ABSTRACT
Waterlogging due to excessive rainfall has become a factor limiting flax production in southern China. This has led to morphology alteration, and biomass and yield reduction in field crop production. Flax variety Zhongyama 1 was planted in sand culture, and the adaptive mechanisms of the responses to waterlogging in the fast growth and harvest stage were determined. According to the results, height and total biomass decreased significantly under waterlogged conditions during the fast growth period; furthermore, in the harvest stage, flax height increased significantly, whereas the technical length, fork diameter, and weight decreased significantly. For gene expression, the gene of glucose, glutathione metabolism, was up-regulated in shoot, and the gene of phenylpropanoid metabolic, lignin, was up-regulated in root. The results suggested that the synthesis and degradation of lignin is involved in flax resistance to waterlogging, especially in terms of phenylpropanoid biosynthesis, glycolysis, and metabolism of plant hormone signal transduction. Furthermore, unpaired electrons flowing through the electron transport chain may react with oxygen to produce ROS and hamper plant growth, development, and survival.
摘要
降雨过多导致的水涝已成为限制中国南方亚麻生产的一个重要因素。它导致了在亚麻田间生产中的形态变化, 以及生物量、产量下降。本研究通过砂培试验, 研究了中亚麻1号在快速生长期和收获期对水涝的适应性机制。研究发现, 水涝导致亚麻株高和总生物量在快速生长期显著下降; 亚麻株高在收获期显著增加, 而工艺长度、茎粗和生物量显著下降。在基因表达方面, 水涝处理下葡萄糖、谷胱甘肽代谢基因在地上部的表达量显著上调, 苯丙素代谢基因、木质素基因在根部的表达量显著上调。本研究的结果表明, 木质素的合成和降解与亚麻的耐涝性有关, 尤其是在苯丙烷生物合成、糖酵解和植物激素信号转导。此外, 流经电子传输链的未配对电子可能与氧气反应产生活性氧 (ROS), 从而阻碍亚麻的生长、发育和存活。
Introduction
Waterlogging is a major natural hazard in the agricultural industry (Li et al. Citation2022). According to estimations by the Food and Agriculture Organization (FAO) of the United Nations and the International Union of Soil Sciences (IUSS), cultivated land under frequent flooding stress accounts for approximately 12% of the total cultivated land area globally, which is associated with an approximately 20% reduction in crop yields (Ahmed et al. Citation2002). China is a largely agricultural country that experiences frequent flooding and natural disasters (Qi and Zhang Citation2022). According to the Flood Control and Drought Relief Office (2018), flood disasters in 2017 affected 51,964.70 km2 of various crops in China; the affected land area was 27,811.90 km2. Furthermore, the yield of seed crops has been reduced by 29.97 m tons due to flood disasters (Xiao Citation2018). Therefore, it is necessary to improve plant resistance to waterlogging (Bhusal et al. Citation2022; Wang et al. Citation2017).
The effects of waterlogging stress first appear in the root (Nielsen, Lynch, and Weiss Citation1997). As one of the primary adaptations to waterlogging stress, adventitious roots can instantly replace the outermost cells that die from hypoxia (Li et al. Citation2021). Adventitious root tip cells have a high capacity for cell division and physiological activity, markedly improving the porosity of internal root tissue and O2 uptake and transport (Bispo and Vieira Citation2022). It is conducive to the nutrition fixation of plants in soil under flooding stress (Arslan Ashraf Citation2012; Lin et al. Citation2019). Furthermore, signal transmission plays a crucial role in water-logging resistance (Na et al. Citation2018).
Interactions among ABA, GA3, IAA (Ullah et al. Citation2019), and other hormones are directly related to the response of plant morphology to waterlogging. Waterlogged conditions significantly alter physiological and biochemical activities in plants, leading to considerable changes in plant growth and morphogenesis (Liu et al. Citation2018). Under waterlogging stress, the development of new plant leaves in the shoot is inhibited, and symptoms such as leaf wilting, rollback, drooping, and leaf surface lesions emerge, with leaf abscission exacerbated. Furthermore, long-term flooding stress has been reported to damage the chloroplast ultrastructure and light system II reaction center, reduce light energy conversion efficiency and leaf photosynthesis, and to severely affect the plant growth and development (Li et al. Citation2021). However, little research has been conducted on flax flooding stress, particularly in the context of waterlogging.
Southern China provides large tracts of land for the growth of flax because of the large number of winter fallow fields (Liu et al. Citation2018). However, excessive rainfall can result in stagnant farmland water (Shuqiao Citation2020), which severely affects flax yield and quality (Li et al. Citation2018). Flax (Linum usitatissimum L.) is a Linum I, Linaceae herbaceous dicotyledonous plant (Bjelková, Genčurová, and Griga Citation2011). Also known the “queen of fiber,” it is the earliest natural plant fiber used by humans and has a long history of cultivation. Flax farming originated in Ethiopia, the Mediterranean Basin, the Middle East, India, Central Asia, and China (Guo et al. Citation2020). Based on its usage, flax can be classified into fiber, oil, and oil fiber. Flax is used extensively in industry, medicine, and textiles, as it is naturally produced (Guo et al. Citation2020). The material is soft and has environmental protection, wear resistance, moisture resistance, and comfort properties (Rozhmina et al. Citation2022). Flax can be used as a raw material for coatings, floors, and synthetic rubbers. And the oil of flax is rich in the human essential acids α-linolenic acid and γ-linolenic acid, and is both nutritious and edible (Goyal et al. Citation2014; Gao et al. Citation2017).
Waterlogging has become the main limiting factor of flax production in southern China. A previous study revealed that waterlogging stress affects the economic characteristics of flax, especially, plant height; under waterlogging conditions, the technical length of the plant is reduced, and peroxidase (POD) activity increases at first and then decreases (Zhang et al. Citation2007). Despite what is known about the impact of waterlogging on flax growth, only a few studies have investigated how it impacts lignin development. Lignin development is the limiting factor for fiber quality and growth; however, the relationship between waterlogging resistance and lignin development remains unclear. The present study used transcriptomics and quantitative RT-PCR to study the physiological responses of flax to waterlogged conditions and thereby elucidate the mechanisms underlying the resistance to waterlogging stress. The present study demonstrates a relationship between waterlogging and lignin development.
Materials and methods
Plant material and growth conditions
The experiment was conducted in an open-ventilated greenhouse under natural light and ambient temperature and relative humidity in Southern China (28°12′56″ N, 112°53′49″ E). The experimental site has a moist subtropical monsoon climate with an annual average temperature of 17.2°C, and an annual average rainfall of 1361.6 mm. Previous studies have shown that the flax variety Zhongyama 1 is well adapted for Southern China; therefore, Zhongyama 1 was selected in the present study (Guo et al. Citation2020; Xin et al. Citation2014). The seeds were grown in plastic pots (15 cm × 30 cm) with sand culture, and watered uniformly with Hoagland’s nutrient solution. They were either subjected to waterlogging (experimental group, with the height of water close to the stem). In order to maintain the treatment of waterlogging, check water level and promptly supply with distilled water daily) or grow normally (CK group) during their fast-growth period (almost 40 d after seedling). The flax plants were sampled after 2 weeks or at the harvest stage (Wu et al. Citation2019).
Analysis of physiological characteristics
During the fast-growth period (almost 40 d after seedling), flax height was measured using a graduated scale. For total biomass, all samples were weighed after cleaning and drying firstly. Whole plants were oven-dried to a constant weight by at 105°C for 30 min followed by slow drying at 70°C and then weighed again to determine dry weight. In the harvest stage, flax height was measured using a graduated scale again, and the length between the cotyledon scar and the first upper branch was measured as technical length. Whole plants were oven-dried to a constant weight at 105°C for 30 min followed by slow drying at 70°C and then weighed to determine dry weight. System diameter was measured using a Vernier caliper. The analysis was performed using six replicates (Smykalova et al. Citation2010; Wu et al. Citation2019).
SPAD measurements
Chlorophyll content was determined using a SPAD-502 leaf chlorophyll meter (Minolta Camera Co., Ltd., Osaka, Japan). The analysis was performed using six replicates (Clement et al. Citation2018).
RNA extraction and sequencing
After 1 h of waterlogging, roots and shoots were sampled. Samples were washed with distilled water, dried with filter paper first, flash frozen in liquid N2, and stored in 1.5 mL tubes at −80°C until use. RNA was extracted from flax during the fast-growth period using TRIzol reagent (Invitrogen, Waltham, MA, USA). cDNA was synthesized using M-MLV reverse transcriptase (Promega Corporation, Madison, WI, USA), according to the manufacturer’s instructions.
Transcriptome assays were performed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). A total of 1 μg RNA per sample was used as the input material (Chang et al. Citation2020). Sequencing libraries were generated using the NEBNext® UltraTM RNA Library Prep Kit for Illumina® (New England Biolabs, Inc., Ipswich, MA, USA) according to the manufacturer’s instructions, and index codes were added to attribute sequences to each sample (Hua et al. Citation2018).
After the library was built, Qubit 2.0 was used for preliminary quantitative analysis. The library was diluted to 1 ng/μL, and an Agilent 2100 Bioanalyzer was used to detect the insert size of the library. Q-PCR was used to detect the effective concentration of the library (effective concentration of library N > 2 nM) to ensure library quality (Guo et al. Citation2017).
Reads mapping to the reference genome and quantification of gene expression level
All clean reads were aligned to the flax genome database. The annotations of flax genes were downloaded from the flax genome website (http://www.phytozome.org/flax.php) directly. Index of the reference genome was built using Bowtie v2.0.6 and paired-end clean reads were aligned to the reference genome using TopHat v2. HTSeq v0.5.3 was used to count the reads numbers mapped to each gene. Then, RPKM (reads per kilobase of exon model per million mapped reads) of each gene was calculated based on the length of the gene and read counts mapped to the gene (Guo et al. Citation2021).
Differential expression level analysis and functional annotation of differentially expressed genes (DEGs)
Differential expression analysis of four groups (SCK -vs- RCK, SWL -vs- RWL, RCK -vs- RWL, SCK -vs- SWL) was performed using the DESeq R package (1.18.0). Gene Ontology (GO) enrichment analysis of differentially expressed genes (DEGs) was implemented using the GOseq R package, in which gene length bias was corrected. GO terms with corrected P-value < 0.05 were considered significantly enriched by differentially expressed genes. KOBAS software was used to test the statistical enrichment of differential expression genes in the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of DEGs (Guo et al. Citation2021).
Quantitative RT-PCR
Relative gene expression in plant roots was determined via a qRT-PCR run on a LightCycler instrument (Roche Molecular Systems, Inc., Branchburg, NJ, USA) using the SYBR Green Real-Time PCR Master Mix Kit (TOYOBO Co., Ltd., Osaka, Japan) under the following conditions: 95°C for 2 min, followed by 45 cycles of 95°C for 10 s, 60°C for 10 s, and 72°C for 20 s. Gene primer sequences were designed using Primer Premier (ver 6.0, PREMIER Biosoft, San Francisco, CA, USA). Relative gene expression was calculated using the 2−△△Ct method (Livak and Schmittgen Citation2001; Zhang et al. Citation2018). β-tubulin was used as a reference gene (Huang, Lee, and Tai Citation2009). The analysis was performed using six replicates.
△Ct = (mean CtTarget − mean Ctβ-tubulin)
△△Ct = (△CtTarget − △Ctβ-tubulin)
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 22 (IBM Corp., Armonk, NY, USA). Data were compared using a two-way Analysis of Variance (ANOVA) and Tukey’s HSD post-hoc test at a significance level of p < 0.05. Data are presented as the arithmetic mean ± standard error of the mean of the indicated replicates.
Results
Waterlogging affected leaf blade yellowing and flax growth
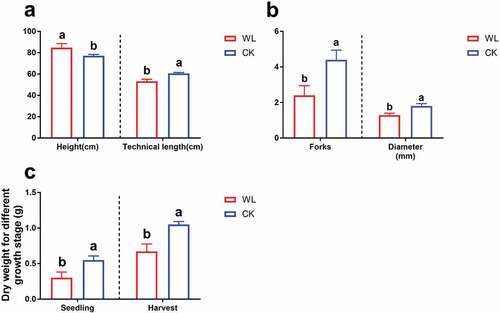
To study the mechanism of resistance to waterlogging in flax, we first investigated the characteristics of flax during the fast-growth period. The SPAD decreased significantly with an increase in waterlogging (). The height, total biomass, and SPAD of the waterlogged plants were significantly lower than those of the CK group ().
Figure 1. The phenotype for flax in fast growth period under water logging condition. (a) Phenotype. (b) SPAD. (c) Height. (d) Total biomass for per plant.
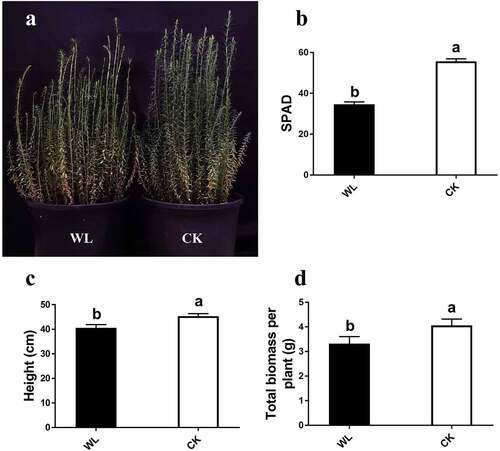
In the harvest stage, the height of the waterlogged plants was significantly higher than that of the controls. Technical length and fork diameter were significantly lower under waterlogged conditions (). Subsequently, we analyzed the total biomass in the seedling and harvest stages; similar trends were shown in the different growth stages, and the difference between the two increased gradually with plant growth (). The results suggested that waterlogging inhibited flax growth significantly.
Sequencing and mapping of differentially expressed genes
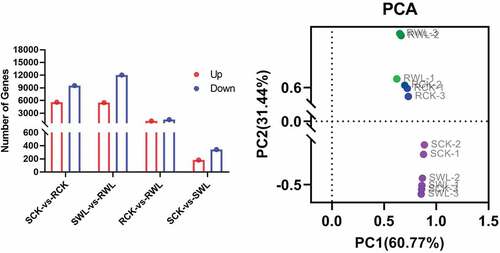
We performed transcriptome assays and found more than 47.9–60.6 million reads from the 12 libraries, out of which more than 72.20% of the genes were known (Fig. S1a). Correlation analysis showed that the correlation between genes found in SWL (shoot, waterlogging conditions) and SCK (shoot, normal conditions) was closer than that between genes found in RWL (root, waterlogging condition) and RCK (root, normal condition) (Fig. S1b).
Genes that showed significant differences in expression were identified between pairwise comparisons of SCK vs. RCK, SWL vs. RWL, RCK vs. RWL, and SCK vs. SWL. For SCK vs. RCK, 5594 upregulated and 9527 downregulated differentially expressed genes (DEGs) were identified. For SWL vs. RWL, 5499 upregulated genes and 12,037 downregulated DEGs were identified. For RCK vs. RWL, 1177 upregulated genes and 1485 downregulated DEGs were identified. For SCK vs. SWL, 183 upregulated genes and 343 downregulated DEGs were identified (). The data suggest that RCK vs. RWL had significant differences in gene expression compared to SCK vs. RCK ().
Gene Ontology and metabolic pathway enrichment analysis of differentially expressed genes
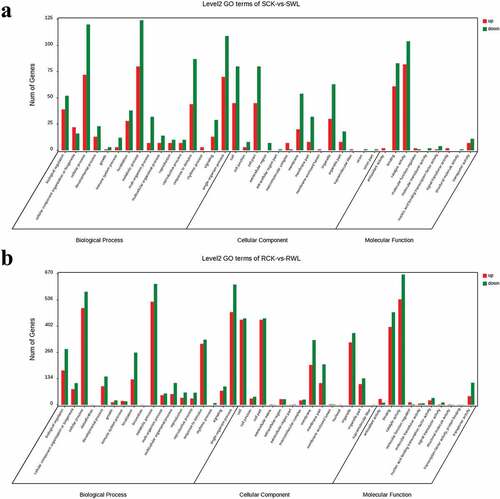
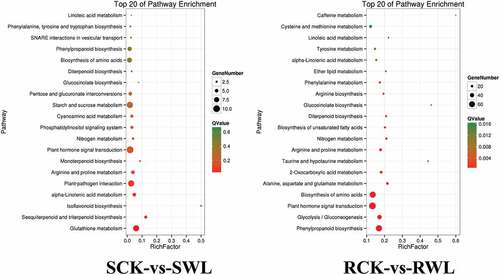
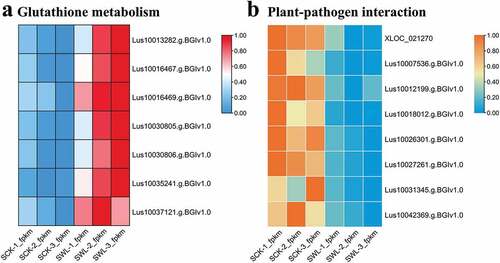
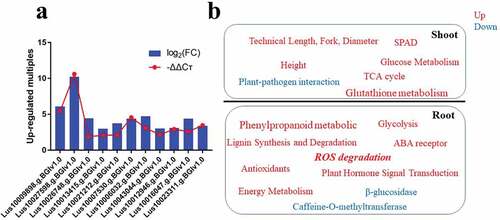
The top 30 significantly enriched categories for the DEGs are shown in . In the shoot, more DEGs were enriched in the biological process than in the other two ontologies, particularly in cellular, metabolic, and single-organism processes (). In the root, fewer DEGs were enriched in molecular function than in the other two ontologies. Cellular, metabolic, and single-organism processes were the most enriched DEGs among the ontologies of biological processes. For the ontologies of cellular components, cells, cell parts, and organelles were enriched in most DEGs (). We screened different genes using KEGG enrichment analysis. The terms “Glutathione metabolism” and “Plant–pathogen interaction” were significantly enriched in the shoot tissue in the SCK vs. SWL group. In roots, “Phenylpropanoid biosynthesis,” “Plant hormone signal transduction,” and “Biosynthesis of amino acids” were significantly enriched in the RCK vs. RWL group, especially “Phenylpropanoid biosynthesis” ().
Differentially expressed genes are involved in shoot resistance to waterlogging
Based on the KEGG enrichment analysis, we further studied the terms “Glutathione metabolism” and “Plant–pathogen interaction.” Under waterlogged conditions, genes related to glutathione metabolism, including Lus10013282.g.BGIv1.0, Lus10016467.g.BGIv1.0, Lus10016469.g.BGIv1.0, Lus10030805.g.BGIv1.0, Lus10030806.g.BGIv1.0, Lus10035241.g.BGIv1.0, and Lus10037121.g.BGIv1.0, were highly expressed (). The FPKM of some genes involved in plant–pathogen interactions, such as XLOC_021270, Lus10007536.g.BGIv1.0, Lus10012199.g.BGIv1.0, Lus10018012.g.BGIv1.0, Lus10026301.g.BGIv1.0, Lus10027261.g.BGIv1.0, Lus10031345.g.BGIv1.0, and Lus10042369.g.BGIv1.0, were downregulated under waterlogged conditions ().
Differentially expressed genes are involved in root resistance to waterlogging
We studied the differentially expressed genes in the RWL and RCK groups. In the root, we studied the genes involved in the term “Phenylpropanoid biosynthesis.” Under waterlogged conditions, the FPKM of phenylpropanoid biosynthesis-related genes, such as Lus10009898.g.BGIv1.0, was higher than that in the CK group (). We studied all phenylpropanoid biosynthesis genes and found 21 genes that were significantly upregulated under waterlogged conditions. Under the term “ethylene receptor,” Lus10013415.g.BGIv1.0 and Lus10021212.g.BGIv1.0 were significantly upregulated under waterlogging conditions.
Figure 7. The FPKM of phenylpropanoid biosynthesis in root under water-logging conditions. (a) 21 up-regulated genes. (b) Most significantly up-regulated genes.
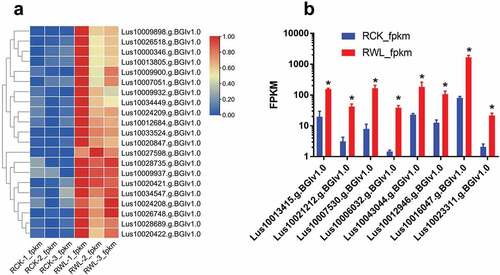
Next, we compared the expression of the lignin-forming anionic peroxidase (Lus10009898.g.BGIv1.0) (Dasgupta et al. Citation2014), trans-cinnamate 4-monooxygenase-like (Lus10027598.g.BGIv1.0) (Liu et al. Citation2017), peroxidase superfamily protein-related (Lus10026748.g.BGIv1.0) (Brigelius-Flohé and Flohé Citation2020), ethylene receptor-related (Lus10013415.g.BGIv1.0 and Lus10021212.g.BGIv1.0) (Bakshi et al. Citation2018), abscisic acid receptor PYL4-like (Lus10007530.g.BGIv1.0) (Belda-Palazon et al. Citation2016), 6-phosphofructokinase-related (Lus10006032.g.BGIv1.0 and Lus10043044.g.BGIv1.0) (Frese et al. Citation2014), hexokinase-related (Lus10012946.g.BGIv1.0) (Wolf et al. Citation2016), phosphofructokinase alpha subunit-related (Lus10016047.g.BGIv1.0), and gibberellin 2-oxidase-related (Lus10023311.g.BGIv1.0) genes between the waterlogged and CK plants (Wang et al. Citation2021; Yuan et al. Citation1997). This study showed that the transcription of the 11 genes was consistent with the RT-PCR results (). Therefore, this study suggests that the up-regulation of TCA cycle, glutathione metabolism, and glucose metabolism, and the down-regulation of plant–pathogen interaction jointly promoted the technical length, fork diameter, and height growth in flax shoots, under waterlogged conditions. In the root, the up-regulation of phenylpropanoid metabolic, lignin synthesis and degradation, glycolysis, ABA receptor, antioxidants, energy metabolism, and plant hormone signal transduction, and the down-regulation of β-glucosidase and caffeine-O-methyltransferase jointly promoted resistance to waterlogging stress. Aerenchyma formation in flax roots is a synchronous process of lignin synthesis and degradation under waterlogged conditions ().
Discussion
Waterlogging inhibited flax growth
We first studied the plant phenotype to explore the mechanism of resistance to waterlogged conditions in flax. Under waterlogged conditions, the SPAD was significantly lower than in the CK group (Lee et al. Citation2021). Waterlogging may impede the root’s nutrient absorption function and adversely impact the shoot’s nutrient supply (Dixon et al. Citation2019). Under waterlogged conditions, flax height was significantly lower than in CK, leading to lower total biomass during the fast-growth period () (Raineri et al. Citation2022).
A greater height is better for obtaining energy from sunlight and provides more organic matter to roots, helping to ameliorate the effects of waterlogging (Arslan Ashraf Citation2012). At the harvest stage, under waterlogged conditions, technical length, fork and stem diameter, and weight were significantly lower than in CK (Li et al. Citation2022). However, the height under waterlogged conditions was significantly higher than in CK ().
Waterlogging promotes flax shoot metabolism
In the present study, the FPKM of the genes involved in glutathione metabolism, including Lus10013282.g.BGIv1.0, Lus10016467.g.BGIv1.0, Lus10016469.g.BGIv1.0, Lus10030805.g.BGIv1.0, Lus10030806.g.BGIv1.0, Lus10035241.g.BGIv1.0, and Lus10037121.g.BGIv1.0, were highly expressed under waterlogged conditions (). Shoots are the main factories for nutrition synthesis and play a key role in resistance to waterlogging (Poitout et al. Citation2018). Glutathione, an important intracellular regulatory metabolite, is not only a coenzyme of glyceraldehyde phosphate dehydrogenase but also a coenzyme of glyoxalase and pyruvate dehydrogenase (Bahmani et al. Citation2019). It participates in the tricarboxylic acid cycle and glucose metabolism in vivo. It can activate various enzymes, such as thiol (SH) enzyme-coenzymes, to promote carbohydrate, fat, and protein metabolism (Cyle et al. Citation2020). Meanwhile, genes involved in “plant–pathogen interaction” were significantly downregulated under waterlogging conditions, which may reduce energy loss (Ke et al. Citation2019), promote more efficient metabolism under waterlogged conditions, reduce the harmful effect of energy capture deficiency due to SPAD and height decrease.
Waterlogging enhances lignin synthesis and degradation efficiency in the flax root
The genes involved in “plant hormone signal transduction” were significantly enriched in the RCK group compared to those in the RWL group (). A total of 21 genes involved in phenylpropanoid biosynthesis were significantly upregulated under waterlogged conditions (). Reactive oxygen species, as free radicals, possess one or more unpaired electrons; the unpaired electrons are not in a stable configuration. These radicals react with other cellular molecules to produce more free radicals (Arslan Ashraf Citation2012). The results indicate that the synthesis and degradation of lignin play a key role in waterlogging resistance and ROS degradation, especially in terms of phenylpropanoid biosynthesis, glycolysis, and plant hormone signal transduction metabolism.
The root is an organ in direct contact with water (Pang et al. Citation2021). Being the central organ for nutrition absorption, morphosis, signing, and developmental capacity, its play vital roles in adaptation to waterlogging (Bispo and Vieira Citation2022). Although oxygen is very important for plants on earth, its reduction due to any reason could result in the generation of ROS (reactive oxygen species) and disrupt cellular metabolic processes in plants (Arslan Ashraf Citation2012). Phenylpropane metabolism is an essential secondary metabolic pathway in plants (Wu et al. Citation2019; Xun et al. Citation2020). Metabolites, such as lignin, pollen, anthocyanin, and organic acids, play a crucial role in regulating plant adaptive growth (Rao et al. Citation2018). Phenylpropanoid metabolism in medicinal plants is associated with the synthesis of numerous medicinally active ingredients (Wu et al. Citation2019). Almost all natural active ingredients containing phenylpropanoid skeletons, such as flavonoids, terpenes, and phenols, are directly or indirectly synthesized by the phenylpropanoid metabolic pathways (Xun et al. Citation2020). A few secondary metabolites produced by phenylpropanoid metabolic pathways can also be secreted from plant roots into the surrounding soil, thereby affecting plant growth and resistance to biotic or abiotic stress by altering the ecology of root microbes (Xiong et al. Citation2017). Thus, phenylpropane metabolism may be involved in root development.
The expression of genes related to lignin synthesis increased significantly under waterlogged conditions, and the expression of β-glucosidase and caffeine-O-methyltransferase decreased considerably. The expression of lignin peroxidase and other degradation enzymes was significantly upregulated, and that of trans-cinnamate 4-monooxygenase (Liu et al. Citation2017), which is related to lignin synthesis, was also upregulated. Hormone homeostasis plays a key role in the adaptation of plants to waterlogging stress (Yu et al. Citation2019).
Conclusions
As a natural hazard for the flax industry, waterlogging has become the factor limiting lignin development in South China. This study revealed that synthesis and degradation of lignin may reduce the harmful effects of waterlogging, and unpaired electrons flowing through the electron transport chain may react with oxygen to produce ROS and hamper plant growth, development, and survival. However, the connection between lignin and ROS degradation needs to be explored in the future to elucidate further the underlying mechanisms of the responses of flax to waterlogging.
Highlights
Waterlogging markedly inhibited the growth of flax.
Differentially expressed genes in flax are involved in shoot and root resistance to waterlogging.
Waterlogging enhanced lignin synthesis and degradation efficiency in the flax root.
Lignin synthesis and degradation may affect the resistance of flax to waterlogging, especially by regulating phenylpropanoid biosynthesis, glycolysis, and plant hormone metabolism.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahmed, S., E. Nawata, M. Hosokawa, Y. Domae, and T. Sakuratani. 2002. Alterations in photosynthesis and some antioxidant enzymatic activities of mungbean subjected to waterlogging. Plant Science 163 (1):117–14. doi:10.1016/S0168-9452(02)00080-8.
- Arslan Ashraf, M. 2012. Waterlogging stress in plants: A review. African Journal of Agricultural Reseearch 7 (13):1976–81. doi:10.5897/ajarx11.084.
- Bahmani, R., D. Gwan Kim, J. Na, and S. Hwang. 2019. Expression of the tobacco non-symbiotic class 1 hemoglobin gene Hb1 reduces cadmium levels by modulating Cd transporter expression through decreasing nitric oxide and ROS level in arabidopsis. Frontiers in Plant Science 10 (February):1–19. doi:10.3389/fpls.2019.00201.
- Bakshi, A., S. Piy, J. C. Fernandez, C. Chervin, T. Hewezi, and B. M. Binder. 2018. Pethylene receptors signal via a noncanonical pathway to regulate abscisic acid responses. Plant Physiology 176 (1):910–29. doi:10.1104/pp.17.01321.
- Belda-Palazon, B., L. Rodriguez, M. A. Fernandez, M. C. Cruz Castillo, E. M. Anderson, C. Gao, M. Gonzalez-Guzman, M. Peirats-Llobet, Q. Zhao, N. De Winne, et al. 2016. FYVE1/FREE1 interacts with the PYL4 ABA receptor and mediates its delivery to the vacuolar degradation pathway. The Plant Cell 28 (9):2291–311. doi:10.1105/tpc.16.00178.
- Bhusal, N., A. Adhikari, M. Lee, A. Han, A. R. Reum Han, and H. S. Kim. 2022. Evaluation of growth responses of six gymnosperm species under long-term excessive irrigation and traits determining species resistance to waterlogging. Agricultural and Forest Meteorology 323 (June):109071. doi:10.1016/j.agrformet.2022.109071.
- Bispo, T. M., and E. A. Vieira. 2022. Assimilatory deficit and energy regulation in young handroanthus chrysotrichus plants under flooding stress. Journal of Plant Research 135 (2):323–36. doi:10.1007/s10265-022-01370-3.
- Bjelková, M., V. Genčurová, and M. Griga. 2011. Accumulation of cadmium by flax and linseed cultivars in field-simulated conditions: A potential for phytoremediation of Cd-contaminated soils. Industrial Crops and Products 33 (3):761–74. doi:10.1016/j.indcrop.2011.01.020.
- Brigelius-Flohé, R., and L. Flohé. 2020. Regulatory phenomena in the glutathione peroxidase superfamily. Antioxidants and Redox Signaling 33 (7):498–516. doi:10.1089/ars.2019.7905.
- Chang, W., H. Zhao, S. Yu, J. Yu, K. Cai, W. Sun, X. Liu, X. Li, M. Yu, S. Ali, et al. 2020. Comparative transcriptome and metabolomic profiling reveal the complex mechanisms underlying the developmental dynamics of tobacco leaves. Genomics 112 (6):4009–22. doi:10.1016/j.ygeno.2020.07.005.
- Clement, G., M. Moison, F. Soulay, M. Reisdorf-Cren, and C. Masclaux-Daubresse. 2018. Metabolomics of laminae and midvein during leaf senescence and source-sink metabolite management in brassica napus L. leaves. Journal of Experimental Botany 69 (4):891–903. doi:10.1093/jxb/erx253.
- Cyle, K. T., A. R. Klein, L. Aristilde, C. E. Martínez, and N. Y. Zhou. 2020. Ecophysiological study of paraburkholderia Sp. strain 1N under soil solution conditions: Dynamic substrate preferences and characterization of carbon use efficiency. Applied and Environmental Microbiology 86 (24). doi: 10.1128/aem.01851-20.
- Dasgupta, M. G., B. S. George, A. Bhatia, O. Prakash Sidhu, and S. Ranganathan. 2014. Characterization of withania somnifera leaf transcriptome and expression analysis of pathogenesis - related genes during salicylic acid signaling. PLoS One 9 (4):e94803. doi:10.1371/journal.pone.0094803.
- Dixon, E., I. Rabanser, M. Dzieciol, B. Zwirzitz, M. Wagner, E. Mann, B. Stessl, and S. U. Wetzels. 2019. Reduction potential of steam vacuum and high-pressure water treatment on microbes during beef meat processing. Food Control 106 (March):106728. doi:10.1016/j.foodcont.2019.106728.
- Frese, M., S. Schatschneider, J. Voss, F. J. Vorhölter, and K. Niehaus. 2014. Characterization of the pyrophosphate-dependent 6-phosphofructokinase from Xanthomonas campestris pv. campestris. Archives of Biochemistry and Biophysics 546:53–63. doi:10.1016/j.abb.2014.01.023.
- Gao, Z., X. Hu, Q. Li, G. Xu, and Z. Wang. 2017. Discussion on standard of high-quality linseed oil. Journal of Shanxi Agricultural Sciences 45 (12):2049–51. doi:10.3969/j.issn.1002-2481.2017.12.35.
- Goyal, A., V. Sharma, N. Upadhyay, S. Gill, and M. Sihag. 2014. Flax and flaxseed oil: An ancient medicine & modern functional food. Journal of Food Science and Technology 51 (9):1633–53. doi:10.1007/s13197-013-1247-9.
- Guo, Y., C. Qiu, S. Long, P. Chen, D. Hao, M. Preisner, H. Wang, and Y. Wang. 2017. Digital gene expression profiling of flax (Linum Usitatissimum L.) stem peel identifies genes enriched in fiber-bearing phloem tissue. Gene 626 (January):32–40. doi:10.1016/j.gene.2017.05.002.
- Guo, Y., C. Qiu, S. Long, H. Wang, and Y. Wang. 2020. Cadmium accumulation, translocation, and assessment of eighteen Linum usitatissimum L. cultivars growing in heavy metal contaminated soil. International Journal of Phytoremediation 22 (5):490–96. doi:10.1080/15226514.2019.1683714.
- Guo, Y., L. Wen, J. Chen, G. Pan, Z. Wu, Z. Li, Q. Xiao, Y. Wang, C. Qiu, S. Long, et al. 2021. Comparative transcriptomic analysis identifies key cellulose synthase genes (CESA) and cellulose synthase-like genes (CSL) in fast growth period of flax stem (Linum usitatissimum L.). Journal of Natural Fibers 00 (00):1–16. doi:10.1080/15440478.2021.1993510.
- Huang, C. H., F. L. Lee, and C. J. Tai. 2009. The β-tubulin gene as a molecular phylogenetic marker for classification and discrimination of the saccharomyces sensu stricto complex. Antonie van Leeuwenhoek, International Journal of General and Molecular Microbiology 95 (2):135–42. doi:10.1007/s10482-008-9296-1.
- Hua, Y. P., T. Zhou, Q. Liao, H. X. Song, C. Y. Guan, and Z. H. Zhang. 2018. Genomics-assisted identification and characterization of the genetic variants underlying differential nitrogen use efficiencies in allotetraploid rapeseed genotypes. G3: Genes, Genomes, Genetics 8 (8):2757–71.
- Ke, Y., Y. Kang, M. Wu, H. Liu, S. Hui, Q. Zhang, X. Li, J. Xiao, and S. Wang. 2019. Jasmonic acid-involved OsEDS1 signaling in rice-bacteria interactions. Rice 12 (1):1–12. doi:10.1186/s12284-019-0283-0.
- Lee, E. J., K. Y. Kim, J. Zhang, Y. Yamaoka, P. Gao, H. Kim, J. U. Hwang, M. C. Suh, B. Kang, and Y. Lee. 2021. Arabidopsis seedling establishment under waterlogging requires ABCG5-mediated formation of a dense cuticle layer. The New Phytologist 229 (1):156–72. doi:10.1111/nph.16816.
- Lin, C. C., Y. T. Chao, W. C. Chen, H. Y. Ho, M. Y. Chou, Y. R. Li, Y. L. Wu, H. A. Yang, H. Hsieh, C. S. Lin, et al. 2019. Regulatory cascade involving transcriptional and N-end rule pathways in rice under submergence. Proceedings of the National Academy of Sciences of the United States of America 116 (8):3300–09. doi:10.1073/pnas.1818507116.
- Li, W., H. C. Ren, J. Zuo, and H. L. Li Ren. 2018. Early summer southern China rainfall variability and its oceanic drivers. Climate Dynamics 50 (11–12):4691–705. doi:10.1007/s00382-017-3898-0.
- Li, Y., L. C. Shi, J. Yang, Z. H. Qian, Y. X. He, and M. W. Li. 2021. Physiological and transcriptional changes provide insights into the effect of root waterlogging on the aboveground part of pterocarya stenoptera. Genomics 113 (4):2583–90. doi:10.1016/j.ygeno.2021.06.005.
- Liu, X. Y., H. Na Yu, S. Gao, Y. Feng Wu, A. Xia Cheng, and H. Xiang Lou. 2017. The isolation and functional characterization of three liverwort genes encoding cinnamate 4-hydroxylase. Plant Physiology and Biochemistry 117:42–50. doi:10.1016/j.plaphy.2017.05.016.
- Liu, C., Z. Xue, D. Tang, Y. Shen, W. Shi, L. Ren, G. Du, Y. Li, and Z. Cheng. 2018. Ornithine δ-aminotransferase is critical for floret development and seed setting through mediating nitrogen reutilization in rice. The Plant Journal 96 (4):842–54. doi:10.1111/tpj.14072.
- Liu, L., X. Xu, Y. Hu, Z. Liu, and Z. Qiao. 2018. Efficiency analysis of bioenergy potential on winter fallow fields: A case study of rape. The Science of the Total Environment 628–629:103–09. doi:10.1016/j.scitotenv.2018.02.016.
- Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25 (4):402–08.
- Li, J., T. Xie, Y. Chen, Y. Zhang, C. Wang, Z. Jiang, W. Yang, G. Zhou, L. Guo, J. Zhang, et al. 2022. High-throughput unmanned aerial vehicle-based phenotyping provides insights into the dynamic process and genetic basis of rapeseed waterlogging response in the field. Journal of Experimental Botany 73 (15):5264–78. doi:10.1093/jxb/erac242.
- Li, T., W. Yuan, Q. S, and J. Shi. 2021. Selection of reference genes for gene expression analysis in liriodendron hybrids’ somatic embryogenesis and germinative tissues. Scientific Reports 11 (1):1–12. doi:10.1038/s41598-021-84518-w.
- Na, Y., J. Li, Z. Han, C. Min, and Y. Shen. 2018. Research Progress of Jasmonate- Responsive Transcription Factors in Regulating Plant Secondary Metabolism 43 (5). doi: 10.19540/j.cnki.cjcmm.20180109.001.
- Nielsen, K. L., J. P. Lynch, and H. N. Weiss. 1997. Fractal geometry of bean root systems: Correlations between spatial and fractal dimension. American Journal of Botany 84 (1):26–33. doi:10.2307/2445879.
- Pang, Z., J. Chen, T. Wang, C. Gao, Z. Li, L. Guo, J. Xu, and Y. Cheng. 2021. Linking plant secondary metabolites and plant microbiomes: A review. Frontiers in Plant Science 12 (March). doi: 10.3389/fpls.2021.621276.
- Poitout, A., A. Crabos, I. Petřík, O. Novák, G. Krouk, B. Lacombe, and S. Ruffel. 2018. Responses to systemic nitrogen signaling in Arabidopsis roots involve trans-zeatin in shoots. The Plant Cell 30 (6):1243–57. doi:10.1105/tpc.18.00011.
- Qi, X., and Z. Zhang. 2022. Assessing the urban road waterlogging risk to propose relative mitigation measures. The Science of the Total Environment 849:157691. doi:10.1016/j.scitotenv.2022.157691.
- Raineri, J., L. Caraballo, N. Rigalli, M. Portapila, M. Elena Otegui, and R. Lía Chan. 2022. Expressing the sunflower transcription factor HaHB11 in maize improves waterlogging and defoliation tolerance. Plant Physiology 189 (1):230–47. doi:10.1093/plphys/kiac054.
- Rao, G. S., P. Deveshwar, M. Sharma, S. Kapoor, and K. Venkateswara Rao. 2018. Evolvement of transgenic male-sterility and fertility-restoration system in rice for production of hybrid varieties. Plant Molecular Biology 96 (1–2):35–51. doi:10.1007/s11103-017-0678-5.
- Rozhmina, T., A. Samsonova, A. Kanapin, and M. Samsonova. 2022. An account of fusarium wilt resistance in flax Linum usitatissimum: The disease severity data. Data in Brief 41:107869. doi:10.1016/j.dib.2022.107869.
- Shuqiao, L. 2020. Southern China sees abnormally heavier rainfall season in 2020. China Meteorological News Press 2020: 07–27.
- Smykalova, I., M. Vrbova, E. Tejklova, M. Vetrovcova, and M. Griga. 2010. Large scale screening of heavy metal tolerance in flax/linseed (Linum usitatissimum L.) tested in vitro. Industrial Crops and Products 32 (3):527–33. doi:10.1016/j.indcrop.2010.06.027.
- Ullah, I., B. Oudh Al-Johny, K. M. S. AL-Ghamdi, H. A. A. Al-Zahrani, Y. Anwar, A. Firoz, N. AL-Kenani, and M. Ali Ahmed Almatry. 2019. Endophytic bacteria isolated from Solanum nigrum L., alleviate cadmium (Cd) stress response by their antioxidant potentials, including SOD synthesis by SodA gene. Ecotoxicology and Environmental Safety 174 (September 2018):197–207. doi:10.1016/j.ecoenv.2019.02.074.
- Wang, A. F., M. Roitto, T. Lehto, S. Sutinen, J. Heinonen, G. Zhang, and T. Repo. 2017. Photosynthesis, nutrient accumulation and growth of two Betula species exposed to waterlogging in late dormancy and in the early growing season. Tree Physiology 37 (6):767–78. doi:10.1093/treephys/tpx021.
- Wang, X., M. Wah Li, F. Ling Wong, C. Yee Luk, C. Yik Lok Chung, W. Shing Yung, Z. Wang, M. Xie, S. Song, G. Chung, et al. 2021. Increased copy number of gibberellin 2-oxidase 8 genes reduced trailing growth and shoot length during soybean domestication. The Plant Journal 107 (6):1739–55. doi:10.1111/tpj.15414.
- Wolf, A. J., C. N. Reyes, W. Liang, C. Becker, K. Shimada, M. L. Wheeler, H. Cheol Cho, N. I. Popescu, K. Mark Coggeshall, M. Arditi, et al. 2016. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell 166 (3):624–36. doi:10.1016/j.cell.2016.05.076.
- Wu, Z., J. Luo, Y. Han, Y. Hua, C. Guan, and Z. Zhang. 2019. Low nitrogen enhances nitrogen use efficiency by triggering NO₃− uptake and its long-distance translocation. Journal of Agricultural and Food Chemistry 67 (24):6736–47. doi:10.1021/acs.jafc.9b02491.
- Wu, L. Y., Z. Tao Fang, J. Ke Lin, Y. Sun, Z. Zheng Du, Y. Ling Guo, J. Hong Liu, Y. Rong Liang, and J. Hui Ye. 2019. Complementary ITRAQ proteomic and transcriptomic analyses of leaves in tea plant (Camellia sinensis L.) with different maturity and regulatory network of flavonoid biosynthesis. Journal of Proteome Research 18 (1):252–64. doi:10.1021/acs.jproteome.8b00578.
- Wu, Z., L. Yang, L. Jiang, Z. Zhang, H. Song, X. Rong, and Y. Han. 2019. Low concentration of exogenous ethanol promoted biomass and nutrient accumulation in oilseed rape (Brassica napus L.). Plant Signaling & Behavior 14 (12):1–7. doi:10.1080/15592324.2019.1681114.
- Xiao, M. 2018. Summary of flood disaster in China in 2017. Flood and Drought Disaster 28 (8):60–66. doi:10.16867/j.issn.1673-9264.2018135.
- Xin, D., Q. Caisheng, C. Xinbo, L. Songhua, G. Yuan, H. Dongmei, and W. Yufu. 2014. Influence of potassium fertilizer on lodging resistance of flax b Linum usitatissimum L. Y. Plant Fiber Sciences in China 36 (4):4–8.
- Xiong, D., S. Lu, J. Wu, C. Liang, W. Wang, W. Wang, J. Ming Jin, and S. Yan Tang. 2017. Improving key enzyme activity in phenylpropanoid pathway with a designed biosensor. Metabolic Engineering 40 (11):115–23. doi:10.1016/j.ymben.2017.01.006.
- Xun, Z., X. Guo, L. Yaling, X. Wen, C. Wang, and Y. Wang. 2020. Quantitative proteomics analysis of tomato growth inhibition by ammonium nitrogen. Plant Physiology and Biochemistry 154:129–41. doi:10.1016/j.plaphy.2020.05.036.
- Yuan, W., D. L. Tuttle, Y. Jiang Shi, G. S. Ralph, and W. A. Dunn. 1997. Glucose-induced microautophagy in pichia pastoris requires the α-subunit of phosphofructokinase. Journal of Cell Science 110 (16):1935–45. doi:10.1242/jcs.110.16.1935.
- Yu, J., N. Fan, R. Li, L. Zhuang, Q. Xu, and B. Huang. 2019. Proteomic profiling for metabolic pathways involved in interactive effects of elevated carbon dioxide and nitrogen on leaf growth in a perennial grass species. Journal of Proteome Research 18 (6):2446–57. doi:10.1021/acs.jproteome.8b00951.
- Zhang, X., X. Ding, Y. Ji, S. Wang, Y. Chen, J. Luo, Y. Shen, and L. Peng. 2018. Measurement of metabolite variations and analysis of related gene expression in Chinese liquorice (Glycyrrhiza uralensis) plants under UV-B irradiation. Scientific Reports 8 (1):1–17. doi:10.1038/s41598-018-24284-4.
- Zhang, X., Z. Xue, D. Hao, C. Qiu, H. Huang, and Y. Wang. 2007. Primary research on the physiological mechanisms of flax in waterlogging stress. Plant Fiber Sciences in China 2007 (03):169–72.