ABSTRACT
Creatinine O-phosphate (COP) containing phosphorus and nitrogen elements was used as eco-friendly flame-retardant for lyocell/cotton blended fabric (L/C fabric). The flame retardant fabrics are prepared by a simple and effective dip-dry-cure method. Fourier infrared spectroscopy (FTIR), scanning electron microscope (SEM), and X-ray photoelectron spectroscopy (XPS) analyses certified that COP has been successfully grafted onto the surface of the L/C fabric and distributed evenly. Compared with the control fabric, the thermogravimetric analysis (TG) results showed that the char residue rate of the flame-retardant fabric at 800°C increased by 229% (air atmosphere), indicating that it has excellent char-forming ability. Besides, cone calorimetry results showed that the PHRR and THR values of the treated L/C fabric were reduced by 88.83% and 76.65%, respectively. The volatile pyrolysis products of the treated L/C fabric were determined by thermogravimetric analysis coupled with Fourier transform infrared analysis (TG-IR). In general, these results indicated that the L/C fabric treated with COP had high flame retardancy, excellent washing resistance and thermal stability.
摘要
采用含磷和氮元素的磷酸肌酐(COP)作为环保阻燃剂,利用一种简单有效的浸干固化方法制备的莱赛尔/棉混纺织物(L/C织物。傅里叶红外光谱(FTIR)、扫描电子显微镜(SEM)和X射线光电子能谱(XPS)分析证明COP已成功接枝到L/C织物表面并均匀分布。与对照织物相比,热重分析(TG)结果表明,阻燃织物在800°C(空气气氛)下的焦炭残留率增加了229%,表明其具有优异的焦炭形成能力。此外,锥形量热分析结果表明,处理后的L/C织物的PHRR和THR值分别降低了88.83%和76.65%。通过热重分析和傅里叶变换红外分析(TG-IR)测定了处理后的L/C织物的挥发性热解产物。总之,这些结果表明,经COP处理的L/C织物具有高阻燃性、优异的耐水洗性和热稳定性。
Introduction
Lyocell/cotton blended fabric (L/C fabric) combines the advantages of lyocell and cotton fabric, which is popular for its smoothness, good draping properties, less distortion and wrinkling, and less linting (Şardağ, Toprak, and Aniş Citation2019), leading to a gradual expansion of their textile applications (Ke et al. Citation2020). However, both lyocell and cotton are composed mainly of cellulose which is a polysaccharide carbohydrate that burns rapidly when exposed to flame, for example, the LOI value of cotton fabric is only about 18%. Textiles prepared from cellulose are prone to fire spread after burning, which seriously threatens people’s lives safety and limits their development in interior home, vehicle interior, fire fighting and other fields. In addition, many cellulose fabrics are often treated with various organic substances during the weaving, dyeing and printing processes, which increases the amount of toxic smoke released from their combustion. Therefore, it is essential to implement a suitable flame retardant finishing for L/C fabric (Lou et al. Citation2008).
Halogen flame retardant is one of the most widely used traditional flame retardants, but they are very harmful to the environment. Obviously, the development of eco-friendly, nontoxic, efficient and biodegradable flame retardant fabrics is a top priority (Hansen et al. Citation2020). Phosphorus-nitrogen intumescent flame retardant is one of the research and development hotspots in the field of flame retardancy in recent years. It has the advantages of halogen-free, low smoke, low toxicity, anti-melt dripping and non-corrosive gas, which is in line with the future development direction of flame retardants (Chen and Wang Citation2009). However, there are still many flame retardants that produce formaldehyde during the preparation or combustion process, which also causes great harm to the human body and the environment (Wu and Yang Citation2006). Tian, Wan and others use organic compounds containing nitrogen, silicon and formaldehyde, phosphorous acid to synthesize flame retardants, and the treated fabrics still have flame retardancy after 30-50 washes (Tian et al. Citation2019; Wan et al. Citation2019). Here, there is an urgent need to explore a textile with excellent flame retardant properties. It is important is that little or no toxic substances (such as formaldehyde) are released in the production and use process.
Red phosphorus has a high phosphorus content, and a good flame retardant effect can be achieved by using a small amount. But it is flammable in the air, easy to absorb moisture, and releases harmful gases when burned, so its application is subject to many restrictions. Phosphoric acid is a medium-strong acid, and the proportion of phosphorus is as high as 31%. Thus, the use of phosphoric acid alone as a flame retardant will cause a large loss of mechanical strength of fabrics (Velencoso et al. Citation2018). Wan et al. used guanidinoacetic acid to synthesize a phosphorus-containing high-efficiency flame retardant. The flame retardant treated cotton fabric had an LOI value of 29.5% after 50 washing cycles. However, guanidinoacetic acid does not contain phosphorus, and needs to use formaldehyde as a “bridge” to increase the phosphorus content of flame retardants (Jia et al. Citation2017). Creatinol-O-phosphate (COP), 1-(2-hy droxy-ethyl)-1-methylguanidine dihydrogen phosphate, is a compound containing both phosphate and guanidine and is widely used in food additives and nutritional supplements. It can prolong muscle endurance, increase muscle strength and explosive power, improve athletic performance (Melloni et al. Citation1979), prevent and treat cardiac fatigue and myocardial infarction, and it is well tolerated by the body. (Olsen et al. Citation1993). According to the study, COP has good thermal stability and char formation (Jiang et al. Citation2019), but so far it has hardly been used in the field of flame retardants.
In this paper, in order to improve the flame retardancy of L/C fabric, COP containing phosphoric acid and guanidine groups was used as flame retardant to impart flame retardancy and wash resistance to L/C fabric through pad-dry-cure process. The surface chemical structure, chemical element composition and overall morphology characteristics of treated L/C fabric were studied in detail by FTIR, XPS, SEM. The flame retardancy and combustion properties of treated L/C fabric were characterized by TG, cone calorimetry, vertical flammability tests and other analytical methods. In addition, the flame retardant mechanism of COP-based flame retardant fabric was explored by TG-IR.
Experimental
Materials
Black plain lyocell/cotton fabric (L/C fabric, 50/50, 232.25 g/m2) was provided by Tianjin Polytechnic University (Tianjin, China). Creatinine O-phosphate (COP), urea, and dicyandiamide were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). Phosphoric acid was purchased from Tianjin Fengchuan Chemical Reagent Co., Ltd. (Tianjin, China). All reagents are used as is, no further purification is required. The water used for the flame retardant liquid was distilled water, which was home-made in the laboratory, and the flushing water is tap water with pH of 7.3.
Preparation of flame retardant lyocell/cotton fabric
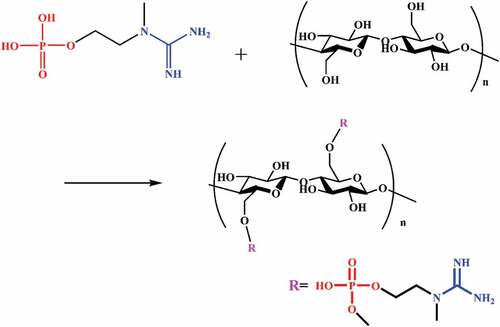
The L/C fabrics were placed in a 1 wt% NaOH solution at 70°C for 30 min, which was used to remove oil and impurities for the convenience of subsequent fabrics modification. The formula of the flame retardant finishing liquid is shown in and the bath ratio is 1:20. All L/C fabrics to be treated were soaked in the flame retardant solution at 70°C for 1 h. Then, all fabrics were processed by laboratory padder to comply a wet pick-up rate of about 100%. After soaking and squeezing, all fabrics were cured at 180°C for 8 min. The soaking, squeezing and curing process of all fabrics was repeated twice in succession. Finally, all flame retardant L/C fabrics (FR-L/C fabrics) were washed with tap water to remove some of the unbound compounds, and dried at 70°C for 1 h. The reaction equation is shown in .
Table 1. The components of flame retardant finishing liquid.
Characterization
The FT-IR assessment of COP, control fabric and treated L/C fabric for chemical structure were conducted by using Nicolet iS50 FT-IR spectrometer (Thermo Fisher Scientific Inc., China) in the range of 4000–400 cm−1 with a resolution of 2.0 cm−1.
X-ray Photoelectron Spectroscopy (K-alpha, Thermofisher) was used to analyze the components of control fabric and treated L/C fabric.
The ZEISS Gemini scanning electron microscope (SEM) was used to observe the surface morphology of the control, flame-retardant-treated, and burned L/C fabric at a beam voltage of 5kV.
TG analysis of control and FR-L/C fabrics was studied by STA449F3 thermogravimetric analyzer. In air atmosphere, the temperature range is 30 ºC-800 ºC, and the heating rate is 10 ºC/min.
The cone calorimetry was used to study the burning performance of a square fabric sample (100 mm × 100 mm) in a horizontal configuration. According to the requirements of GB/T16172–2007/ISO 5660-1: 2002, the heater power is 35 kW/m2.
The vertical flammability was examined according to the ASTM D6413–99 standard test method on a YG8 15B vertica fabric FR tester (Nantong Sansi electromechanical Science & Technology Co., Ltd., China).
The limiting oxygen index (LOI) values of the fabric samples (150 mm × 58 mm) were measured according to GB/T 5454–1997 standard using ASTMD2863 (oxygen index method) fabric flame retardancy tester.
TG-IR of samples was performed using a combination of the Netzsch STA449F3 thermal analyzer and the STA449F3 spectrometer. The sample mass was 15 mg, the gas flow rate was 30 mL/min, and the temperature range was 30–800 ºC (N2).
Results and discussion
Characteristics of control fabric and FR-L/C fabric
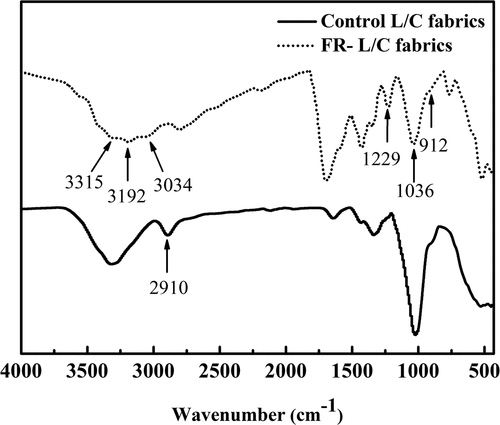
displays the FTIR of COP, control fabric and FR-L/C fabric. The control fabric showed the -OH stretch band at 3000–3500 cm−1. By contrast, the -OH absorption peak intensity of the treated fabric decreased, and the chemically treated fabric showed the 1229 and 1036 cm−1 bands of tensile vibration corresponding to the P=O and P-OH groups (Liu et al. Citation2018a). This corresponds to the spectral line of COP. Simultaneously, More importantly, the absorption shock peak of P-O-C is clearly observed at 912 cm−1 (Ren et al. Citation2020). The treated fabric had an obvious peak at 3192 cm−1, which prove that the urea was connected to the fabric through hydrogen bonding. This may indicate that there was a bond between the fabric and the COP, and after treatment, COP had been successfully grafted to its surface.
Surface chemical composition
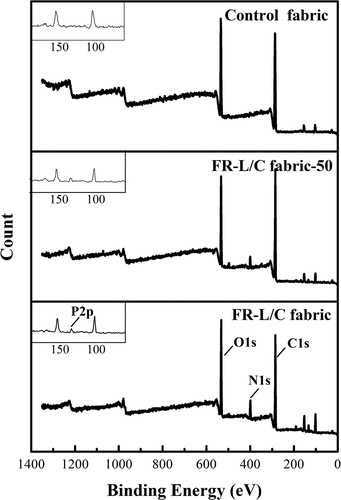
XPS analysis can clearly observe the content of each effective flame retardant element on the textile surface. The spectra and surface elemental compositions (at%) of control fabric, FR-L/C fabric, FR-L/C fabric after 50 washed cycles were summarized in and . For all samples, there were two strong peaks at 286 and 533 eV, corresponding to C1s and O1s, respectively. For modified samples, there were two new characteristic peaks, N1s (15.57%) and P2p (1.40%), appeared at 400 and 134 eV.
Table 2. Chemical compositions of control and FR-L/C fabrics.
After 50 washes of the fabric, the content of N and P on the surface of the fabric decreased, and the ratio of the content of N to P decreased from 11.1 to 4.9. This trend was due to some of the ammonium roots on the flame retardant were in a free state and were easily dislodged during the washing process, while COP was firmly attached to the fabric through the P-O-C chemical bond and was not easy to fall off during the washing process. It indicated that the formation of chemical bonds was beneficial to improve the washing resistance of flame retardant fabrics.
Surface morphology
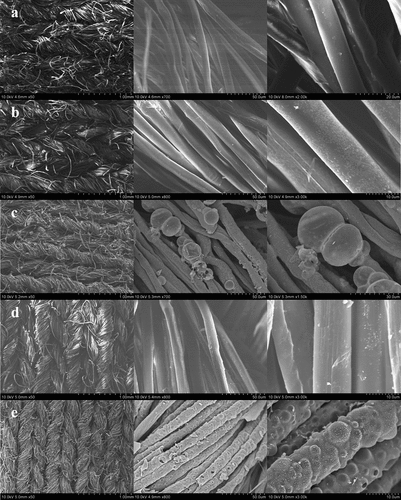
lists SEM images of the blank fabric and the flame-retardant fabric samples before and after burning at different magnifications. can clearly show the smooth surface of the control sample, and a few defects were caused by NaOH during the pretreatment. As shown in , the fiber surface of the modified fabric was rough, and attachments can be observed, but there were no obvious defects and defects on the fiber indicating that the modification did not affect the soft feel of the original fabric. The surface morphology of the burnt coke residue of FR fabric and fabric after 50 washing cycles are shown in . After burning, the fiber did not shrink or break significantly, and there were many bubbles and bulges on the surface, but the overall shape and structure of the fiber were retained. This was because phosphoric acid, pyrophosphoric acid, etc. formed by the phosphorus-containing substances in the COP under heating conditions. These substances promoted the carbonization of cellulose and formed a hard and dense protective char layer on the surface of fibers. During the combustion process, nitrogen-containing gas source generated a large amount of incombustible ammonia gas (Liu et al. Citation2017). These gases keep hitting the outer char layer, causing the char layer to form bubbles of different sizes and shapes. The noncombustible gas and carbon layers impeded the transfer of heat and combustible gases between the fabric and the heat source, interrupted the energy exchange and effectively protected the substrate(Liu et al. Citation2020a). In summary, the FR-L/C fabric exhibit excellent flame retardancy.
Thermogravimetric analysis
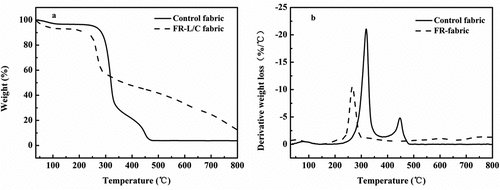
TG analysis used to study the thermal degradation of COP, contro fabricl and FR-L/C fabric. The TG and DTG curves and the key data are shown in and .
Table 3. Data of TG and DTG curves for untreated and treated fabric.
In the air environment, the mass of the control fabric started to decrease rapidly at 283°C, with a mass loss of about 54 wt% at the end of the first degradation phase (335°C) and only 3.80 wt% remaining at 800°C. The T5% of the treated fabric was only 71°C, much lower than the control fabric. The starting temperature of the first degradation stage was 212°C, Tmax is 266°C, and weight loss was 31.5%. The mass gradually decreased to 27.5% when the temperature increased from 212°C to 621°C. During this period, some aliphatic chars were aromatized and CO and CO2 were produced. Finally, only 12.51 wt% residue remained at 800°C.
In the DTG curve, the important finding was that the temperature at which the FR-L/C fabric reached maximum weight loss was significantly lower than that of the control fabric. These phenomena further indicate that with the grafting of flame retardants, the thermal decomposition process of the L/C fabric is changed and the char-forming property is improved(Liu et al. Citation2018a).
Flammability and washing durability
displays the vertical flammability test results of the control fabric (a), treated fabric without COP (b), treated fabric without phosphoric acid (c), FR-L/C fabric (d), and fabric that have passed 30 and 50 washing cycles (e, f) in sequence. As shown in , the control sample burned rapidly and completely after exposure. Although the charcoal residue of the control sample without COP addition was intact after burning, it was thin enough to transmit light. The control sample with COP added but no phosphoric acid had an after-flame time of 2 s, and the length of charcoal residue reached 130 mm. The FR-L/C fabric was not ignited, and the part in contact with the flame can form a complete char layer. Although the coke residue of the flame retardant sample was slightly deformed after burning, it still retaining the original shape of the fabric. The flame-retardant fabric was not ignited, and the char residue length was only 45 mm. The LOI value of FR fabric can reach 54%. The fabric was not ignited after 50 washes, although the charcoal residue increased to 90 mm and its LOI value decreased by 12.7%. This is because after the flame retardant treatment, COP reacts chemically with the -OH on the cellulose to form a P-O-C bond, which is not easy to fall off during washing (Su et al. Citation2021). The results of combustion test and LOI test show that FR-L/C fabric has a certain degree of washing resistance (Liu et al. Citation2018b).
Combustion properties
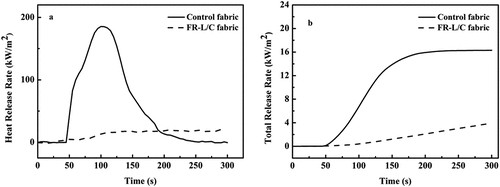
The cone calorimeter can measure the burning performance of FR-L/C fabric, and the results are shown in and . During the test, the flame retardant fabric did not burn, and the time to ignition of the control fabric was 43 s. Simultaneously, it was obvious that the heat release rate (HRR) of flame-retardant fabric was almost a horizontal line, and the slope of total heat release (THR) was also very small, and the THR value rose slowly. The data showed that the peak heat release rate (PHRR) and THR of flame retardant fabric were 20.7 kW/m2 and 3.9 MJ/m2, respectively, which were 88.8% and 76.6% lower than those of untreated fabric. In our previous researchers, we used the layer-by-layer self-assembly method to finish egg proteins and phytic acid onto the surface of cotton fabric, and the optimized coated sample was equipped with 22.6% and 66.8% reduction in PHRR and THR (Liu et al. Citation2020b). Also, we prepared flame-retardant lyocell fabric using a homemade tris(2-aminoethyl)aminophosphate as a raw material by the pad-dry-cure method, and the PHRR and THR of the optimized finished fabric were reduced by 74.8% and 70.6% (Zhang et al. Citation2020). Therefore, compared these literature reports, COP showed a greater advantage in suppressing the heat release during the combustion process of L/C fabric. In addition, the fire hazard degree of the material can be estimated by the fire growth rate (FGR), which is equal to the ratio of PHRR and time to peak heat release rate. The decrease of FGR value of treated L/C fabric represented that its fire safety had been improved. Before and after the flame-retardant treatment, the CO2/CO ratio of the fabric decreased from 23 to 2.6. The main reason is that the release of noncombustible gases from COP reduced the amount of free radicals (H· and OH·) in the flame and prevents their complete combustion to CO2 (Sun et al. Citation2021).
Table 4. Combustion data obtained by cone calorimetry for control fabric and FR-L/C- fabric.
TG-FTIR analysis
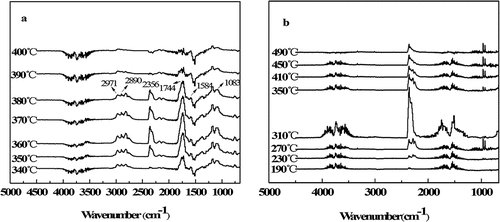
The TG-IR technology can directly obtain the structural information of the volatile products, so the qualitative analysis of the pyrolysis products of the control fabric and treated fabric can be carried out by this technology (Zhang et al. Citation2020), and the results are shown in . For the blank samples, the absorption shock peaks of the hydroxyl group of cellulose and the small molecule gas H2O with increasing temperature appeared at 1200–1800 cm−1 and 3400–4000 cm−1, and the absorption oscillation peak of the small molecule gas CO2 appeared at 2356 cm−1 . The absorption bands of other decomposition products can also be observed in the spectrum, such as the vibration absorption peaks of -CH bonds derived from hydrocarbon compounds at 2971 and 2890 cm−1. As well as the C-H at 1744 cm−1 and the C-O-C at 1083 cm−1, these small molecule volatiles were considered to be flammable levoglucan and furan derivatives (Ren et al. Citation2020). In summary, the main degradation products include flammable substances (such as H2O and CO2) and flammable substances (such as hydrocarbons, carbonyl compounds and ethers).
For the treated sample (), the presence of flame retardants changed the degradation mode of the fabric, and the vibration absorption peaks of the -CH bond at 2971 and 2890 cm−1 disappeared. The peak intensities of C-H (1744 cm−1) and C-O-C (1083 cm−1) were significantly lower than the original fabric. The absorption peaks of CO2 and H2O appeared at a lower temperature (230°C), and the strength was higher than that of the original fabric. The absorption peaks of the nonflammable small molecule gas NH3 produced by the N-containing structure appear at 964 and 935 cm−1. These results show that COP is beneficial to the dehydration and carbonization of fabric, while inhibiting the generation of combustible volatiles while promoting the release of noncombustible volatiles.
Flame retardant mechanism analysis
From the above analysis, it can be seen that COP is a compound containing phosphoric acid and guanidine groups. The flame retardant modification of L/C fabric is mainly based on the intumescent flame retardant mode. Flame retardants work in both condensation and gas phase. In the condensation stage, the phosphoric acid group in the COP is decomposed into small molecular weight components such as P·, PO·, PO2· and HPO2· in the flame. These free radicals can interact with the hydrogen and hydroxyl radicals in the gas flame zone. The role of free radicals in the flame into stable molecules. Simultaneously, the polyphosphoric acid released by the flame retardant on the fabric surface promotes the dehydration of the fabric into char to form a protective layer. In this way, the transfer of oxygen and heat between the substrate and the outside world is blocked. In the gas phase, nitrogen-containing COP and urea will release incombustible gases during the decomposition process, such as NH3, which continuously hit the char layer to form expansion bulges. These gases effectively dilute the concentration of combustible gases and slow down the rate of combustion. Finally, when burned, flame retardant fabric release more CO2, H2O, aliphatic hydrocarbons and other gases, and fewer aldehydes and ketones, compared to untreated fabric.
Conclusion
A novel phosphorus-nitrogen flame retardant lyocell/cotton blended fabric was successfully chemically treated through a dip-dry-cure method with COP, and good flame retardancy and washing resistance were obtained. The chemical structure of the flame retardant fabric was characterized by FTIR, and the morphological structure and elemental composition of the control fabric and FR-L/C fabric were analyzed by SEM and XPS, respectively.The TG results showed that the flame retardant fabric had excellent char formation ability, and the residual char of the flame retardant fabric at 800°C was significantly increased (12.51 wt%, under air) compared to the control fabric. Cone calorimetry tests showed that the PHRR and THR of the modified sample were decreased by 88.83% and 76.65%, respectively. In summary, the flame retardancy of FR-L/C fabric grafted with COP was significantly improved. Thus, the COP-based flame retardant system provides an innovative and simple strategy for the preparation of flame retardant cellulose fibers, and also broadens the scope of application of L/C fabric in various special protective fields.
Highlights
The lyocell/cotton blended fabric was surface treated with nontoxic and eco-friendly creatinine O-phosphate (COP).
The flame retardant imparted excellent fire safety to the lyocell/cotton blended fabric.
The flame retardant acted in both the gas phase and the condensed phase to prevent the flame from spreading further.
Acknowledgments
The authors are very thankful for the financial support provided by the National Key Research and Development Program of China (No. 2017YFB0309004) and Quanzhou Science and Technology Bureau (No. 2019C104).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chen, L., and Y. Z. Wang. 2009. A review on flame retardant technology in China. Part I: Development of flame retardants. Polymers for Advanced Technologies 21:1–12. doi:10.1002/pat.1550.
- Hansen, K. M., P. Fauser, K. Vorkamp, and J. H. Christensen. 2020. Global emissions of dechlorane plus. The Science of the Total Environment 742:140677. doi:10.1016/j.scitotenv.2020.140677.
- Jia, Y., Y. Lu, G. Zhang, Y. Liang, and F. Zhang. 2017. Facile synthesis of an eco-friendly nitrogen–phosphorus ammonium salt to enhance the durability and flame retardancy of cotton. Journal of Materials Chemistry A 5 (20):9970–81. doi:10.1039/c7ta01106g.
- Jiang, L. K., P. F. Zhang, L. Y. Dong, and K. Liu. 2019. Thermal degradation kinetic study of creatinol phosphate and model selection. Journal of Thermal Analysis and Calorimetry 138 (5):3031–37. doi:10.1007/s10973-019-08337-y.
- Ke, G. Z., Z. H. Xiao, X. Y. Jin, L. X. Yu, J. Q. Li, and H. X. Zhang. 2020. Wrinkle recovery angle enhancement and tensile strength loss of 1,2,3,4-butanetetracarboxylic acid finished lyocell fabrics. Textile Research Journal 90 (17–18):2097–108. doi:10.1177/0040517520912035.
- Liu, Y. S., Y. B. Guo, Y. L. Ren, Y. Wang, X. Guo, and X. H. Liu. 2020a. Phosphorylation of sodium copper chlorophyll enables color-fasten and durable flame retardant wool fibers. Polymer Degradation and Stability 179:109286. doi:10.1016/j.polymdegradstab.2020.109286.
- Liu, Y., Q. Q. Wang, Z. M. Jiang, C. J. Zhang, Z. F. Li, H. Q. Chen, and P. Zhu. 2018b. Effect of chitosan on the fire retardancy and thermal degradation properties of coated cotton fabrics with sodium phytate and APTES by LBL assembly. Journal of Analytical and Applied Pyrolysis 135:289–98. doi:10.1016/j.jaap.2018.08.024.
- Liu, X. H., Q. Y. Zhang, B. W. Cheng, Y. L. Ren, Y. H. Zhang, and C. Ding. 2017. Durable flame retardant cellulosic fibers modified with novel, facile and efficient phytic acid-based finishing agent. Cellulose 25 (1):799–811. doi:10.1007/s10570-017-1550-0.
- Liu, X. H., Y. G. Zhang, B. W. Cheng, Y. L. Ren, Q. Y. Zhang, C. Ding, and B. Peng. 2018a. Preparation of durable and flame retardant lyocell fibers by a one-pot chemical treatment. Cellulose 25 (11):6745–58. doi:10.1007/s10570-018-2005-y.
- Liu, X. H., Y. G. Zhang, B. Peng, Y. L. Ren, B. W. Cheng, C. Ding, X. W. Su, J. He, and S. G. Lin. 2020b. Flame retardant cellulosic fabrics via layer-by-layer self-assembly double coating with egg white protein and phytic acid. Journal of Cleaner Production 243:118641. doi:10.1016/j.jclepro.2019.118641.
- Lou, C. W., C. W. Lin, Y. S. Chen, C. H. Yao, Z. S. Lin, C. Y. Chao, and J. H. Lin. 2008. Properties evaluation of tencel/cotton nonwoven fabric coated with chitosan for wound dressing. Textile Research Journal 78 (3):248–53. doi:10.1177/0040517507089747.
- Melloni, G. F., G. M. Minoja, G. F. Lureti, L. Merlo, F. Pamparana, and B. Brusoni. 1979. Acute clinical tolerance of creatinol O-phosphate. Arznmittelforschung 29 (9a):1447–49.
- Olsen, J. I., P. Rossini, M. P. Schweizer, M. Bernardi, V. Moretti, L. RE, and L. Rossini. 1993. A 31P NMR spectroscopy study of Xenopus laevis heart perfused in vitro with creatinol-O-phosphate, phosphocreatine, adenosine triphosphate, fructose diphosphate and ouabain. Pharmacological Research 28 (2):135. doi:10.1006/phrs.1993.1116.
- Ren, Y. L., Y. S. Liu, Y. Z. Wang, X. Guo, and X. H. Liu. 2020. Preparation of durable and flame retardant lyocell fabrics by using a biomass-based modifier derived from vitamin C. Cellulose 27 (11):6677–89. doi:10.1007/s10570-020-03218-2.
- Şardağ, S., T. Toprak, and P. Aniş. 2019. The effects of the combined process of enzymatic bleach clean-up, enzymatic defibrillation and dyeing on the comfort and physical properties of tencel/cotton knitted fabrics. Textile Research Journal 90 (9–10):1118–29. doi:10.1177/0040517519886555.
- Su, X. W., C. Z. Cheng, Y. B. Zheng, X. H. Liu, Y. L. Ren, J. He, S. G. Lin, and B. W. Cheng. 2021. A novel biomass vitamin B6-based flame retardant for lyocell fibers. Cellulose 28 (5):3201–14. doi:10.1007/s10570-021-03681-5.
- Sun, L., H. X. Wang, W. N. Li, J. J. Zhang, Z. Zhang, Z. Lu, P. Zhu, and C. H. Dong. 2021. Preparation, characterization and testing of flame retardant cotton cellulose material: Flame retardancy, thermal stability and flame-retardant mechanism. Cellulose 28 (6):3789–805. doi:10.1007/s10570-020-03632-6.
- Tian, P. X., Y. Lu, D. F. Wang, G. X. Zhang, and F. X. Zhang. 2019. Solvent-free synthesis of silicon–nitrogen–phosphorus flame retardant for cotton fabrics. Cellulose 26 (11):6995–7007. doi:10.1007/s10570-019-02554-2.
- Velencoso, M. M., A. Battig, J. C. Markwart, B. Schartel, and F. R. Wurm. 2018. Molecular firefighting—how modern phosphorus chemistry can help solve the challenge of flame retardancy. Angewandte Chemie International Edition 57 (33):10450–67. doi:10.1002/anie.201711735.
- Wan, C. Y., P. X. Tian, M. S. Liu, G. X. Zhang, and F. X. Zhang. 2019. Synthesis of a phosphorus˗nitrogen flame retardant endowing cotton with high whiteness and washability. Industrial Crops and Products 141:111738. doi:10.1016/j.indcrop.2019.111738.
- Wu, W. D., and C. Q. Yang. 2006. Comparison of different reactive organophosphorus flame retardant agents for cotton: Part I. The bonding of the flame retardant agents to cotton. Polymer Degradation and Stability 91 (11):2541–48. doi:10.1016/j.polymdegradstab.2006.05.010.
- Zhang, Q. Y., X. H. Liu, Y. L. Ren, Y. H. Zhang, and B. W. Cheng. 2020. Fabrication of a high phosphorus–nitrogen content modifier with star structure for effectively enhancing flame retardancy of lyocell fibers. Cellulose 27 (14):8369–83. doi:10.1007/s10570-020-03333-0.