?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present study focuses on the possibility of using Ageratina Adenophora (AA) fiber as reinforcement in structural polymer composite materials because of its eco-friendly nature as well as lightweight. Due to high specific strength and modulus of fiber, reinforced polymer composites tend to be a viable option replace many metallic structures. By nature, synthetic fibers become a risk for health as it leads to cancer. Suitable applications replace conventional and synthetic materials, in cases where energy conservation and very little weight is required. Ageratina Adenophora stem fiber undergoes comprehensive characterization analysis such as Thermal Gravimetric Analysis, Fourier-transform infrared spectroscopy, X-ray diffraction, Scanning Electron Microscopy, Physico-chemical analysis. The fibers were extracted from Ageratina Adenophora plant stem. Ageratina Adenophora Stem Fiber (AASF) contains high cellulose content of 65.7% and very little wax. Good thermal stability of the fiber up to 244°C was obtained from thermogravimetric analysis, which is indicated within its polymerization process temperature. The results of this investigation encourage its applications to be used in industries for manufacturing fiber reinforced composites.
摘要
本研究的重点是使用腺角蛋白(AA)纤维作为结构聚合物复合材料的增强材料的可能性,因为其具有环保性和轻质性. 由于纤维增强聚合物的高比强度和模量,复合材料往往是替代许多金属结构的可行选择. 从本质上讲,合成纤维会导致癌症,从而对健康构成威胁. 在需要节能和非常轻的情况下,合适的应用替代了传统和合成材料. Ageratina Adenophora茎纤维经过全面的表征分析,如热重量分析、傅里叶变换红外光谱、X射线衍射、扫描电子显微镜、物理化学分析. 这些纤维是从腺角蛋白植物茎中提取的. Ageratina Adenophora茎纤维(AASF)含有65.7%的高纤维素含量和极少的蜡. 热重分析表明,纤维在244°C以下具有良好的热稳定性,这在其聚合过程温度范围内. 这项研究的结果鼓励其应用于制造纤维增强复合材料的工业.
Introduction
Due to increased consumption of petroleum products in recent times, increased environmental law showed the way to research on renewable, recyclable, and biodegradable composite materials (Gopinath et al. Citation2021; Manivel et al. Citation2022; Sanjay et al. Citation2018; Thirumurugan et al. Citation2021). There are several advantages of employing composite materials which are low cost, suitable specific strength, low density, high thermal and acoustic insulating properties, compared with conventional reinforced materials (Madhu et al. Citation2018; Mahalingam Citation2022; Muruganrama et al. Citation2022; Mylsamy et al. Citation2020; Nagappan et al. Citation2021; Ramesh et al. Citation2022; Ramkumar et al. Citation2022; Vinod et al. Citation2020). The origin, maturity, and technique applied to extract fibers highly influence the mechanical properties of that fiber (Sanjay et al. Citation2018). On comparing conventional reinforced material with natural fibers, the natural fibers are renewable, biodegradable, environment friendly, cost efficient with high specific strength (Bhuvaneshwaran et al. Citation2021; Sanjay et al. Citation2022). Ageratina Adenophora is a plant most commonly available in southern and south-eastern Asia and commonly known as Crofton weed. It is native to Mexico, where it is a common weed (Wang and Wang Citation2006). In this study, fiber of Ageratina Adenophora Stem Fiber (AASF) is taken for examination and compared with other discovered natural fibers; to examine and notice the chemical, mechanical, thermal properties, and SEM Analysis. Identifying the fibers in bio-composite material society has become a challenge for researchers. The researcher’s attention turns toward natural fiber with an exhibit of potential properties of reinforcement materials either thermoset or thermoplastic matrix Coir (Dicker et al. Citation2014), flax (Goutianos et al. Citation2006), jute (Ahmed and Vijayarangan Citation2007), sisal (Li, Mai, and Ye Citation2000), hemp (Madsen et al. Citation2007). The biggest challenge for researchers in bio-composite material community is identifying natural fibers, due to their narrow range. Data from the last five years of study shows that cellulose-based natural fibers such as Coccinia Indica (Balu et al. Citation2020), Calotropis gigantean (Ganeshan et al. Citation2018), Lavender (Eyupoglu and Merdan Citation2021) Calotropis Procera (Yoganandam et al. Citation2020) have been recommended cellulose based fibers as an alternative to reinforced new composites. For study purpose, Urtica dioica fiber was chosen and it was reported that this fiber can be used to fabricate environment-friendly natural fiber reinforced composites (Bodros and Baley Citation2008). Natural fibers are widely used in the automotive sector (Thakur, Thakur, and Gupta Citation2014). Natural banana fiber reinforced with epoxy resin composite was determined to be as eco-friendly material such as household telephone stand and also utilized for manufacturing household appliances with minimum cost (Sapuan and Maleque Citation2005). From recent research papers, natural composite is deemed to be important. In this study, the experiment is conducted to get surface morphology, thermo-physico properties, chemical composition, and mechanical properties of Ageratina Adenophora stem fiber.
Materials and experiments
Fiber extraction
The Ageratina Adenophora stem (matured) is found and obtained from Butwal, which is in Rupandehi, Nepal. Ageratina Adenophora plants are normally perennial herbaceous shrub and are native to Mexico (Wang and Wang Citation2006). Ageratina Adenophora stands at a height of 3–6.5 feet. (a‒1) shows the extracted AA stem fiber’s and microbial degradation process. In this method fibers were taken from the stem. The stem was soaked in water for 21 days, and allowed for microbial degradation. After degradation process, the dipped stem was washed with running fresh water and dried by keeping in open air for 7 days. After washing, the dried AA stem fibers were taken out through combing process and, metal teeth brush carried out combing process (Bhuvaneshwaran et al. Citation2019; Sanjay et al. Citation2019; Sisti et al. Citation2018).
Chemical composition, physical, and mechanical property
To determine lignin, hemicelluloses, and cellulose of AA fiber, standard test methods were used (Doree Citation1950). Balance method was selected to evaluate the density of AASF (Indran, Raj, and Sreenivasan Citation2014). The wax content is examined as per the common protocol (Conrad Citation1944). ASTM standard E1755–01 is used to examine the content of ash. According to ASTM D3822 M14 method, the fibers (n = 25) undergo tensile strength test. shows the single fiber tensile test setup, with the help of 1kN load cell, Instron 5500 R testing machine was used at room temperature condition and, 50 mm gauge length specimen is subjected to a crosshead acceleration of 2 mm/min.
Fourier transform infrared (FTIR) spectroscopy
FTIR spectrometer was used to determine the existence of free functional groups on the AASF (Perkin Elmer RXI FTIR tester). The wave spectral output functions were recorded from the span of 4000–400 cm−1 by using 32 scans per second and were acquired in transmittance mode.
X-ray diffraction (XRD) analysis
The crystallinity of AASF can be found using Powder X-ray diffraction technique. X’PERT PRO diffractometer was used for analysis with condition viz. tension of 40kV, current 30 mA generator setting with Cu anode substance at 25°C temperature with 0.05 (2θ step size). The diffracted intensity obtained was among 10–80 with CuKα radiation wavelength of 0.152 nm.
Thermogravimetric-difference thermogravimetric (TG-DTG) analysis
The thermal stability characteristics of the AASF are examined with the help of Thermogravimetric (TG and DTG) analysis. An analysis was done using STA, NETZSCH models of GERMANY to compute heat transformation and loss of mass. At the rate of 20 ml/min, nitrogen gas is mixed with AASF. To measure the temperature of the sample, thermocouple method is used by crushing 10 mg of AASF and placing it in alumina crucible for better coupling of samples. The rate of heating of the fiber is measured step by step starting with the atmospheric temperature to 600°C, that is, the heating rate is 10°C/min over a range of temperatures.
Surface morphology of AASF by scanning electron microscopy (SEM) and optical microscope
To visualize the surface of AASF with a voltage of 30kV scanning electron microscope is used and vacuum quantity obtained is 1.5 × 10−3Pa. and to avoid gathering of electrical charges during evaluation, the specimens were sprayed with fine gold layer. Olympus make model B×51 Optical Microscope was used to determine the diameter of the fiber.
Results and discussions
Chemical, physical, and mechanical properties analysis of AASF
Biodegradability, mechanical, fire resistance, and resilience are properties that were highly influenced by chemicals of cellulose in fibers. Due to contribution of high cellulose content about 65.17 wt.% of AASF, the mechanical properties of the fiber are enhanced exponentially (Senthamaraikannan et al. Citation2018). The existence of hemicellulose content, leads to deterioration of micro fibrils and also decreases the strength of the fiber. The hemicellulose content found in AASF is 12.5 wt.%. The fiber structure, morphology and properties were changed due to the lignin presence of about 12.5 wt% (Mohanty, Misra, and Hinrichsen Citation2000). In the composite interfacial bonding among the fiber and polymer, the presence of wax content plays a vital role (Bright et al. Citation2021; Khan et al. Citation2021). The minimal wax of 0.5 wt.% of AASF is lower with Cortaderia selloana grass and Calotropis procera, and so on, and during the manufacturing of composite materials, the bonding is greatly enhanced. Physical properties of AASF are suitable for reinforcing substance in manufacturing composites. AASF fiber is compared with E-glass fiber to find its density that it affords in achieving weight reducing for structural applications. shows the physical property and, chemical composition of AASF and compared with various natural fibers. A mechanical property of AASF depends on chemical composition, cell wall structure of the fiber and the percentage of the cellulose amount in the fiber. The tensile strength of the fibers with 50 mm gauge length is tested by using Universal testing machine and, the results are presented in and compared with various natural fibers.
Table 1. Chemical compositions, density of raw AASF and compared with different natural fibers.
Table 2. Mechanical, crystalline, and thermal properties of raw AASF and various natural fibers.
FTIR analysis
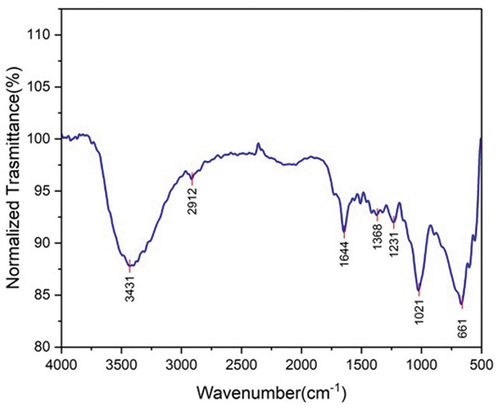
shows the result of FTIR analysis used to find the functional groups present in AASF on the prepared samples. From , FTIR’s spectrum for raw AASF, the peaks were allocated for Si-O bonding between 406.34 cm−1 and 512 cm−1 (Boopathi, Sampath, and Mylsamy Citation2012). The peak is indicated by the presence of sulfur and lignin. Cellulose bonding is indicated from the peak at 806.11 cm−1. The presence of C=O stretch is highlighted by the band around 1643.7 cm−1 for hemicellulose of acetyl groups (Bhuvaneshwaran et al. Citation2021). The peak at 1774.85 cm−1 represents the aldehydic groups of lignin and the carbonyl stretching C-O for acetyl group in hemicellulose. In the case of hemp fibers, the carbonyl region was predicted at 1710 cm−1 (Pracella et al. Citation2006) typical band for C-H stretching vibration from CH and CH2 in hemicellulose and cellulose constituent is foreseen at the peak 2515.15 cm−1 (Fiore, Scalici, and Valenza Citation2014). At the peak of 3400 cm−1, the O-H stretching vibrations and hydrogen bonds of hydroxyl group occurs.
XRD analysis
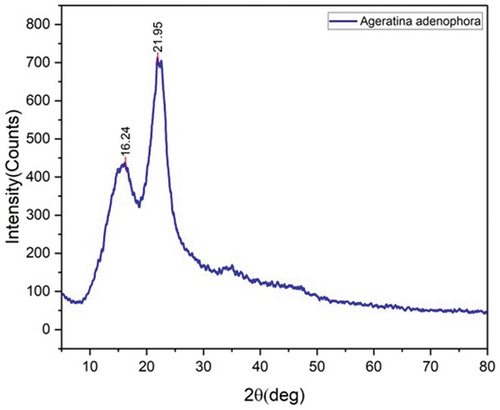
shows X-Ray diffraction spectrum of AASF. The peak 2θ = 21.95° (200) clearly indicates the cellulose content. Commonly non-cellulose materials like wax, lignin, and so on, were shown at peak 2θ = 16.24° (100). Crl (Crystallinity) is used to measure the crystalline of cellulose. The expression used to determine Crl is as follows (Segal et al. Citation1959) of AAS fiber
Where
Crl : Relative degree of the crystallinity
I002 : Maximum Intensity
IAM: Minimum Intensity
40.29% Crl is found for AASF, Higher Crl tends to be brittle, while at the same time higher Crl gives higher strength to fibers (Manimaran et al. Citation2018). The crystallite size (L) of AASF is founded by using Scherer’s equation (Vijay et al. Citation2019). The value of L is 17.01 nm which is significantly larger than that of pithecellobium dulce fiber (3.46 nm) and near to Albizia lebbeck (16.57 nm). A large crystal size of the fiber means condensed surface area as a result lower water and chemical absorption of fibers (Manimaran et al. Citation2018). presents the crystallite size of various natural fibers and crystallinity index.
Where
: wave length of the radiations
Β: field width at half maximum(FWHM)
K: Scherer’s constant (0.94)
TG-DTG analysis
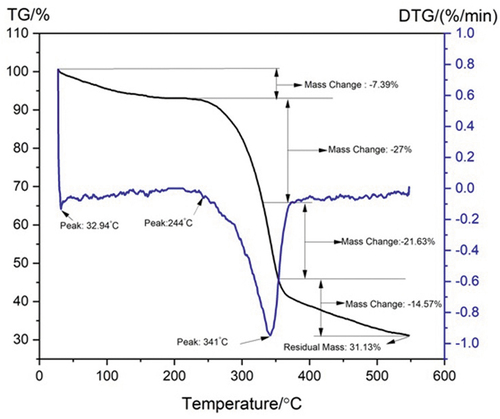
The thermal stability of the AAS fiber is evaluated using thermal analyzer. AASF begins to decompose at 108.70°C, resulting in a weight loss of approximately 7.39% due to evaporation of water within the fiber. The DTG and TG curves in represent thermal stability at 244°C, and from that an acceptable peak observed at 244°C falls between 200°C and 300°C. This is equivalent to the thermal sterilization of hemicelluloses contained therein. A prominent peak at 341°C with a weight loss of 48.62% was indicated from the degradation of α-cellulose. From the thermal analysis of AA fiber, it withstands temperature up to 244°C, so it can be used as reinforcement material in polymer composites. Such as the thermoplastics resin working temperature below 170°C (Manimaran et al. Citation2018).
The thermal stability of different natural fibers was present in . The complex aromatic ring component of the lignin structure contains several branches and thus decomposition occurs at minimal weight loss rate within its whole temperature varies from room temperature to higher than 600°C (De Rosa et al. Citation2010). Oxidative depletion in burnt remains may result in remaining two peak values such as 341°C and 496°C. Very high temperatures are required for larger crystalline structure (Yang and Kokot Citation1996). From the thermal analysis, AA fiber withstand temperature up to 244°C, so it can be used as reinforcement material in polymer composites. Such as the thermoplastics resin working temperature below 170°C. Peaks were observed at 321°C, 376°C, 298°C, 331°C, and 321°C for jute, hemp, Coccina india, bamboo fiber, Prosopis juliflora, respectively (Yao et al. Citation2008).
SEM analysis of AASF
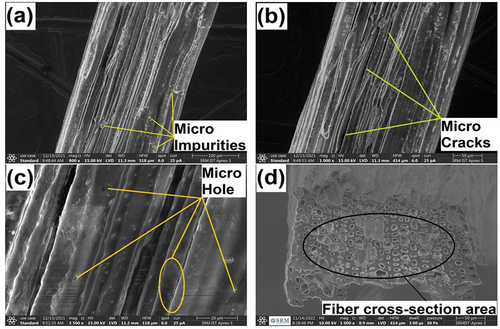
(a‒6) shows high-resolution SEM images which are used to determine the structure of AAS fiber surface and shows the impurities, micro cracks, and micro holes, respectively, along with longitudinal direction of fiber. These holes and cracks in polymer matrix could make high contact area in fiber. SEM image present with colored micro particles may be seen due to the presence of amorphous constituents in fiber such as cellulose, lignin, and hemicellulose (Gopi Krishna, Kailasanathan, and Nagaraja Ganesh Citation2020).
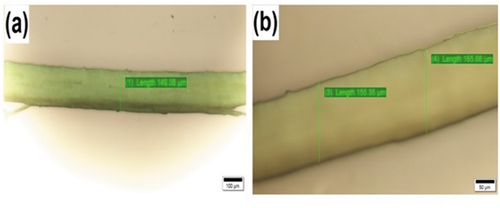
The existence of impurities is indicated by white layer on the surface of fiber. This type of samples were treated chemically to eliminate the impurities that would result in enhancing the interfacial adhesion with polymer matrices. (Fiore, Scalici, and Valenza Citation2014). ) shows the optical microscope images. The diameter of the AAS fibers was measured using optical microscope, and it shows the range to be 148.34–165.58 µm.
Conclusion
The findings of this study highlight the main need of natural stem fiber for the preparation of polymer composite. Since AA stem fiber contains a high amount of lignin content, which contributes a greater rigidity, and its lower density may allow it to be fabricate light weight composite laminates. Since AA stem fiber has desirable tensile properties, it offers a viable alternative material to conventional fibers such as glass and carbon on polymer matrixes. The relatively lower elongation and higher strength are given to AASF by the good value of Crl and size of crystallize size. Thermogravimetric investigation revealed that the thermal stability of AAS fiber is up to 244°C. In the future study, we will develop AAS fiber reinforced polymer composite laminates and which will be tested to look at its thermal and mechanical properties.
Highlights
Extraction and characterization of new natural cellulose fiber from the stem of Ageratina Adenophora (AA) plant.
Chemical composition analyses revealed that fibers were high in cellulose content and less in wax content.
XRD, FTIR, TGA, and SEM were used to characterize the fibers.
Chemical compositions and densities of raw Ageratina Adenophora stem fiber (AASF) and several natural fibers are shown in .
. Present the mechanical, crystalline, and thermal properties of raw Ageratina Adenophora stem fiber (AASF) and various natural fibers.
Acknowledgments
The author would like to thank SRM Institute of Science and Technology, Kattankulathur, Chennai, India, for providing their facilities to prepare samples. In addition, we would like to acknowledge South India Textile Research Association, Coimbatore, Tamil Nadu, India and PSG COE INDUTECH, Coimbatore, Tamil Nadu, India for providing their lab facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ahmed, K. S., and S. Vijayarangan. 2007. Experimental characterization of woven jutefabric-reinforced isothalic polyester composites. Journal of Applied Polymer Science 104 (4):2650–12. doi:10.1002/app.25652.
- Balu, S., P. S. Sampath, M. Bhuvaneshwaran, G. Chandrasekar, A. Karthik, and S. Sagadevan. 2020. Dynamic mechanical analysis and thermal analysis of untreated Coccinia indica fiber composites. Polimery 65 (5):357–62. doi:10.14314/polimery.2020.5.3.
- Bhuvaneshwaran, M., P. S. Sampath, S. Balu, and S. Sagadevan. 2019. Physicochemical and mechanical properties of natural cellulosic fiber from Coccinia Indica and its epoxy composites. Polimery 64 (10):656–64. doi:10.14314/polimery.2019.10.2.
- Bhuvaneshwaran, M., S. P. Subramani, S. K. Palaniappan, S. K. Pal, and S. Balu. 2021. Natural cellulosic fiber from Coccinia Indica stem for polymer composites: Extraction and characterization. Journal of Natural Fibers 18 (5):644–52. doi:10.1080/15440478.2019.1642826.
- Bodros, E., and C. Baley. 2008. Study of the tensile properties of stinging nettle fibres (Urtica dioica). Materials letters 62 (14):2143–45. doi:10.1016/j.matlet.2007.11.034.
- Boopathi, L., P. S. Sampath, and K. Mylsamy. 2012. Investigation of physical, chemical and mechanical properties of raw and alkali treated Borassus fruit fiber. Composite Part B: Engineering 43 (8):3044–52. doi:10.1016/j.compositesb.2012.05.002.
- Bright, B. M., B. J. Selvi, S. Abu Hassan, M. M. Jaafar, S. Suchart, M. R. Sanjay, and B. Kurki Nagaraj. 2021. Characterization of natural cellulosic fiber from cocos nucifera peduncle for sustainable biocomposites. Journal of Natural Fibers 19: 9373–83. Advance online publication. doi:10.1080/15440478.2021.1982827.
- Conrad, C. M. 1944. Determination of wax in cotton fiber, a new alcohol extraction method. Industrial Engineering Chemistry, Analytical Edition 16 (12):745–48. doi:10.1021/i560136a007.
- De Rosa, I. M., J. M. Kenny, D. Puglia, C. Santulli, and F. Sarasini. 2010. Morphological, thermal and mechanical characterization of okra (Abelmoschus esculentus) fibres as potential reinforcement in polymer composites. Composites Science and Technology 70 (1):116–22. doi:10.1016/j.compscitech.2009.09.013.
- Dicker, M. P., P. F. Duckworth, A. B. Baker, G. Francois, M. K. Hazzard, and P. M. Weaver. 2014. Green composites: A review of material attributes and complementary applications. Composites: Part A, Applied Science and Manufacturing 56:280–89. doi:10.1016/j.compositesa.2013.10.014.
- Doree, C. 1950. The methods of cellulose chemistry. 2nd ed. New York: Springer US.
- Eyupoglu, S., and N. Merdan. 2021. Physicochemical properties of new plant based fiber from lavender stem. Journal of Natural Fibers 19: 9248–58. Advance online publication. doi:10.1080/15440478.2021.1982816.
- Fiore, V., T. Scalici, and A. Valenza. 2014. Characterization of a new natural fiber from Arundo donax L. as potential reinforcement of polymer composites. Carbohydrate Polymers 106:77–83. doi:10.1016/j.carbpol.2014.02.016.
- Ganeshan, P., B. NagarajaGanesh, P. Ramshankar, and K. Raja. 2018. Calotropis gigantea fibers: A potential reinforcement for polymer matrices. International Journal of Polymer Analysis and Characterization 23 (3):271–77. doi:10.1080/1023666X.2018.1439560.
- Gopi Krishna, M., C. Kailasanathan, and B. Nagaraja Ganesh. 2020. Physico-chemical and morphological characterization of cellulose fibers extracted from sansevieria roxburghiana Schult. & Schult. F Leaves. & Schult. F Leaves. Journal of Natural Fibers 19: 3300–16. Advance online publication. doi:10.1080/15440478.2020.1843102.
- Gopinath, R., P. Billigraham, T. P. Sathishkumar, M. R. Sanjay, and S. Siengchin. 2021. Characterization of Sida acuta fiber and its polymer composites with effect of fly ash. Journal of Natural Fibers Advance online publication. doi:10.1080/15440478.2021.1967833.
- Goutianos, S., T. Peijs, B. Nystrom, and M. Skrifvars. 2006. Development of flax fibre based textile reinforcements for composite applications. Applied Composite Materials 13 (4):199–215. doi:10.1007/s10443-006-9010-2.
- Indran, S., and R. Edwin. 2015. Characterization of new natural cellulosic fiber from Cissus quadrangularis stem. Carbohydrate polymers 117 (6):392–99. doi:10.1016/j.carbpol.2014.09.072.
- Indran, S., R. E. Raj, and V. S. Sreenivasan. 2014. Characterization of new natural cellulosic fiber from Cissus quadrangularis root. Carbohydrate Polymers 110:423–29. doi:10.1016/j.carbpol.2014.04.051.
- Khan, A., R. Vijay, D. L. Singaravelu, M. R. Sanjay, S. Siengchin, F. Verpoort, K. A. Alamry, and A. M. Asiri. 2021. Characterization of natural fibers from Cortaderia selloana grass (pampas) as reinforcement material for the production of the composites. Journal of Natural Fibers 18 (11):1893–901. doi:10.1080/15440478.2019.1709110.
- Li, Y., Y. W. Mai, and L. Ye. 2000. Sisal fibre and its composites: A review of recent developments. Composites Science and Technology 60 (11):2037–55. doi:10.1016/S0266-3538(00)00101-9.
- Madhu, P., M. R. Sanjay, P. Senthamaraikannan, S. Pradeep, S. S. Saravanakumar, and B. Yogesha. 2018. A review on synthesis and characterization of commercially available natural fibers: Part-I. Journal of Natural Fibers 16 (8):1132–44. doi:10.1080/15440478.2018.1453433.
- Madsen, B., P. Hoffmeyer, A. B. Thomsen, and H. Lilholt. 2007. Hemp yarn reinforced composites–i. Yarn characteristics. Composites: Part A, Applied Science and Manufacturing 38 (10):2194–203. doi:10.1016/j.compositesa.2007.06.001.
- Mahalingam, J. 2022. Mechanical, thermal, and water absorption properties of hybrid short coconut tree primary flower leaf stalk fiber/glass fiber-reinforced unsaturated polyester composites for biomedical applications. Biomass Conversion and Biorefinery Advance online published. doi: 10.1007/s13399-022-02958-4.
- Manimaran, P., M. R. Sanjay, P. Senthamaraikannan, S. S. Saravanakumar, S. Siengchin, G. Pitchayyapillai, and A. Khan. 2021. Physico-chemical properties of fiber extracted from the flower of celosia argentea plant. Journal of Natural Fibers 18 (3):464–73. doi:10.1080/15440478.2019.1629149.
- Manimaran, P., M. R. Sanjay, P. Senthamaraikannan, B. Yogesha, C. Barile, and S. Siengchin. 2018. A new study on characterization of Pithecellobium dulce fiber as composite reinforcement for lightweight applications. Journal of Natural Fibers 17 (3):359–70. doi:10.1080/15440478.2018.1492491.
- Manivel, S., N. Pannirselvam, R. Gopinath, and T. P. Sathishkumar. 2022. Physico-mechanical, chemical composition and thermal properties of cellulose fiber from hibiscus vitifolius plant stalk for polymer composites. Journal of Natural Fibers 19 (13):6961–76. doi:10.1080/15440478.2021.1941484.
- Maran, M., R. Kumar, P. Senthamaraikannan, S. S. Saravanakumar, S. Nagarajan, M. R. Sanjay, and S. Siengchin. 2022. Suitability evaluation of Sida mysorensis plant fiber as reinforcement in polymer composite. Journal of Natural Fibers 19 (5):1659–69. doi:10.1080/15440478.2020.1787920.
- Mohanty, A. K., M. A. Misra, and G. I. Hinrichsen. 2000. Biofibres, biodegradable polymers and biocomposites: An overview. Macromolecular Materials and Engineering 276 (1):1–24. doi:10.1002/(SICI)14392054(20000301)276:1<1:AIDMAME1>3.0.CO;2-W.
- Muruganrama, T., J. Mahalingam, S. Dharmalingam, and S. Natarajan. 2022. Investigation of static and dynamic mechanical properties of short Palmyra palm leaf stalk fiber (PPLSF) reinforced polymer composites. Journal of Natural Fibers 19 (5):1908–24. doi:10.1080/15440478.2020.1840478.
- Mylsamy, B., S. K. Palaniappan, S. P. Subramani, S. K. Pal, and B. Sethuraman. 2020. Innovative characterization and mechanical properties of natural cellulosic Coccinia Indica fiber and its composites. Materials Testing 62 (1):61–67. doi:10.3139/120.111451.
- Nagappan, S., S. P. Subramani, S. K. Palaniappan, and B. Mylsamy. 2021. Impact of alkali treatment and fiber length on mechanical properties of new agro waste Lagenaria Siceraria fiber reinforced epoxy composites. Journal of Natural Fibers 19 (13):6853–64. doi:10.1080/15440478.2021.1932681.
- Pracella, M., C. Chionna, I. Angusillesi, Z. Kulinski, and E. Piorkowska. 2006. Functionalization, compatibilization and properties of polypropylene composites with hemp fibers. Composites Science and Technology 66 (13):2218–30. doi:10.1016/j.compscitech.2005.12.006.
- Ramesh, M., C. Deepa, L. R. Kumar, M. R. Sanjay, and S. Siengchin. 2022. Life-cycle and environmental impact assessments on processing of plant fibres and its bio-composites: A critical review. Journal of Industrial Textiles 51 (4S):5518S–42. doi:10.1177/1528083720924730.
- Ramkumar, T., K. Hariharan, M. Selvakumar, and M. Jayaraj. 2022. Effect of various surface modifications on characterization of new natural cellulosic fiber from coconut tree secondary flower leaf stalk fiber (CSF). Journal of Natural Fibers 19: 13362–75. Advance online publication. doi:10.1080/15440478.2022.2091715.
- Sanjay, M. R., P. Madhu, M. Jawaid, P. Senthamaraikannan, S. Senthil, and S. Pradeep. 2018. Characterization and properties of natural fiber polymer composites: A comprehensive review. Journal of Cleaner Production 172:566–81. doi:10.1016/j.jclepro.2017.10.101.
- Sanjay, M. R., S. Siengchin, J. Parameswaranpillai, M. Jawaid, and T. Ozbakkaloglu. 2022. Lignocellulosic fiber reinforced composites: Progress, performance, properties, applications, and future perspectives. Polymer Composites 43 (2):645–91. doi:10.1002/pc.26413.
- Sanjay, M. R., S. Siengchin, J. Parameswaranpillai, M. Jawaid, C. I. Pruncu, and A. Khan. 2019. A comprehensive review of techniques for natural fibers as reinforcement in composites: Preparation, processing and characterization. Carbohydrate polymers 207:108–21. doi:10.1016/j.carbpol.2018.11.083.
- Sapuan, S. M., and M. A. Maleque. 2005. Design and fabrication of natural woven fabric reinforced epoxy composite for household telephone stand. Materials & Design 26 (1):65–71. doi:10.1016/j.matdes.2004.03.015.
- Segal, L., J. J. Creely, A. E. Martin, and C. M. Conrad. 1959. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Textile Research Journal 29 (10):786–94. doi:10.1177/004051755902901003.
- Senthamaraikannan, P., M. R. Sanjay, K. S. Bhat, N. H. Padmaraj, and M. Jawaid. 2018. Characterization of natural cellulosic fiber from bark of Albizia amara. Journal of Natural Fibers 16 (8):1124–31. doi:10.1080/15440478.2018.1453432.
- Sisti, L., G. Totaro, M. Vannini, and A. Celli. 2018. Retting process as a pretreatment of natural fibers for the development of polymer composites. In Lignocellulosic composite materials. Cham: Springer. doi:10.1007/978-3-319-68696-7_2.
- Thakur, V. K., M. K. Thakur, and R. K. Gupta. 2014. Review: Raw natural fiber-based polymer composites. International Journal of Polymer Analysis and Characterization 19 (3):256–71. doi:10.1080/1023666X.2014.880016.
- Thirumurugan, R., M. Jayaraj, D. Shanmugam, and T. Ramkumar. 2021. Characterization of new natural cellulosic fiber from coconut tree primary flower leaf stalk fiber (CPFLSF). Journal of Natural Fibers 18 (11):1844–56. doi:10.1080/15440478.2019.1701608.
- Vijay, R., D. L. Singaravelu, A. Vinod, I. D. Franklin Paul Raj, M. R. Sanjay, and S. Siengchin. 2019. Characterization of novel natural fiber from saccharum bengalense grass (Sarkanda). Journal of Natural Fibers 17 (12):1739–47. doi:10.1080/15440478.2019.1598914.
- Vinod, A., M. R. Sanjay, S. Suchart, and P. Jyotishkumar. 2020. Renewable and sustainable biobased materials: An assessment on biofibers, biofilms, biopolymers and biocomposites. Journal of Cleaner Production 258:120978. doi:10.1016/j.jclepro.2020.120978.
- Wang, R., and Y. Z. Wang. 2006. Invasion dynamics and potential spread of the invasive alien plant species Ageratina adenophora (Asteraceae) in China. Diversity & Distributions 12 (4):397–408. doi:10.1111/j.1366-9516.2006.00250.x.
- Yang, P., and S. Kokot. 1996. Thermal analysis of different cellulosic fabrics. Journal of Applied Polymer Science 60 (8):1137–46. doi:10.1002/(SICI)1097-4628(19960523)60:8<1137:AID-APP6>3.0.CO;2-M.
- Yao, F., Q. Wu, Y. Lei, W. Guo, and Y. Xu. 2008. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polymer Degradation and Stability 93 (1):90–98. doi:10.1016/j.polymdegradstab.2007.10.012.
- Yoganandam, K., P. Ganeshan, B. Nagaraja Ganesh, and K. Raja. 2020. Characterization studies on calotropis procera fibers and their performance as reinforcements in epoxy matrix. Journal of Natural Fibers 17 (12):1706–18. doi:10.1080/15440478.2019.1588831.