 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In the presented research work, the impact of two harvesting dates, temperature, and precipitation on the content of essential oils in inflorescences of three hemp cultivars in fresh and dry plant matter was assessed. Plants were harvested at full flowering and at full hemp seeds maturity. The highest content of essential oils in the dry matter of inflorescences was characteristic of the “Białobrzeskie” cultivar in full flowering, and the smallest of the ”Zołotonowska 13” cultivar, regardless of the harvest date. When the harvest was delayed from full flowering to full maturity, the content of essential oils in the fresh mass of the inflorescence increased. On the basis of the obtained results, it was concluded that the content of essential oils in hemp in the dry matter of inflorescence is determined mainly by the cultivar, while the harvest date and the influence of various weather factors modify these values. On the other hand, the content of essential oils in the fresh mass of the inflorescence depends mainly on the harvest date – the closer to full maturity, the higher their content.
摘 要
在本研究工作中,评估了两个收获日期、温度和降水量对三个大麻品种花序中新鲜和干燥植物物质中精油含量的影响. 植物在充分开花和大麻种子完全成熟时收获。花序干物质中精油含量最高的是开花期的“Białobrzeskie”品种,最小的是“Zoł; otonowska 13”品种,无论收获日期如何. 当收获从完全开花推迟到完全成熟时,花序新鲜质量中的精油含量增加. 根据所获得的结果,得出结论,大麻中的精油在花序干物质中的含量主要由品种决定,而收获日期和各种天气因素的影响改变了这些值. 另一方面,花序新鲜质量中精油的含量主要取决于收获日期-越接近完全成熟,其含量越高.
1. Introduction
Hemp (Cannabis sativa L.) is a plant species belonging to the family Cannabaceae) family and the genus of hemp (Cannabis) (Backer et al. Citation2009; Strzelczyk, Lochynska, and Chudy Citation2021). This plant can be applied in many industries: textile, cosmetics, automotive, pulp and paper, pharmaceutical, food, construction, and seed. Hemp can also be used in other industries, e.g., toy manufacturing, disinfection mats, and animal substrates (Andre, Francois Hausman, and Guerriero Citation2016; Bócsa, Karus, and Lohmeyer Citation2000; Frankowski et al. Citation2021; Kaniewski et al. Citation2017; Lekavicius et al. Citation2015).
A great versatility of hemp enables using almost every part of this plant for commercial purposes. One of the examples may be the use of hemp`s essential oils.
A lot of research was conducted on the content of cannabinoids, resin, and seed oil from different cultivars of Cannabis sativa L.; however, few studies pertaining to the chemical composition and pharmacology of the essential oils obtained from fresh inflorescences were done, and even less research pertained to the influence of various factors on essential oils content in the plant and its applicability (Gulluni et al. Citation2018).
In this context, hemp essential oil is a niche product, potentially of interest to pharmaceutical, cosmetic, and agrochemical companies. Nowadays the production of hemp essential oil is becoming increasingly important due to its valuable health-promoting properties (Fiorini et al. Citation2020; Nagy et al. Citation2019).
Hemp essential oil is a complex mixture of many volatile compounds, mainly monoterpenes, sesquiterpenes, and other terpenoid-like substances (Bertoli et al. Citation2010; Johannes et al. Citation2001; Pieracci et al. Citation2021). The main chemical components are myrcene, β-caryophyllene, limonene, α-pinene, β-pinene, terpinolene, and α-humulene (Hillig Citation2004; Ross and ElSohly Citation1996).
The specific properties of these substances exert antidepressant, relaxing, anxiolytic, sedative, antibacterial, and antioxidant effects (Gulluni et al. Citation2018; Stasiłowicz et al. Citation2021; Vitorović et al. Citation2021).
Hemp essential oil may be used in the production of beer and wine and as an additive to refreshing drinks (Markowska et al. Citation2021; Ovidi et al. Citation2022). The aromatic properties of the oil have been used in the production of cosmetics (creams, lotions, soaps, perfumes, tonics, masks) and aromatherapy (Kaniewski et al. Citation2015; Kaniewski, Konczewicz, and Cierpucha Citation2000; Kędzia et al. Citation2014a, Citation2014b; Meier and Mediavilla Citation1998; Truta et al. Citation2009)
Hemp essential oil is also included in dermatological preparations, as it has a strong antibiotic effect against yeast-like fungi. However, the antibiotic effect is weaker in the relation to gram-positive bacteria and dermatophytes, and the lowest in relation to gram-negative bacteria and mold fungi (Kędzia et al. Citation2014b; Mehdizadeh and Moghaddam Citation2018).
These properties are used in dermatological cosmetics, in treatment of atopic dermatitis, urticaria, eczema, and psoriasis (Martins et al. Citation2022). It was reported that the addition of hemp preparations for oral hygiene, as well as soaps and powders is being considered (Khan, Warner, and Wang Citation2014). Also, it is known that monoterpenes and sesquiterpenes support the treatment of chronic respiratory diseases (Mediavilla and Steinemann Citation1997).
Additionally, hemp terpenes have proven to exhibit bacteriostatic activity (McPartland Citation1997; Mehdizadeh and Moghaddam Citation2018; Nissen et al. Citation2010), mainly against Gram-positive bacteria (Staphylococcus sp. and Streptococcus sp.) (Kaniewski, Konczewicz, and Cierpucha Citation2000).
Moreover, hemp essential oils repel insects (compounds: limonen and β-pinene, terpenol, borneol) (Kaniewski et al. Citation2015; Kaniewski, Konczewicz, and Cierpucha Citation2000; Mediavilla and Steinemann Citation1997; Pate Citation1999). However, an alternating hemp with other less toxic plants constitute on food of insects (McPartland Citation1997). The leaves and female flowers were shown to be appropriate food for Spilosoma obliqua caterpillars, but when the monotonous Cannabis diet was introduced, the caterpillars died after 20 days (Deshmukh Citation1979). Hemp compounds have been used to produce plant protection products that are valuable and irreplaceable in organic farming (Russo and Guy Citation2006; Russo and Taming Citation2011). The attempts have also been made to use hemp oil against potato blight (Phytophthora infestans) (Mont) (Mediavilla and Steinemann Citation1997). As early as in 1885, it was noted that the vicinity of fibrous hemp protected cotton plantations against the potato pest (Alabama argillacea) (Hübner), against the Colorado potato beetle (Leptinotarsa decemlineata) (Say) and cabbage plantations against cabbage butterfly (Pieris brassicae L.) (McPartland Citation1997; McPartland, Connell Clarke, and Paul Watson Citation2000). Active substances from hemp also act as a deterrent against the larvae of wheat bulb fly (Delia coarctata Fall.) in wheat crops and grubs of the Maybeetle (Melolontha melolontha L.), nematodes (Mojumdar et al. Citation1989) and Hemipterain particular, apple aphid (Aphis pomi DeG.) and rosy apple aphid (Dysaphis plantaginea Pass.); two-spotted spider mite (Tetranychus urticae Koch) and carmine spider mite (Tetranychus cinnabarinus Bois.) (Dorna et al. Citation2010). Moreover, it has been revealed that the use of a water emulsion based on hemp essential oil reduces the development of carrot fungal pathogens (Daucus carota L.).
The essential oil is obtained from hemp leaves, which account for 20–25% of the weight of the whole plant (Bócsa, Karus, and Lohmeyer Citation2000), and they are also used in the food industry for the production of teas (Knezevic et al. Citation2021). The teas made of hemp leaves are very popular in many EU countries (Bócsa, Karus, and Lohmeyer Citation2000) as well as in the United States and Canada.
Due to the great importance hemp mineral oil, and its versatile application in the industry, the factors determining the content of the essential oils are worth further and thorough research. The aim of this work was to assess the impact of environmental factors like two harvesting dates, temperature, and precipitation on the content of essential oils in inflorescences of three hemp cultivars, in fresh and dry plant matter.
2. Materials and methods
The field experiments were conducted in the period of 2018–2020 at the Experimental Farm of the Institute of Natural Fibres and Medicinal Plants – National Research Institute (INF&MP-NRI) in Pętkowo, Poland (52°12′40″N 17°15′31″E). The experiments were carried out on proper black earth soil with an arable layer of 35–40 cm, classified in Polish classification as class IIIa.
The research material was monoecious fibrous hemp of the following cultivars: ”Białobrzeskie,” “Zenit,” and ”Zołotonowska 13.” The “Białobrzeskie” cultivar is the first monoecious hemp cultivar registered in Poland in 1967, bred at the INF&MP-NRI in Poznań. “Zenit” and “Zołotonowska 13” are monoecious cultivars of fibrous hemp adapted to cultivation in Central and Northern Europe; the first is of Romanian origin, the second is Ukrainian.
The experiments were set up as two-factorial, with a randomized block design, in a split-plot design in four replications. The experimental factors were: first-order factor – hemp cultivar (A); second-order factor – harvest time (B).
The area of plots was 12 m2, and 5 m2 were intended for harvesting. In each year of the experiment, the hemp seeds (nuts), were sown in the amount of 180 germinating nuts/m2, with row spacing every 15 cm, to a depth of 4 cm.
The harvest was carried out on two dates: full flowering (BBCH 6.65) of the inflorescences and full maturity of nuts (BBCH 8.85). Developmental stages were determined based on the BBCH scale (Mishchenko et al. Citation2017). The observations of the development phases were carried out in all periods of plant vegetation, from sowing to harvesting. The plant density of each cultivar was determined twice: 2 weeks after full emergence and at full plant maturity, on an area of 1 m2.
In accordance with the recommendations of good agricultural practice, each year winter plowing was carried out on the experimental field to a depth of 25–30 cm. The liming of the soil was carried out before setting up the experiments, i.e., in 2016 – for the experiment carried out in 2018, in 2017 – for the experiment carried out in 2019 and in 2018 – for the experiment sown in 2020.
Dolomitic lime was applied in the amount of 3 t⋅ha−1. Every spring before sowing, mineral fertilization was conducted ().
Table 1. Mineral fertilization.
In spring, before sowing, in order to provide the plants with optimal conditions for development, cultivation was performed with a pre-sowing tillage unit. Before starting the experiments, the analysis of the forecrop as well as the pH and content of minerals in the soil was performed.
The course of weather conditions in each year of the research made it possible to sow seeds in the first week of May. Chemical weeding was not applied before sowing and during the vegetation period ().
Table 2. The course of hemp vegetation in the various years of the experiment.
The analysis of weather conditions was carried out on the basis of observations made at the Central Agricultural Plant Variety Research Center in Środa Wielkopolska – COBORU (2018–2020) and the INF&MP Experimental Plant in Pętkowo (2018–2020) at the local observation and measurement point.
The analysis of weather conditions was carried out on the basis of average daily air temperatures and total daily precipitation. For a decade and for perennial average, the hydrothermal coefficients by Selyaninov were calculated, using the formula: K = (Mo × 10)/Dt × days, where: Mo – the sum of precipitation in the decade/perennial, Dt – average daily temperature.
2.1. Sampling, preparation, and analysis of samples for the content of essential oils in hemp inflorescences
Top sections of inflorescences with a length of 30 cm were collected. The determination of the content of essential oils was performed for each experimental object on collective samples from four repetitions. Two-kilogram samples of inflorescences of each cultivar were divided into two parts. A half of the collected material was subjected to slow, natural drying (the final moisture of the plant material) was 7%, using humimeter FLH (Schaller, Warsaw, Poland), and the remaining part was sent to the laboratory on the day of harvest. In laboratory, the samples were prepared on the same day. The content of essential oils in inflorescences was determined in fresh and dry matter at two harvest dates: full flowering and full nuts (seeds) maturity.
The determination of the essential oil content consisted of the aqueous distillation of a sample weighing 50 g with the Deryng apparatus (Baj et al. Citation2015), collecting the distillate in a graduated tube and reading the total volume of the organic phase on the microscale. The analysis was carried out in two parallel repetitions and results were converted into 100 g of raw material, expressing the content of essential oils in percent weight per volume.
Main shoots and nuts (samples from the second harvest date) were removed from the fresh hemp inflorescences intended for testing, and the rest was ground. The water content of the samples was determined according to the ISO 939 standard. A 50 g sample (weighed with an accuracy of 0.01 g) was placed in a 2000 ml round-bottom flask and poured over with 500 ml of distilled water. After mixing of the contents of the flask, the material was rinsed from the walls with 500 ml of distilled water. The flask was connected to the Deryng apparatus according to the method recommended by Polish Pharmacopoeia VI (Polish Pharmacopoeia VI Citation2002), the receiver was filled with the water and heated for 3 h, counting from the moment of the boiling of the contents of the flask and distilling the first drop of oil. The intensity of the heating was regulated so that 3–4 ml of fluid per minute flowed into the receiver. After the set time, the heat source was removed, the oil was placed on a microscale and the result was read after 30 min. The oil content (OE) was calculated according to the following formula and expressed in milliliters per 100 g of dry product:
where: – oil volume (ml),
– sample weight (g),
– water content (%).
The mean value was adopted as the final result for each of the tested combinations from two testing’s according to the formula:
where: - arithmetic mean,
- value of a single measurement,
- number of measurements.
2.2. Statistical evaluation of the results
The obtained results were statistically evaluated using the analysis of variance for the orthogonal factorial experiments and the split-plot analysis of variance and the correlation and regression calculation for the factorial experiments using the STATPAKU software (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.). The significance of the differences was estimated at the level of α = 0.05 using the Tukey’s test. Pearson correlation coefficient was calculated for linear variables as described (Ibourki et al. Citation2021).
The values of hydrothermal coefficients for decades in the individual months of hemp vegetation indicate that the water supply of plants varied significantly ().
Table 3. Average air temperatures for decades and individual months during the vegetation period.
Table 4. Precipitation totals for decades and individual months during the vegetation period.
Table 5. Selyaninov hydrothermal coefficient for decades and individual months.
The amount of precipitation during the vegetation period in 2018 was high (311.7 mm) and it accounted for 98.48% of the perennial precipitation (2000–2020).
In 2019, the different weather conditions prevailed (). Summing up, it should be stated that the 2019 year was dry, and the total rainfall during the hemp vegetation period was 189.2 mm, accounting for 69.0% of the average perennial precipitation.
A small amount of water during the hemp vegetation period did not reduce the yield due to the fact that these plants easily adapt to the prevailing, often unfavorable weather conditions, due to hemp’s deep, pile root system.
In 2020, the course of the weather during the vegetation period was not favorable for hemp ().
The amount of precipitation during the hemp vegetation period was 294.4 mm, which is 94.5% of this period in the perennial period. A lack of water during the emergence period, strong gusts of wind combined with abundant rainfall caused lodging and breaking of plants. All these unfavorable meteorological conditions had a negative effect on the size and quality of the hemp yield.
In accordance with the adopted methodology, the first harvest was carried out in 2018 in the first decade of August, and in the following years (2019 and 2020) of the experiments, harvest was carried out in the second decade of August. At that time, the full flowering of the inflorescences was observed in of all the cultivars tested.
The vegetation period of hemp in the climatic conditions of Poland ranges from 80 to about 140 days, depending on the cultivar (Struik et al. Citation2000). In the discussed experiments, this period ranged from 120 days in the first year of the study up to 138 in the last year of the study.
3. Results and discussions
3.1. The abundance of minerals in soil
The abundance of minerals in soil varied between the years (). The content of phosphorus, potassium, and magnesium in the soil was highest in 2020, while the lowest content of phosphorus was recorded in 2019, and the content of potassium and magnesium, in 2018. The pH of the soil also varied from slightly acidic (pH 6.5) in 2019 to alkaline (pH 7.9 and 8.1) in 2018 and 2020.
Table 6. The forecrop, soil pH, and abundance in phosphorus, potassium, and magnesium.
3.2. The content of essential oils in the dry matter (DM) of the inflorescences
When comparing the cultivars, it can be observed that the “Białobrzeskie” cultivar (0.68%) had the highest content of essential oils, and the “Zolotonowska 13” (0.16%) - the lowest content. The result is significantly different based on the LSD value (). It should be emphasized that the significant differentiation of essential oil content was noted between all the cultivars studied. Similar effect was reported for Morocco’s cultivars of hemp, where a clear differentiation of essential oil content was observed for each tested cultivar (Bakali et al. Citation2022). On the other hand, the harvest dates did not significantly modify the essential oil content in DM of the hemp inflorescences. What is more in similar studies on other plant families, a wide variations in wild chamomile (Cladanthus mixtus (L.) Chevall) were found in peer-reviewed literature depending on several factors like geographical area, phenology, and techniques used (Zeroual et al. Citation2021b).
Figure 1. Influence of a cultivar on the content of essential oils in the dry matter of hemp inflorescences.
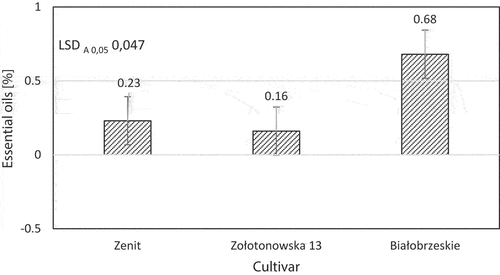
The statistical analysis of variance confirmed that the content of essential oils in the DM of the inflorescences also significantly depended on the interaction of the cultivar with the harvest date.
The demonstrated interaction of factors resulted from the fact that the content of the essential oils in the “Białobrzeskie” cultivar at full flowering was significantly higher than in the case of the harvest carried out at full maturity ().
Figure 2. Influence of cultivar and harvest date on the content of essential oils in the dry matter of hemp inflorescences.
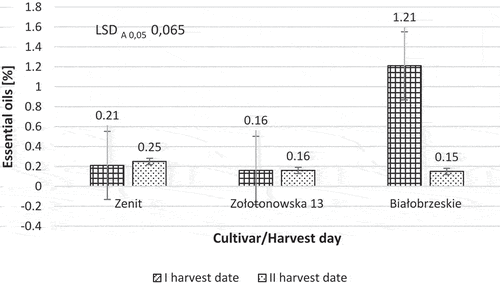
However, the delay in the harvest date did not have a significant impact on the content of essential oils in dry matter of inflorescences in the “Zolotonowska 13” and “Zenit”’ cultivars.
It was shown that the productivity of hemp essential oil depends on the cultivar and harvest date, especially on the inflorescences yield. The concentration of essential oils in the inflorescences is of secondary importance (Burczyk et al. Citation2009, Citation2011).
However, these authors did not find any significant influence of sowing density, time of day or the level of nitrogen fertilization on the content of essential oils. In turn, Meier and Mediavilla (Citation1998) emphasize that the choice of cultivar, sowing density and inter-row spacing, as well as the harvest date determine the yield of essential oils, and the quality of essential oils depends on: the cultivar, date and technique of harvesting (more favorable manual harvesting), and also on the kind apparatus for the distillation of essential oils. As it was also shown in other studies essential oils content appears to be related to cultivar and adoption of early harvest date for inflorescence harvest date resulted in good quantitative and qualitative yields of essential oils from monoecious cultivars (Baldini et al. Citation2018, Vuerich et al. Citation2019).
The other factor influencing the content of the essential oils, described in the literature is the effect of sowing time. For Thailand hemp, the flowering time of all sowing time-treatments was identical. The plant height and diameter has declined from the earliest planting date. The earlier sowing date has enabled a longer vegetative period, which resulted in producing a longer stem. The flowering of hemp ended plant height growth. A late planting reduced stem length and time needed for plants to end their growth, however it did not affected the content of the essential oils (Sengloung, Kaveeta, and Nanakorn Citation2009). The studies on optimizing sowing time were also performed in Italy, using two monoecious and two dioecious genotypes, in two subsequent years. Sowing time, was reported to be crucial for the particular cultivar. Optimal sowing time, for the dioecious cultivar, was observed between the end of April and May. On the contrary, before and after that period was optimal for the monoecious cultivar (Cosentino et al. Citation2012). The hemp yield was also reported to be influenced by genotype–environment interaction like the sowing date. The early French cultivars, such as Fedora and Felina, proved to be the best performing and stable for seed yield and both increased their yield in correspondence to delayed sowing times, opening up the possibility of cultivating hemp as a second crop (Ferfuia et al. Citation2021).
Other research have showed that the comparison of the composition of essential oil and cannabinoids in 11 hemp phenotypes from three cultivars, distinguished based on morphological distinctness, has showed that based on the content of cannabinoids and essential oil, the cultivars have significantly and positively differed from each other, making some of them important for the hemp industry (Eržen et al. Citation2021). The above mentioned experiments suggest that the content of essential oil might greatly depend on the cultivar, and that the optimization of growth in each climatic conditions, might be of great importance. Hence, there is a necessity of performing the experiments confirming this thesis.
3.2.1. The content of essential oils in the fresh matter (FM) of inflorescences
The content of essential oils in the fresh mass of hemp inflorescences increased significantly when the harvest was delayed from the full flowering to full maturity of the plants (). This increase amounted to 0.058% points.
Figure 3. Influence of the harvest date on the content of essential oils in the fresh mass of hemp inflorescences.
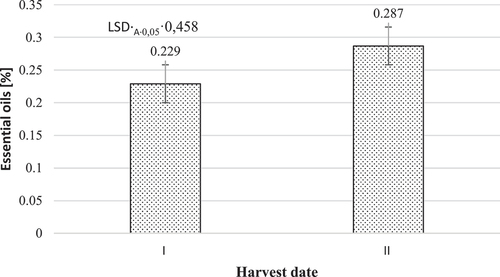
Based on the identified articles (Abdollahi et al. Citation2020; Baj et al. Citation2015; Bakali et al. Citation2022; Eržen et al. Citation2021; Pieracci et al. Citation2021), it can be concluded that most of the works in which essential oil content was studied were done on dried plant material and it is not possible to compare the obtained results with others.
3.3. Statistical characteristics of the content of essential oils in the DM of inflorescences
In the environmental conditions prevailing in the years of research, the highest stability of the content of essential oils in the DM of the hemp inflorescences was proved for the “Zolotonowska 13” cultivar, and by the variation in this content was from 0.2700% to 0.1850%, coefficient was 15.64% ().
Table 7. Statistical characteristics of the content of essential oils in the dry matter of inflorescences of the studied hemp cultivars (Pętkowo 2018–2020).
The content of essential oils in the dry matter of inflorescences of the “Zenit” cultivar turned out to be the most variable in these years, for which the coefficient of variation was 29.12%. The maximum values of the essential oils’ content in the “Zenit” and “Białobrzeskie” cultivars were higher than in the “Zolotonowska 13” cultivars and in both cases amounted to 0.2350%.
On the other hand, the highest average content of oils in dry matter was obtained from the “Zolotonowska 13” cultivar (0.2308%), and for the remaining cultivars the average content ranged from 0.1617% for “Zenit” and 0.1792% for”Białobrzeskie.”
There are several studies in the literature (Abdollahi et al. Citation2020; Eržen et al. Citation2021; Meier and Mediavilla Citation1998; Nissen et al. Citation2010; Vuerich et al. Citation2019) on the dependence of essential oil extraction efficiency on hemp genotype, while there are no data available on the dependence on crop year.
3.4. Statistical characteristics of the content of essential oils in the FM of inflorescence
The content of essential oils in the FM of hemp inflorescence in cultivars “Zenit” and “Zolotonoska 13” were the most stable in the years of the research, as evidenced by the low and similar values of the coefficients of variation, which were, respectively, CV = 17.95; CV = 17.60.
In the case of the “Białobrzeskie” cultivar, it was revealed that it was most dependent on growing conditions (CV = 24.69%). For the “Zenit” and “Białobrzeskie” cultivars, the highest, and at the same time equal, maximum values of essential oils were recorded. They amounted to 0.33%. For the same cultivars, the highest average content of the essential oils in the FM of fluorescence was proved ().
Table 8. The statistical characteristics of the content of essential oils in the fresh matter of the inflorescences of the tested hemp cultivars (Pętkowo 2018–2020).
3.5. Correlation of the content of essential oils in DM and FM of fluorescence with the weather conditions prevailing during the research period
Among the cultivars studied, only for the “Białobrzeskie” cultivar a beneficial effect of temperature on the content of essential oils in DM of inflorescences was proved, and the correlation coefficient was 0.7928 ().
Table 9. The correlation between the content of essential oils in dry matter and in fresh mass of inflorescences of the tested hemp cultivars and the average temperature during the vegetation period (Pętkowo 2018–2020).
Whereas no such relationship was found for the remaining cultivars. In turn, for the “Zolotonowska 13” cultivar, a negative effect of temperature on the concentration of essential oils in the FM of the raw material was observed, and the correlation coefficient was −0.7958. On the basis of the conducted studies, no other relationships in this regard were found.
For the two tested cultivars: “Zenit”’ and “Białobrzeskie,” the negative effect of the sum of precipitation during the vegetation period on the content of essential oils in the dry matter of the hemp inflorescences was shown and the correlation coefficients were, respectively: −0.6454 and −0.5385 ().
Table 10. Correlation between the content of essential oils in dry and fresh matter of the inflorescences of the tested hemp cultivars and the sum of precipitation during the growing season (Pętkowo 2018–2020).
The content of essential oils in the fresh mass of the hemp inflorescences of the studied cultivars did not depend on the sum of precipitation during the vegetation period, which is indicated by low values of correlation coefficients.
Here Table 10The correlation coefficients of the same cultivar, for the traits “dry matter” and “fresh mass” are radically different. The reason of this result might be the ratio of the oil content to the amount of fresh and dry tissue. At high temperature, plants have a lowered turgor, so the content of the dry mass, to the whole, fresh plant, is higher, so the values of the correlation coefficient are closer to one. Whether they are negative or positive values depends on whether there is a correlation (positive values) or not (negative values). So the cultivars with a high content of oils will have higher correlation values and with a plus sign (because such a correlation takes place). Those with low content will take values close to zero, or even negative, because there is no correlation (). Whereas in conditions of high humidity, plants accumulate water, the turgor is high, so the ratio of dry matter content to the total plant content is lower, i.e., the correlation will be opposite. As a result, in cultivar that produces a lot of oils, this relationship will decrease, because the amount of water in the plant will increase, and the relationship will take the opposite value (). For example, for the Białobrzeskie cultivar, which showed the highest content of oils, in the case of high temperatures, the content of oils for the whole plant will be particularly high – hence the positive correlation (). When the same cultivar has a high turgor, i.e., a high water content in the tissues, i.e., a changed ratio of water to dry matter components, the correlation is the opposite, i.e., a negative sign (). Conversely, for the Zolotonowska 13 variant, which gives the fewest oils, at high temperatures and low turbulence, the content of oils will be proportionally lower than in the Białobrzeskie variant, which means that the correlation values are lower and their orientation is negative, i.e., negative (). On the other hand, the Zolotonowska 13 cultivar, in conditions of high humidity and high turbulence, the correlation may be positive because the ratio of dry matter to fresh plant is not disturbed (). These dependencies may be intensified depending on how much water a given cultivar accumulates in the cells. The more water a plant accumulates in cells, the wider the range of results will be observed and the correlation values will be opposite.
A similar study but on essential oil extraction yield was conducted, where the statistically significant difference was found in percentage content between the genotypes studied, year of cultivation, and an interaction between genotype and year (Pieracci et al. Citation2021). Also, what is important, in another study, concerning Origanum compactum, in reference to an oil extraction results, a significant variations were highlighted between the two techniques used. It was reported that the microwave extraction showed its superiority for almost all chemical compounds, the percentage of total compounds, and the essential oil yield. In contrary, the Clevenger hydrodistillation resulted in the best scores of p-cymene, β-linalool, and α-thujene, absent in the microwave extraction (Zeroual et al. Citation2021a).
Conclusions
The “Białobrzeskie” cultivar, fully flowering, had the highest content of essential oils. The lowest content of essential oils, regardless of the harvest date, was found in “Zolotonowska 13.” Moreover, it was proved that the content of essential oils in the dry matter of inflorescences significantly depended on the cultivar and interaction of the cultivar with the harvest date. The content of essential oils in the fresh mass of hemp inflorescences increased significantly when the harvest was delayed from full flowering to full plant maturity. The “Zolotonowska 13” cultivar was characterized by the highest stability of the content of essential oils in the dry matter of inflorescences.
For the “Białobrzeskie” cultivar, as the average temperature of the vegetation period increased, the average content of essential oils in the dry matter of inflorescences increased as well.
Highlights
The total precipitation during the growing season had no effect on the content of essential oils in the fresh mass of the cannabis inflorescences.
The impact of two harvesting times, temperature, and precipitation on the content of essential oils in inflorescences of three hemp cultivars in fresh and dry plant matter was assessed.
It was shown that the content of essential oils in the dry matter of inflorescences significantly depended on the cultivar and interaction of the cultivar with the harvest date, which, together with various weather factors, modify (shape) these values.
The content of essential oils in the fresh mass of hemp inflorescences increased significantly when the harvest was delayed from full flowering to full plant maturity.
Ethical approval
We confirm that all the research meets ethical guidelines and adheres to the legal requirements of the study country. The research does not involve any human or animal welfare related issues.
Acknowledgments
Mr Grzegorz Oleszak for providing technical and substantive inputs, and MSc. Eng. Natalia Kryszak for providing technical support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abdollahi, M., F. Sefidkon, M. Calagari, A. Mousavi, and M. Fawzi Mahomoodally. 2020. Impact of four hemp (Cannabis sativa L.) varieties and stage of plant growth on yield and composition of essential oils. Industrial Crops and Products 155:112793. doi:10.1016/j.indcrop.2020.112793.
- Andre, C. M., J. Francois Hausman, and G. Guerriero. 2016. Cannabis Sativa: The plant of the thousand and one molecules. Frontiers in plant science 7 (FEB2016):1–17. doi:10.3389/fpls.2016.00019.
- Backer, D., B. D. Benjamin, P. Lebrun, L. Theunis, N. Dubois, L. Decock, A. Verstraete, P. Hubert, and C. Charlier. 2009. Innovative development and validation of an HPLC/DAD method for the qualitative and quantitative determination of major cannabinoids in cannabis plant material. Journal of Chromatography B 877 (32):4115–24. Elsevier. doi:10.1016/j.jchromb.2009.11.004.
- Baj, T., E. Sieniawska, R. Kowalski, M. Wesołowski, and B. Ulewicz-Magulska. 2015. Effectiveness of the deryng and Clevenger-type apparatus. Acta Poloniae Pharmaceutica - Drug Research 72 (3):507–15.
- Bakali, E., E. H. S. Ismail, A. Boutahar, M. Kadiri, and A. Merzouki. 2022. A comparative phytochemical profiling of essential oils isolated from three hemp (Cannabis sativa L.) cultivars grown in central-northern morocco. Biocatalysis and Agricultural Biotechnology 42 (November 2021):102327. Elsevier Ltd. doi:10.1016/j.bcab.2022.102327.
- Baldini, M., C. Ferfuia, B. Piani, A. Sepulcri, G. Dorigo, F. Zuliani, and C. Danuso. 2018. The performance and potentiality of monoecious hemp (Cannabis sativa L.) cultivars as a multipurpose crop. Agronomy 8:162. doi:10.3390/agronomy8090162.
- Bertoli, A., S. Tozzi, L. Pistelli, and L. G. Angelini. 2010. Fibre hemp inflorescences: From crop-residues to essential oil production. Industrial Crops and Products 32 (3):329–37. doi:10.1016/j.indcrop.2010.05.012.
- Bócsa, I., M. Karus, and D. Lohmeyer. 2000. Der Hanfanbau: Botanik, Sorten, Anbau Und Ernte, Märkte Und Produktlinien. Munster, Germany: Landwirtschaftsverlag GmbH.
- Burczyk, H., L. Grabowska, M. Strybe, and W. Konczewicz. 2009. Effect of sowing density and date of harvest on yields of industrial hemp. Journal of Natural Fibers 6 (2):204–18. Taylor & Francis. doi:10.1080/15440470902972588.
- Burczyk, H., R. Kaniewski, W. Konczewicz, N. Kryszak, and J. Turowski. 2011. Efficiency of hemp essentials oil depending on sowing density and time of inflorescence harvest. In Renewable resources and biotechnology for material application, materials science and technologies, ed. G. E. Zaikov, F. Pudel, and G. Spychalski, 31–39. New York: Nova Science Publishers, Inc.
- Cosentino, S. L., G. Testa, D. Scordia, and V. Copani. 2012. Sowing time and prediction of flowering of different hemp (Cannabis sativa L.) genotypes in Southern Europe. Industrial Crops and Products 37 (1):20–33. doi:10.1016/j.indcrop.2011.11.017.
- Deshmukh, P. D. 1979. Larval survival of diacrisia obliqua walker on several plant species. Indian Journal of Entomology 41 (1):5–12.
- Dorna, H., R. Kaniewski, M. Jarosz, J. Banach, and D. Szopińska. 2010. Health and germination of carrot seeds treated with aqueous extract from hemp (Cannabis sativa L.). Progress in Plant Protection 50 (1)):373–77. (Instytut Ochrony Roślin (Institute of Plant Protection)).
- Eržen, M., I. J. Košir, M. Ocvirk, S. Kreft, and A. Cerenak. 2021. Metabolomic analysis of cannabinoid and essential oil profiles in different hemp (Cannabis sativa L.) Phenotypes. Plants 10 (966):1–14. doi:10.3390/plants10050966.
- Ferfuia, C., F. Zuliani, F. Danuso, B. Piani, C. Cattivello, G. Dorigo, and M. Baldini. 2021. Performance and stability of different monoecious hemp cultivars in a multi-environments trial in North-Eastern Italy. Agronomy 11 (7):1–15. doi:10.3390/agronomy11071424.
- Fiorini, D., S. Scortichini, G. Bonacucina, N. G. Greco, E. Mazzara, R. Petrelli, J. Torresi, F. Maggi, and M. Cespi. 2020, March. Cannabidiol-enriched hemp essential oil obtained by an optimized microwave-assisted extraction using a central composite design. ( Elsevier) Industrial Crops and Products 154:112688. doi: 10.1016/j.indcrop.2020.112688.
- Frankowski, J., A. Wawro, J. Batog, and H. Burczyk. 2021. New polish oilseed hemp cultivar Henola – cultivation, properties and utilization for bioethanol production. Journal of Natural Fibers 0 (0):1–13. Taylor & Francis. doi:10.1080/15440478.2021.1944439.
- Gulluni, N., R. Tania, I. Loiacono, G. Lanzo, L. Gori, C. Macchi, F. Epifani, N. Bragazzi, and F. Firenzuoli. 2018. Cannabis essential oil: A preliminary study for the evaluation of the brain effects. In Evidence-Based complementary and alternative medicine: ECAM, 1–11. London: Hindawi Limited.
- Hillig, K. W. 2004. A chemotaxonomic analysis of terpenoid variation in cannabis. Biochemical Systematics and Ecology 32 (10):875–91. doi:10.1016/j.bse.2004.04.004.
- Ibourki, M., S. Gharby, D. Guillaume, E. Hassan Sakar, A. Laknifli, A. El Hammadi, and Z. Charrouf. 2021. Profiling of mineral elements and heavy metals in argan leaves and fruit by-products using inductively coupled plasma optical emission spectrometry and atomic absorption spectrometry. Chemical Data Collections 35 (August):100772. Elsevier B.V. doi:10.1016/j.cdc.2021.100772.
- Johannes, N., K. Zitterl-Eglseer, S. G. Deans, and C. M. Franz. 2001. Essential oils of different cultivars of Cannabis sativa L. and their antimicrobial activity. Flavour and fragrance journal 16 (4):259–62. doi:10.1002/ffj.993.
- Kaniewski, R., D. Kalemba, L. Ciechonski, and I. Pniewska. 2015. Składniki olejku eterycznego z konopi siewnych Cannabis sativa L. Len i Konopie.Biuletyn Informacyjny Polskiej Izby Lnu i Konopi 25: 34–38.
- Kaniewski, R., W. Konczewicz, and W. Cierpucha. 2000. New trends in harvesting processing and utilizing of hemp. Włókna Naturalne: Natural Fibres XLIV:77–93.
- Kaniewski, R., I. Pniewska, A. Kubacki, M. Strzelczyk, M. Chudy, and G. Oleszak. 2017. Cannabis (Cannabis sativa L.) - valuable plant useful and medicinal. Postępy Fitoterapii 2:139–44. doi:10.25121/PF.2017.16.2.139.
- Kędzia, B., E. Hołderna-Kędzia, R. Kaniewski, and M. Szarata. 2014a. Investigation on antibiotic activity of native hemp essential oil. Postępy Fitoterapii 3:140–43.
- Kędzia, A., M. Ziółkowska-Klinkosz, B. Kochańska, A. W. Kędzia, and A. Gębska. 2014b. Evaluation of activity essential oil from Cannabis sativa L. against anaerobic bacteria. Postępy Fitoterapii 3:136–40.
- Khan, B. A., P. Warner, and H. Wang. 2014. Antibacterial properties of hemp and other natural fibre plants: A review. BioResources 9 (2):3642–59. doi:10.15376/biores.9.2.Khan.
- Knezevic, F., A. Nikolai, R. Marchart, S. Sosa, A. Tubaro, and J. Novak. 2021. Residues of herbal hemp leaf teas–how much of the cannabinoids remain? Food Control 127: Elsevier:108146. doi: 10.1016/j.foodcont.2021.108146.
- Lekavicius, V., P. Shipkovs, S. Ivanovs, and A. Rucins. 2015. Thermo-insulation properties of hemp-based products. Latvian Journal of Physics and Technical Sciences 52 (1):38–51. doi:10.1515/lpts-2015-0004.
- Markowska, J., E. Polak, A. Drabent, and A. Żak. 2021. Hemp Cannabis sativa L. – types, properties, uses. Zywnosc. Nauka. Technologia. Jakosc/Food. Science Technology. Quality 28 (2):90–105. doi:10.15193/ZNTJ/2021/127/380.
- Martins, A. M., A. L. Gomes, I. Vilas Boas, J. Marto, and H. M. Ribeiro. 2022. Cannabis-based products for the treatment of skin inflammatory diseases: A timely review. Pharmaceuticals 15 (2):210. doi:10.3390/ph15020210.
- McPartland, J. M. 1997. Cannabis as repellent and pesticide. Journal of the International Hemp Association 4 (2):87–92.
- McPartland, J. M., R. Connell Clarke, and D. Paul Watson. 2000. Hemp diseases and pests: Management and biological control: An advanced treatise. Wallingford: CABI.
- Mediavilla, V., and S. Steinemann. 1997. Essential oil of Cannabis sativa L. strains. Journal of International Hemp Association 4:80–82.
- Mehdizadeh, L., and M. Moghaddam. 2018. Essential oils: Biological activity and therapeutic potential. Therapeutic, Probiotic, and Unconventional Foods (January):167–79. doi:10.1016/B978-0-12-814625-5.00010-8.
- Meier, C., and V. Mediavilla. 1998. Factors influencing the yield and the quality of hemp (Cannabis sativa L.) essential oil. Journal of International Hemp Association 5 (1):16–20.
- Mishchenko, S., J. Mokher, I. Laiko, N. Burbulis, H. Kyrychenko, and S. Dudukova. 2017. Phenological growth stages of hemp (Cannabis sativa L.): Codification and description according to the BBCH scale. Žemės Ūkio Mokslai 24 (2). doi:10.6001/zemesukiomokslai.v24i2.3496.
- Mojumdar, V., S. D. Mishra, M. M. Haque, and B. K. Goswami. 1989. Nematicidal efficacy of some wild plants against pigeon pea cyst nematode, heterodera cajani. International Nematology Network Newsletter 6 (2):21–24.
- Nagy, D. U., K. Cianfaglione, F. Maggi, S. Sut, and S. Dall’Acqua. 2019. Chemical characterization of leaves, male and female flowers from spontaneous Cannabis (Cannabis sativa L.) growing in Hungary. Chemistry & Biodiversity 16:3. doi:10.1002/cbdv.201800562.
- Nissen, L., A. Zatta, I. Stefanini, S. Grandi, B. Sgorbati, B. Biavati, and A. Monti. 2010. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 81 (5):413–19. Elsevier. doi:10.1016/j.fitote.2009.11.010.
- Ovidi, E., V. Laghezza Masci, A. Rita Taddei, J. Torresi, W. Tomassi, M. Iannone, A. Tiezzi, F. Maggi, and S. Garzoli. 2022. Hemp (Cannabis sativa L., Kompolti Cv.) and Hop (Humulus Lupulus L., Chinook Cv.) essential oil and hydrolate: HS-GC-MS chemical investigation and apoptotic activity evaluation. Pharmaceuticals 15 (8):976. doi:10.3390/ph15080976.
- Pate, D. W. 1999. Hemp seed: A valuable food source. In Advances in Hemp Research edited by P. Ranalli, 243–55. Binghamton, NY: The Haworth Press, Inc.
- Pieracci, Y., R. Ascrizzi, V. Terreni, L. Pistelli, G. Flamini, L. Bassolino, F. Fulvio, M. Montanari, and R. Paris. 2021. Essential oil of Cannabis sativa L.: Comparison of yield and chemical composition of 11 hemp genotypes. Molecules 26 (13):1–22. doi:10.3390/molecules26134080.
- Polish Pharmacopoeia VI. 2002. Warsaw.
- Ross, S. A., and M. A. ElSohly. 1996. The volatile oil composition of fresh and air-dried buds of Cannabis sativa. Journal of Natural Products 59 (1):49–51. ACS Publications. doi:10.1021/np960004a.
- Russo, E., and G. W. Guy. 2006. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Medical Hypotheses 66 (2):234–46. Elsevier. doi:10.1016/j.mehy.2005.08.026.
- Russo, E. B., and T. H. C. Taming. 2011. Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. British Journal of Pharmacology 163 (7):1476–5381. doi:10.1111/j.1476-5381.2011.01238.x.
- Sengloung, T., L. Kaveeta, and W. Nanakorn. 2009. Effect of sowing date on growth and development of Thai hemp (Cannabis sativa L.). Kasetsart Journal Natural Sciences 43:423–31.
- Stasiłowicz, A., A. Tomala, I. Podolak, and J. Cielecka-Piontek. 2021. Cannabis sativa L. As a natural drug meeting the criteria of a multitarget approach to treatment. International Journal of Molecular Sciences 22 (2):1–31. doi:10.3390/ijms22020778.
- Struik, P. C., S. Amaducci, M. J. Bullard, N. C. Stutterheim, G. Venturi, and H. T. H. Cromack. 2000. Agronomy of fibre hemp (Cannabis sativa L.) in Europe. Industrial Crops and Products 11 (2–3):107–18. doi:10.1016/S0926-6690(99)00048-5.
- Strzelczyk, M., M. Lochynska, and M. Chudy. 2021. Systematics and botanical characteristics of industrial hemp Cannabis sativa L. Journal of Natural Fibers 19:1–23. Taylor & Francis. doi:10.1080/15440478.2021.1889443.
- Truta, E., S. Surdu, C. Maria Rosu, and M. Asaftei. 2009. Hemp-biochemical diversity and multiple uses. Journal of Experimental and Molecular Biology 10(2):1–8. Universitatea “Alexandru Ioan Cuza”.
- Vitorović, J., N. Joković, N. Radulović, T. Mihajilov-Krstev, V. J. Cvetković, N. Jovanović, T. Mitrović, A. Aleksić, N. Stanković, and N. Bernstein. 2021. Antioxidant activity of hemp (Cannabis sativa L.) seed oil in Drosophila Melanogaster larvae under non-stress and H2O2-induced oxidative stress conditions. Antioxidants 10 (6):830. doi:10.3390/antiox10060830.
- Vuerich, M., C. Ferfuia, F. Zuliani, B. Piani, A. Sepulcri, and M. Baldini. 2019. Yield and quality of essential oils in hemp varieties in different environments. Agronomy 9 (7). doi: 10.3390/agronomy9070356.
- Zeroual, A., E. Hassan Sakar, N. Eloutassi, F. Mahjoubi, M. Chaouch, and A. Chaqroune. 2021a. Phytochemical profiling of essential oils isolated using hydrodistillation and microwave methods and characterization of some nutrients in origanum compactum benth from Central-Northern Morocco. Biointerface Research in Applied Chemistry 11 (2):9358–71. doi:10.33263/BRIAC112.93589371.
- Zeroual, A., E. Hassan Sakar, N. Eloutassi, F. Mahjoubi, M. Chaouch, and A. Chaqroune. 2021b. Wild chamomile [Cladanthus Mixtus (L.) Chevall.] collected from central-northern Morocco: Phytochemical profiling, antioxidant, and antimicrobial activities. Biointerface Research in Applied Chemistry 11 (4):11440–57. doi:10.33263/BRIAC114.1144011457.
