However, there can be some shifts in the obtained results due to the use of different mordants. For each used natural dye source, the higher UV protection was obtained via dyeing with iron sulfate, and the sample with the lowest transmittance (UVA: 0.08%; UVB: 0.09%) was observed in the sample dyed with buckthorn berry in the presence of iron ions. The sun’s harmful rays (UVB) transmittance rates of selected samples are listed as follows for the dyeing with buckthorn berries, from the higher to the lower; unmordanted-dyed sample (0.18) > Al-dyed sample (0.16) > Sn-dyed sample (0.14) > Cr-dyed sample (0.13) > Cu-dyed sample (0.12) > Fe-dyed sample (0.09). It was also observed that the used natural dye source has an effect on the obtained UV protection features. So, the so-called sequence for dyeing with buckthorn leaves becomes as follows: Sn-dyed sample (0.17) > unmordanted/Al-dyed sample (0.16) > Cu-dyed sample (0.15) > Cr-dyed sample (0.14) > Fe-dyed sample (0.12). It could be stated that the UV protection properties change depending on the presence and type of mordant materials. The UV protection capacities of the samples dyed with buckthorn leaves were lower than the UV protection capacities of the samples dyed with buckthorn berries. A similar trend was observed in the case of using mordant. But it should be taken into account that, in addition to the direct effect of the used natural dye source and mordants, the obtained colors can also be responsible for the obtained results, as reported previously (Ghazi, Couteau, and Coiffard Citation2010). It can be stated that the UV protection properties of dyed wool fabrics are higher than the leaves of the Buckthorn berry. shows the samples in addition to the UV protection feature results.
Table 1. UV protection feature results of wool fabrics dyed with natural dye sources.
From this, it is clearly understood that clothes that protect from the harmful rays of the sun can be produced using natural materials using buckthorn berries and leaves. It is mentioned in the introduction that there are various natural sources that show UV protective properties by using different natural materials. In their studies, Rather et al. reported different approaches. In one, UV protection was obtained by dyeing woolen fabrics with peanut shells, which was an agricultural waste for sustainable natural dye production (Rather et al. Citation2021a). In the other study, it was stated that the waste water from Cinnamomum camphora natural dye, which shows sustainable UV protective and antioxidant properties in dyeing wool fabric, can be reused (Rather, Zhou, and Li Citation2021b).
Color analysis
Buckthorn is an old natural dye source, and the main dye component can be reported as quercetin, emodin, or kaempferol (Donmez et al. Citation2017; Karadag, Buyukakinci, and Torgan Citation2020; Poulin Citation2018). The bath of blank dyeing, which means it contains only natural dye sources in the dyeing process without the addition of fabric and any mordant, was centrifuged after the dyeing process. By this way, an extraction from the natural sources is managed, which is normally occurring in dyeing processes. After centrifugation, the absorbance of the extracts was taken without dilution, and the diagrams are given below in .
The absorbance curve of the natural dye extracts shown in indicates the color of the solutions. The UV-Vis spectrum of the solution of ground buckthorn leaves and berries extracted in aqueous medium was taken separately. It was previously stated in different studies that the UV-Vis absorption spectrum of a typical flavonoid comprises two maxima between 240–285 nm (band II) and 300–550 nm (band I). It was also reported that the absorption range of anthocyanidins and anthocyanin is between 465 and 560 nm (Andersen and Markham Citation2005), and 437 nm is the maximum UV absorption wavelength for emodin (Sucheta et al. Citation2011; Ţebrencu et al. Citation2015). Likewise, a peak was observed in the range of 400–500 nm. In addition to the absorbance curve of the dye extract, the colors that came out by the use of buckthorn berries and leaves in simultaneous dyeing and mordanting were analyzed and presented in . One of the most important results obtained is the dyeability of wool fabric with buckthorn berries and leaves without using any metal salt. It was found that by solely using these natural sources, the coloration of the wool can be managed, and it was determined that the colors obtained with the change of the natural source also changed. At the end of dyeing, wool fabrics dyed with buckthorn berry and buckthorn leaves without any mordant showed mustard yellow (a*: 8.24, b*: 40.94) and brown-beige (a*: 2.77, b*: 26.62) respectively. In trials carried out without using metal salt, the color efficiency (K/S) values were as follows: buckthorn berry (21.74) > buckthorn leaves (6.63). Among them, it was seen that the highest color efficiency (K/S) can be obtained with buckthorn berry. It is stated that a general trend of an increase in K/S values due to mordanting reveals that the extracted dye molecules can form metal complexes with positively charged mordant ions. At the same time, the L* value was as follows: buckthorn berry (54.03) < buckthorn leaves (65.19). Besides, the hue degree (h°) was as follows; buckthorn berry (78.63) < buckthorn leaves (84.07). In addition to the trials without using metal salt, their colors were examined using five different metal salts as mordanting agents. The colors belonging to dyed samples obtained from the dyeing via mordants are presented in . When the colors obtained are generally evaluated, dark brown, dark yellow, and brown-red colors are seen when buckthorn berry is used. Via the use of buckthorn leaves, brown-yellow tones were brought out ().
Table 2. The CIE L*a*b* and K/S values of the dyed samples.
When the data obtained from dyed samples () with buckthorn berries were examined in detail, the following results can be highlighted. In the case of using iron, copper, aluminum, tin, chromium ions, bitter brown (a*: 3.85, b*: 17.74), brown (a*: 9.4, b*: 35.84), dark yellow (a*: 10.11, b*: 42.51), red brown (a*: 19.18, b*: 53.95), and burgundy (a*: 17.85, b*: 26.36) colors were obtained, respectively. The darkest color tone was obtained from the dyeing combination using buckthorn berry and iron ions (). The ability of some transition metal ions to form coordination complexes allows them to tightly interact with natural dye molecules, resulting in the production of intense color on cloth. The transition metal mordants, such as ferrous sulfate, generate many complexes with the dye molecules, usually octahedral ones with coordination number 6 (Uddin Citation2014). Furthermore, different color efficiencies from wool fabrics dyed with buckthorn berries were obtained by using different mordanting agents. The highest color efficiency (24.65) was obtained from the experiment with tin salt, and the lowest K/S value (4.52) was obtained from the fabric sample dyed with chrome. Besides, the highest L* (49.1) value was obtained from the experiment that used tin salt, and the lowest L* value (31.99) was obtained from the fabric samples that were dyed in the presence of iron ions. Hue degrees (h°) were as follows: chrome (55.9) < tin (70.43) < copper (75.31) < alum (76.62) < iron (77.76).
In dyeing with buckthorn leaves, the colors were changed by the mordants, as in the dyeing with berries. As seen in from the photos of the samples, one of the significant color changes came out of the dyeing with tin chloride. The color became nearly yellow and a*: 3.58 and b*: 46.62 values were measured. In that case, the lightest color has been obtained with an L* value of 70.25. Instead of tin, by using iron-based mordants, the color gets darker and the darkest color among those dyed with the leaves was obtained by the L* value of 41.61. In this case, a* is 1.38 and b* is 12.88. The colors dyed in the presence of KAl(SO4)2.12 H2O and K2Cr2O7 were closer to each other when compared with the samples dyed in the presence of other mordants. This case can be seen from the CIE L*a*b* values of the samples and photos as well. Moreover, for all the leave-based dyed samples, the hue angles were in the range of 82-86° but color efficiencies differed from each other. The highest one was seen in dyeing with copper, and in this case, the color efficiency was 14.99. The lowest K/S (4.52) was seen in dyeing with K2Cr2O7. But as a generalization, it can be said that the K/S of the samples dyed with berries is higher than the ones dyed with leaves, and whereas the darker shades were obtained via the berries.
Color fastness properties
Protein fibers contain many different amino acids with a number of functional groups that provide binding opportunities for all kinds of colorants (Pailthorpe Citation1992). Mordants are widely used to provide better color fastness and to obtain a wide color range from natural dyes with limited color range. The color fastness test results of the wool fabrics dyed using buckthorn berries and leaves were presented in . When the color fastness test results were examined, it was determined that the results obtained from all trial sets were in the range of 4–5 points for the fastness against washing, rubbing, and perspiration. Meanwhile, the light fastness was between 3–5. From the test results reported in , both berries and leaves can be used for obtaining samples with high washing fastness. But in berry-based dyeing, a bit lower fastness has come out. The staining on wool values were 4/5. However, with the use of buckthorn leaves, the washing fastness values were 5 in almost all cases. The same tendency is valid for the rubbing fastness values too.
Table 3. Fastness properties of dyed samples.
In the rubbing fastness test, 5 values were obtained in all samples dyed with buckthorn leaves. But especially for wet rubbing fastness, the samples dyed with berries were a bit lower but still good. The values were 4 and 4/5 and changed depending on the mordant. Perspiration fastness was another tested fastness property. It was seen that for the unmordanted dyed samples in berry-based dyeing, the value was 4 for acidic perspiration in staining on wool. But except in this situation, the values are higher than 4/5 for both tested natural dye sources.
All dyed samples’ artificial light fastness values were obtained from low to medium (3–5). The lowest light fastness, which was 3, was obtained from the fabrics dyed with buckthorn leaves without the use of any mordant. With the use of berries without the use of mordant, the light fastness value was 4. Moreover, it was found that in the case of using solely natural sources, the light fastness values were lower and by the addition of mordants, the light fastnesses were improved by nearly 1–2 points. In terms of light fastness, the mordants had significantly important effects on the obtained results and solely the use of leaves caused the lowest value. In light of the obtained results from the dyeing with buckthorn, it was concluded that both berries and leaves can be useful for coloration and functionalization of the wool. But the use of berries is more commonly known. So, a cop dyeing with the extract of berries without the use of any mordant was conducted for the yarns constituted of Viscose-Acrylic (30/70) as seen in . By this way, it was planned to show the usability of natural dyeing with buckthorn in an industrial aspect. The dyeing process was carried out in the factory environment with the dyeing recipes optimized as a result of the laboratory studies. The berries of the buckthorn plant were used as the dye source. The dyestuffs were extracted from the related plant in a Soxhlet system by water prior to cops dyeing. In the extraction process, a plant amount equal to the weight of the material to be dyed was extracted for 4 hours, and the obtained extract was used directly as a dye bath in the cop dyeing machine.
Table 4. The CIE L*a*b* and K/S values, photo and SEM view of blend yarns dyed with extract of berries in cop dyeing machine.
From , it can be seen that a uniform dyeing with the extract of berries can be obtained in dyeings without using mordants. Meanwhile, as in fabrics, a mustard yellow in a brown shade was obtained. Meanwhile, an SEM view of the surface structure of the dyed sample revealed the smoothness and cleanness of the surface.
Conclusions
Today, various applications are made on clothes to protect the human skin from the harmful rays of the sun. In those applications, non-environmentally friendly and hazardous chemicals can be used. Recently, very intensive research has been carried out on natural resources with UV protection properties. In this study, woolen fabrics were dyed using a natural material derived from a vegetable source (buckthorn), and at the same time, dyed fabrics gained protection properties against harmful UV rays. And thus, this practice became an example of sustainable and environmentally friendly processes. It was observed that good results can be obtained in terms of color fastness values, UV protection properties, and coloration without using metal salt. However, metal salts are very important in terms of UV protection and also for light fastness. When different metal salts are used as mordanting agents, it was seen that although they are the same natural dye sources, very different color tones, color fastness properties, and UV transmittance ratios can be obtained. The most important reason for this difference is thought to be caused by the formation of a complex structure formed by the metal ions and the dye molecule. When these ions are combined with the chemical structure called quercetin found in buckthorn, it has been observed that the UV protection properties are changed. As a result, it could be said that the fabrics treated with buckthorn berries and leaves can act as a protective shield against the harmful sun’s UV rays, and in the meantime, different colors and fastnesses can come out by using different metal salts.
Highlights
Herbal sources can provide alternatives for gaining UV protective properties to textiles.
Textile materials can acquire UV protective properties by dyeing with berries and the leaves of buckthorn.
UV protective properties are obtained simultaneously with different colors and sufficient fastness properties by treating with berries and the leaves of buckthorn.
This method may be a viable way to reduce the use of synthetic UV protectors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Afaq, F., and H. Mukhtar. 2001. Effects of solar radiation on cutaneous detoxification pathways. Journal of Photochemistry and Photobiology B, Biology 63 (1–3):61–14. doi:10.1016/S1011-1344(01)00217-2.
- Ammayappan, L., and S. Jose. 2015. Functional aspects, eco testing and environmental impact of natural dyes. In Handbook of sustainable apparel production, ed. S. S. Muthu, 350. Boca Raton, Florıda: CRC Press.
- Andersen, O. M., and K. R. Markham. 2005. “Flavonoids: Chemistry, biochemistry, and applications”. Boca Raton: CRC press.
- Andreeva, T. I., M. S. Yusubov, E. N. Komarova, and E. I. Korotkova. 2004. Medicinal plants antioxidant activity of cranberry tree (viburnum Opulus L.) bark extract. Pharmaceutical Chemistry Journal 38 (10):548–50. doi:10.1007/s11094-005-0007-1.
- Benli, H. 2017. An investigation of dyeability of wool fabric with red cabbage (Brassica oleracea L. var.) extract. IndustriaTextila 68:108–15.
- Benli, H. 2022. Coloration of cotton and wool fabric by using bio-based red beetroot (Beta Vulgaris L.). Journal of Natural Fiber 19 (10):3753–69. doi:10.1080/15440478.2020.1848725.
- Benli, H., and M. İ. Bahtiyari. 2015. Use of ultrasound in biopreparation and natural dyeing of cotton fabric in a single bath. Cellulose 22 (1):867–77. doi:https://doi.org/10.1007/s10570-014-0494-x.
- Benli, H., and M. İ. Bahtiyari. 2016. Benli and Bahtiyari 2016” is cited in the text and has to be added to the reference list as follows. . Tekstil ve Mühendis 23:189–96. https://dergipark.org.tr/en/pub/teksmuh/issue/24717/261431
- Benli, H., and M. İ. Bahtiyari. 2021. Testing acorn and oak leaves for the UV protection of wool fabrics by dyeing. Journal of Natural Fibers. In press. 10.1080/15440478.2021.1958423
- Böhmer, H., N. Enez, R. Karadag, and C. Known. 2002. “Koekboya: Natural dyes and textiles”. Germany: Remhöb-Verlag. GANDERKESEE.
- Cardon, D. 2007. Natural dyes-sources, tradition, technology and science. London: Archetype Publications Ltd.
- Comlekcioglu, N., A. Aygan, M. Kutlu, and Y. Z. Kocabas. 2017. Antimicrobial Activities of Some Natural Dyes and Dyed Wool Yarn. Iran. J. Chem. Chem. Eng 36:4.
- Dağlıoğlu, Y., İ. Altınok, H. İ̇lhan, and M. Sökmen. 2016. Determination of the acute toxic effect of ZnO-TiO2 nanoparticles in brine shrimp (Artemia salina). Acta Biologica Turcica 29 (1):6–13.
- Davulcu, A., H. Benli, Y. Şen, and M. İ. Bahtiyari. 2014. Dyeing of cotton with thyme and pomegranate peel. Cellulose 21 (6):4671–80. doi:https://doi.org/10.1007/s10570-014-0427-8.
- Deveoğlu, O., and R. Karadağ. 2019. ”A Review on the Flavonoids – a Dye Source”. International Journal of Advances in Engineering and Pure Sciences 31:188–200. https://dergipark.org.tr/en/pub/jeps/issue/48917/476514
- Deveoglu, O., E. Torgan, and R. Karadag. 2012. The characterisation by liquid chromatography of lake pigments prepared from European buckthorn (Rhamnus cathartica L. Pigment & Resin Technology 41:331–38. https://doi.org/10.1108/03699421211274234
- Donmez, E. O., A. A. Akyol, R. Karadağ, E. Torgan, and K. İ̇ren. 2017. Ancient plant remains with special reference to buckthorn, frangula alnus mill., pyrenes from dascyleum, balıkesir, NW Turkey. Acta Societatis Botanicorum Poloniae 86 (1):3520. doi:10.5586/asbp.3520.
- Erdem, A., and M. İ. Bahtiyari. 2018. Ultrasonic-bioscouring and ozone-based bleaching of cotton slivers and coloration of them with natural dye sources. Journal of Cleaner Production journal of Cleaner Production 188:670–77. doi:10.1016/j.jclepro.2018.03.166.
- Gawish, S. M., H. M. Mashaly, H. M. Helmy, A. M. Ramadan, and R. Farouk. 2017. Effect of mordant on UV protection and antimicrobial activity of cotton, wool, silk and nylon fabrics dyed with some natural dyes. Journal of Nanomedicine & Nanotechnology 8:1–9. doi:10.4172/2157-7439.1000421.
- Ghazi, S., C. Couteau, and L. J. M. Coiffard. 2010. What level of protection can be obtained using sun protective clothing? Determining effectiveness using an in vitro method. International Journal of Pharmaceutics 397 (1–2):144–46. doi:https://doi.org/10.1016/j.ijpharm.2010.06.022.
- Giusti, M. M., and T. C. Wallace. 2009. Flavonoids as natural pigments. In Handbook of natural colorants, ed. T. Bechtold and R. Mussak, 257–58. UK: John Wiley and Sons Ltd.
- Grifoni, D., L. Bacci, S. D. Lonardo, P. Pinelli, A. Scardigli, F. Camilli, F. Sabatini, G. Zipoli, and A. Romanicet. 2014. UV protective properties of cotton and flax fabrics dyed with multifunctional plant extracts. Dyes and Pigments 105:89–96. doi:10.1016/j.dyepig.2014.01.027.
- Grifoni, D., L. Bacci, G. Zipoli, L. Albanese, and F. Sabatini. 2011. The role of natural dyes in the UV protection of fabrics made of vegetable fibres. Dyes and Pigments 91 (3):279–85. doi:10.1016/j.dyepig.2011.04.006.
- Grifoni, D., L. Bacci, G. Zipoli, G. Carreras, S. Baronti, and F. Sabatini. 2009. Laboratory and outdoor assessment of UV protection offered by flax and hemp fabrics dyed with natural dyes. Photochemistry and Photobiology 85 (1):313–20. doi:https://doi.org/10.1111/j.1751-1097.2008.00439.x.
- Haji, A., M. K. Mehrizi, and J. Sharifzadeh. 2016. Dyeing of wool with aqueous extract of cotton pods improved by plasma treatment and chitosan: optimization using response surface methodology. Fibers and Polymers 17 (9):1480–88. doi:10.1007/s12221-016-6457-0.
- Han, S., and Y. Yang. 2005. Antimicrobial activity of wool fabric treated with curcumin. Dyes and Pigments 64 (2):157–61. doi:https://doi.org/10.1016/j.dyepig.2004.05.008.
- Hou, X., X. Chen, Y. Cheng, H. Xu, L. Chen, and Y. Yang. 2013. Dyeing and UV-protection properties of water extracts from orange peel. Journal of Cleaner Production 52:410–19. doi:10.1016/j.jclepro.2013.03.004.
- ISO 105-C10. 2006. Textiles-tests for color fastness e part C10: Color fastness to washing with soap or soap and soda, test condition: test a (1), 105–C10. Geneva, Switzerland: International Organization for Standardization.
- ISO 105-B02. 1994. Textiles-tests for color fastness, part B02: Color fastness to artificial light. Brussels, Belgium: International Organization for Standardization.
- ISO 105-E04. 1994. Textiles–tests for color fastness, Part E04: Color fastness to perspiration. Brussels, Belgium: International Organization for Standardization.
- ISO 105-X12. 1993. Textiles-tests for color fastness, part X12: Color fastness to rubbing. Geneva, Switzerland: International Organization for Standardization.
- Jiang, H., R. Guo, R. Mia, H. Zhang, S. Lü, F. Yang, S. Mahmud, and H. Liu. 2022. Eco-friendly dyeing and finishing of organic cotton fabric using natural dye (gardenia yellow) reduced-stabilized nanosilver: Full factorial design. Cellulose 29 (4):2663–79. doi:10.1007/s10570-021-04401-9.
- Karabulut, K., and R. Atav. 2020. Dyeing of cotton fabric with natural dyes without mordant usage part I: Determining the most suitable dye plants for dyeing and UV protective functionalization. Fibers Polym 21 (8):1773–82. doi:10.1007/s12221-020-9365-2.
- Karadag, R., B. Y. Buyukakinci, and E. Torgan. 2020. Extraction and natural CottonDyeing of ValoniaOakandanatolianbuckthornbymicrowaveirradiation. Journal of Natural Fibers 19 (1):159–72. doi:10.1080/15440478.2020.1731907.
- Khan, M. Z., V. Baheti, M. Ashraf, T. Hussain, A. Ali, A. Javid, and A. Rehman. 2018. Development of UV protective, superhydrophobic and antibacterial textiles using ZnO and TiO2 Nanoparticles. Fibers and Polymers 19 (8):1647–54. doi:10.1007/s12221-018-7935-3.
- Khoddami, A., M. A. Wilkes, and T. H. Roberts. Techniques for analysis of plant phenolic compounds. Molecules 2013 (2):2328–75. doi:10.3390/molecules18022328.
- Kociç, A., M. Bizjak, D. Popoviç, G. B. Popariç, and S. B. Stankoviç. 2019. UV protection afforded by textile fabrics made of natural and regenerated cellulose fibres. Journal of Cleaner Production 228:1229–37. doi:10.1016/j.jclepro.2019.04.355.
- Londono, N., A. R. Donovan, H. Shi, M. Geisler, and Y. Liang. 2017. Impact of TiO2 and ZnO nanoparticles on an aquatic microbial community: Effect at environmentally relevant concentrations. Nanotoxicology 11 (9–10):1140–56. doi:10.1080/17435390.2017.1401141.
- Mavrić, Z., B. Tomšič, and B. Simončič. 2018. Recent advances in the ultraviolet protection finishing of textiles. Tekstilec 61 (3):201–20. doi:10.14502/Tekstilec2018.61.201-220.
- Ma, H., P. L. Williams, and S. A. Diamond. 2013. Ecotoxicity of manufactured ZnO nanoparticles – a review. Environmental Pollution 172:76–85. doi:10.1016/j.envpol.2012.08.011.
- McDonald, R. 1997. Recipe prediction for textiles. In Colour physics for industry, ed. R. McDonald, 121–208. Bradford, England: Society of Dyers and Colourists .
- Mia, R., M. M. Islam, T. Ahmed, M. A. Waqar, N. J. Khanam, S. Sultana, M. S. K. Bhuiyan, and M. N. Uddin. 2022. Natural dye extracted from TriadicaSebifera in aqueous medium for sustainable dyeing and functionalizing of viscose fabric. Cleaner Engineering and Technology 8:100471. doi:10.1016/j.clet.2022.100471.
- Mia, R., M. S. Sk, Z. B. S. Oli, T. Ahmed, S. Kabir, and M. A. Waqar. 2021. Functionalizing cotton fabrics through herbally synthesized nanosilver. Cleaner Engineering and Technology 4:100227. doi:10.1016/j.clet.2021.100227.
- Natural dyes market - Global Outlook and Forecast 2019-2024. 2019. Report. 174 Pages. Region: Global. Arizton. https://www.researchandmarkets.com/reports/4752259/natural-dyes-market-global-outlook-and-forecast:text=The%20global%20natural%20dyes%20market,11%25%20during%202018%2D2024.
- Pailthorpe, M. 1992. The theoretical basis for wool dyeing. In Wool Dyeing, ed. D. Lewis, 52–87. Bradford: Society of Dyers and Colourists.
- Poulin, J. 2018. A new methodology for the characterisation of natural dyes on museum objects using gas chromatography–mass spectrometry. Studies in Conservation 63 (1):36–61. doi:10.1080/00393630.2016.1271097.
- Rachel, D. A., and B. M. Z. Hussain. 2019. Healthcare textile dyed natural socks. International Journal of Research Trends Innovation 4 (1): 79–84.
- Rather, L. J., Q. Zhou, A. Ali, Q. Mohd, R. Haque, and Q. Li. 2021a. Valorization of agro-industrial waste from peanuts for sustainable natural dye production: Focus on adsorption mechanisms, UV-protection, and antimicrobial properties of dyed wool fabric. ACS Food Science & Technology 1 (3):427–42. doi:10.1021/acsfoodscitech.1c00005.
- Rather, L. J., Q. Zhou, and Q. Li. 2021b. Re-use of Cinnamomum camphora natural dye generated wastewater for sustainable UV protective and antioxidant finishing of wool fabric: Effect of Fe(II) sulfate. Sustainable Chemistry and Pharmacy 21:100422. doi:10.1016/j.scp.2021.100422.
- Rocchetti, G., M. B. Miras-Moreno, G. Zengin, I. Senkardes, N. B. Sadeer, M. F. Mahomoodally, and L. Lucini. 2019. “UHPLC-QTOF-MS phytochemical profiling and in vitro biological properties of Rhamnus petiolaris (Rhamnaceae)”. Industrial Crops and Products 142: 111856.https://doi.org/10.1016/j.indcrop.2019.111856
- Romani, A., C. Zuccaccia, and C. Clementi. 2006. An NMR and UV–visible spectroscopic study of the principal colored component of stil de grain lake. Dyes and Pigments 71 (3):218–23. doi:10.1016/j.dyepig.2005.07.005.
- Samanta, A. K., and A. Konar. 2011. Dyeing of textiles with natural dyes. In Natural Dyes, ed. A. E. P. Kumbasar, 29–56. Croatia: InTech.
- Sankaran, A., A. Kamboj, L. Samant, and S. Jose. 2021. Synthetic and natural UV protective agents for textile finishing. In Innovative and emerging technologies for textile dyeing and finishing, ed. L. J. Rather, A. Haji, and M. Shabbir, © 2021 Scrivener Publishing LLC 301–24. doi:10.1002/9781119710288.ch11.
- Schuicrer, M. 1997. Practical experience with solar tex products in finishing of sun protection fabrics. Melliand International 3:168.
- Singh, R., A. Jain, S. Panwar, D. Gupta, and S. K. Khare. 2005. Antimicrobial activity of some natural dyes. Dyes and Pigments 66 (2):99–102. doi:https://doi.org/10.1016/j.dyepig.2004.09.005.
- Sivakumar, A., R. Murugan, K. Sundaresan, and S. Periyasamy. 2013. UV protection and self-cleaning finish for cotton fabric using metal oxide nanoparticles. Indian Journal of Fibre& Textile Research 38:285–92.
- Srinivasan, M., and B. M. Gatewood. 2000. Relationship of dye characteristics to UV protection provided by cotton fabric. Textile Chemist and Colorist and American Dyestuff Reporter 32 (4):36–43.
- Sucheta, G. A., K. A. Asha, G. V. Tushar, D. R. Nirmala, and S. P. Jyoti. 2011. Standardization of emodin-an bioactive molecule, using spectral methods. International Journal of Drug Development and Research 3 (3):259–65.
- Tanker, M., and M. Ertan. 1971. Rhamnus Petiolaris Boiss, Bitkisi ve Gövde Kabuklarının Morfolojik ve Anatomik Olarak incelenmesi. J. Fac. Pharm 1. 36.
- Ţebrencu, C. E., R. M. Creţu, G. R. Mitroi, E. Iacob, and E. Ionescu. 2015. Phytochemical evaluation and HPTLC investigation of bark and extracts of Rhamnus Frangula Linn. Phytochemistry Reviews : Proceedings of the Phytochemical Society of Europe 14 (4):613–21. doi:10.1007/s11101-015-9410-8.
- EN 13758-1.2001. Textiles. Solar UV ProtectiveProperties. Part-1: Method of test for apparel fabrics. Brussels: European Committee For Standardization.
- Thakker, A. M., and D. Sun. 2021. Sustainable plant-based bioactive materials for functional printed textiles. The Journal of the Textile Institute 112 (8):1324–58. doi:10.1080/00405000.2020.1810474.
- Uddin, M. G. 2014. Effects of different mordants on silk fabric dyed with onion outer skin extracts. Hindawi Publishing Corporation Journal of Textiles 405626:8. doi:http://dx.doi.org/10.1155/2014/405626.
- Yılmaz, F. 2020. Application of Glycyrrhiza glabra L. root as a natural antibacterial agent in finishing of textile. Industrial Crops and Products 157:112899. doi:10.1016/j.indcrop.2020.112899.
- Yılmaz, F., Ö. Aydınlıoğlu, H. Benli, G. Kahraman, and M. İ. Bahtiyari. 2020b. Treatment of originally coloured wools with garlic stem extracts and zinc chloride to ensure anti‐bacterial properties with limited colour changes. Coloration Technology 136:147–52. doi:10.1111/cote.12444.
- Yılmaz, F., Ö. F. Koçak, F. B. Özgeriş, H. Ş. Selamoğlu, C. Vural, H. Benli, and M. İ. Bahtiyari. 2020a. use of Viburnum Opulus L. (Caprifoliaceae) in dyeing and antibacterial finishing of cotton. Journal of Natural Fibers 17:945–53. doi:10.1080/15440478.2019.1691118.
- Zarkogianni, M., E. Mikropoulou, E. Varella, and E. Tsatsaroni. 2011. Colour and fastness of natural dyes: Revival of traditional dyeing techniques. Coloration Technology 127 (1):18–27. doi:10.1111/j.1478-4408.2010.00273.x.