?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The aim of the present study was to develop eco-friendly antibacterial finish from Ghamra and Apamarga leaf extract and its application on textile. Aqueous and methanol extracts from Ghamra and Apamarga leaf had been applied to cotton fabric using pad dry cure method. Antibacterial efficacy of finished fabric samples were tested against Bacillus cereus and Pseudomonas aeruginosa and counted quantitatively by AATCC-100 test method. For testing the efficacy of finish, the samples were inoculated with selected bacterium and further tested to check their bacterial resistance by calculating the percentage reduction in bacterial count. The observations were taken after 24, 48, 72, and 96 hours of inoculation. The results revealed that samples finished with Ghamra and Apamarga leaf extracts showed 100% reduction after 24 hours of inoculation against both bacterial strains, respectively. After 48 hours, it gradually decreased and after 96 hours it decreased to 94.5% and 94.1% against Bacillus cereus and 94.2% and 94% against Pseudomonas aeruginosa in treated samples, respectively. The results also indicated that samples finished with Ghamra and Apamarga leaf extracts provided excellent ultraviolet protection, i.e. 44.51 and 45.37 as exhibited by higher UPF values. The surface morphological studies using SEM showed some fibrillation.
摘要
本研究的目的是开发以加姆拉和阿玛玛叶提取物为原料的环保型抗菌整理剂及其在纺织品上的应用. 采用垫干固化法将加姆拉叶和阿达玛加叶的水提取物和甲醇提取物应用于棉织物上. 对成品织物样品进行了对蜡样芽孢杆菌和铜绿假单胞菌的抗菌效果测试,并用AACC-100测试方法进行了定量计数. 为了测试面漆的功效,用选定的细菌接种样品,并通过计算细菌计数的减少百分比来进一步测试其细菌耐药性. 在接种24、48、72和96小时后进行观察。结果显示,用Ghamra和Apamarga叶提取物完成的样品在对这两种菌株接种24小时后分别显示出100%的减少. 48小时后,它逐渐降低,96小时后,在处理的样品中,它对蜡样芽孢杆菌和铜绿假单胞菌分别降低到94.5%和94.1%和94.2%和94%. 结果还表明,用Ghamra和Apamarga叶提取物完成的样品提供了极好的紫外线保护,即44.51和45.37,如较高的UPF值所示. 使用SEM进行的表面形态研究显示出一些纤化.
Introduction
The field of textile research has been, off late, inundated with demand of textile products with different kind of functional and performance finishes like wrinkle resistance, water repellence, fade resistance and resistance to microbial invasion. The development of antimicrobial textile finish assumes more importance because of the direct contact of clothes with human body (Sathianarayanan et al. Citation2010). Now cotton is the most widely used and preferred natural fabric because of its properties and functionality but it is highly susceptible to microbial contamination due to its porous and moist nature. Therefore, it becomes necessary to impart antimicrobial finishes to the cotton fabric to resist the growth and proliferation of microbes which are either pathogenic or are responsible for certain deformities in textile material. Antimicrobial finishes carry agents which either restrict the growth or kill microbes to control their adverse effects such as staining, odor, and deterioration. Since microbes are the tiniest of creatures which cannot be seen by the naked eye, consisting of a variety of micro-organisms like bacteria, fungi, algae, and viruses, etc. (Khurshid et al. Citation2015), they might prove harmful to the wearer. At present, there is a wide range of commercial textile finishes/products based on synthetic antimicrobial agents, but these are costly and also create environmental trouble (Patel and Desai Citation2014). Therefore, antimicrobial finishes based on skin friendly, environment friendly, safe, and nontoxic natural antimicrobial agents have gained more attention as compared to synthetic antimicrobial agents in the field of medical and health care.
Nowadays, finishes employing plant sources are gaining popularity because they are herbal in nature, nontoxic, skin friendly, and even the waste parts of the source plants can be utilized. Some plants possess a good type of secondary metabolites that are found in vitro to possess antimicrobial properties. Extracts from different parts of such plants like roots, leaves, stems, flowers, and seeds exhibit antimicrobial properties (Sathianarayanan, Chaudhari, and Bhat Citation2011; Vastrad and Byadgi Citation2018).
Many researchers have also reported the beneficial effects of weeds pertaining to their antimicrobial properties and curing abilities. A weed is commonly defined as a plant that grows out of place, is competitive, persistent and pernicious (Singh and Bist Citation2017; Wittenberg and Cock Citation2001), having no importance but rather posing problems while planting. It is believed that the losses due to the weeds to the cultivated crops are more than either diseases or insects (Jakhar and Dahiya Citation2017). As weeds are resistant to microbial attack as compared to crops, it becomes interesting to investigate weed extracts for their antimicrobial properties (Sharma, Lavania, and Sharma Citation2009; Udayaprakash et al. Citation2011). A variety of weeds available throughout India, possess certain phytochemicals showing antimicrobial activity (Dewick Citation2009).
Tridax procumbens L. is commonly known as Ghamra in Hindi (Cui et al. Citation2020). It belongs to the family Asteraceae. This weed is found everywhere in India, America, Tropical Africa, Asia, and Australia. All plant parts of this weed are found to have noble pharmacological activities. It has been extensively used in Indian traditional medicine as anticoagulant, antifungal, insect repellent, in bronchial catarrh, diarrhea, dysentery and in wound healing (Krishnaswamy and Christina Citation2015; Mankilik, Longdet, and Luka Citation2021). It also prevents falling of hair and leading to hair growth (Mundada and Shivhare Citation2010). The extracts of Tridax procumbens have been reported to have various pharmacological effects. It has many bioactive compounds which show anti-anemic, anti-inflammatory, anti-diabetic, antimicrobial, and anesthetic properties as well as antioxidant activities (Beck et al. Citation2018; Christudas, Kulathivel, and Agastian Citation2012; Ikewuchi, Ikewuchi, and Ifeanacho Citation2015; Kumar, John, and Lakshmi-Narayanan Citation2015; Mir et al. Citation2016, Citation2017; Singh et al. Citation2017). Additionally, it has antitubercular, anticancer, and immune modulatory properties (Bijauliya et al. Citation2022). It also has immense antibacterial and antifungal potential (Ashish and Annasaheb Citation2015; Rajkumari et al. Citation2019).
Achyranthes aspera, belonging to the family Amaranthaceae and commonly recognized as prickly chaff flower in English, is an indigenous medicinal plant in Asia, South America and Africa (Sinan et al. Citation2020). It consists of 160 genera and approximately 2400 species of shrubs, herbs, and climbers (Mankilik, Longdet, and Luka Citation2021). The plant genus Achyranthes consists of about 21 species (Xirui He et al. Citation2017). Traditionally, this plant is used in asthma and cough. It is pungent, anti-periodic, diuretic, anti-phlegmatic, purgative and laxative, useful in dropsy, edema and piles, boils and eruptions of skin, etc. (Mishra Citation2018; Patil and Sharma Citation2013; Srivastav et al. Citation2011; Thapa, Sikha, and Arora Citation2022). Paste of its roots in water is used to cure ophthalmia and opacities of cornea (Saraf and Samant Citation2015). Its leaves are used for the treatment of ophthalmic and other eye infections (Tiwari et al. Citation2023). It also has significant wound healing and antioxidant activities (Edwin et al. Citation2008).
The plant also contains triterpenoid saponins and long-chain alcohols (Balbhadra, Rajnala, and Balbhadra Citation2020; Lakshmi, Roy, and Merlin Citation2020). It shows antibacterial, antifungal, and anthelmintic activities (Ndhlala et al. Citation2015).
The use of these weed plants’ extract as anti-microbial finish will be beneficial for the textile industry and will help in subjugating the industrial waste produced which deteriorates the environment and is hard to treat. Hence, the present study was designed to develop eco-friendly antibacterial finish from Ghamra and Apamarga leaf extract and its application on textile.
Methodology
Collection of raw materials
Medium weight gray cotton fabric was purchased from the local market of Hisar. The fabric was selected on the basis of visual, physical, and chemical properties. For this study, fresh green leaves of Ghamra and Apamarga were obtained from Hisar. Ghamra and Apamarga leaves were shade dried and grinded in a laboratory grinder mixer to form a fine powder.
Extraction process
Maceration and soxhlet extraction methods were used for extraction process. The solvent was selected on the basis of maximum zone of inhibition employing qualitative test method using Well Diffusion Method. The test revealed that aqueous extract of Ghamra and methanolic extract of Apamarga showed maximum zone of inhibition against selected test micro- organisms. Aqueous extract was prepared from Ghamra leaves and methanol extract was prepared from Apamarga leaves. For extraction, dry powder of leaves was separately dissolved in an MLR of 1:16 ratio (Nagpal Citation2017) in a conical flask. The solutions were then kept in a shaking incubator at 37°C for 36 hours and after that the solutions were filtered using Whatman No.1 filter paper. Further, the obtained filtrates were transferred to round bottom flasks for evaporating extra solvent using soxhlet extraction. The process continued till 6–7 hours until a solidified mass was obtained. The obtained extracts were transferred to petri dishes and kept at a temperature of 4°C.
Selection of bacterium
Cotton is a natural cellulosic fiber and is mainly susceptible to cellulose degrading bacteria along with some pathogenic bacterium. For this reason pure cultures of two common human pathogenic bacteria viz., Gram-positive (Bacillus cereus) and Gram-negative (Pseudomonas aeruginosa) were taken for the study.
Application of antibacterial finish
Antibacterial finish of Ghamra and Apamarga leaf extracts was applied on desized and scoured cotton fabric in optimized concentration using pad dry cure method. For application of finish by pad-dry-cure method, the quantity of extracts and cross linking agent was calculated on the basis of weight of the sample. The material-to-liquor ratio was taken as 1:20. The fabric samples were kept in a bath for 30 minutes by maintaining variables like treatment pH and treatment temperature. The fabric samples were placed in a trough (liquor space in mangle) containing the solution of selected leaf extracts (20 g/l concentration) for 5 minutes and passed through the extract solution. Further, the samples were passed through two bowls (rollers) of pneumatic padding mangle with a pressure of 2.5 psi and were uniformly squeezed. The samples were again dipped in the extract solution and passed amid the rollers of the padding mangle to give a wet pickup or maximum take up (two dip two nips operation). The finished samples were further sent for subsequent drying and curing operations. The samples were dried at 80°C for 3 minutes and cured at 120°C for 2 minutes on a lab model curing mangle. A post treatment was given to the finished samples with 8% citric acid as a fixing agent. The samples were then again padded on a two-roller pneumatic padding mangle at a pressure of 2.5 psi, dried and cured at 120°C (Shrivastava Citation2010).
Scanning electron microscopic analysis (SEM)
Scanning electron microscopic analysis was done in order to study the changes in surface morphology with a focus on matrix fusion, state of impregnation, fibre/matrix interfacial interactions and fracture morphologies to highlight the changes after each treatment. The controlled and finished samples were sent to CIRCOT, Mumbai for the SEM analysis. The SEM images of the treated samples were captured at different magnifications, i.e., 800×, 1000× and 1200× using HR FESEM (High-resolution field emission scanning electron microscope) SIGMA VP. The samples were prepared with Gold-Palladium alloy coating and were placed on a flat platform below the electron gun. Electrons were generated from the electron gun which entered the surface of the sample and generated many low energy secondary electrons. The intensity of the secondary electrons was governed by the surface topography of the sample. An image of the sample surface was therefore, constructed by measuring secondary electron intensity as a function of the position of the scanning primary electron beam.
Fourier transform infrared spectroscopy (FTIR) analysis of finished cotton fabric
Infrared spectroscopy has always been a powerful tool for the identification of organic materials. With the development of FTIR, it has become a more popular method for the quantitative analysis of complex mixtures, as well as the investigation of surface and interfacial phenomena. FTIR relies on the fact that the most molecules absorb light in the infra- red region of the electromagnetic spectrum. This absorption corresponds specifically to the bonds present in the molecule. The frequency ranges are measured as wave numbers typically over the range 4000-600 cm−1. The resultant absorption spectrum from the bond natural vibration frequencies indicates the presence of various chemical bonds and functional groups present in the sample. showed the presence of different functional groups at different wavelengths. The controlled and finished samples were sent to CSWRI, Avikanagar (Rajasthan) for the FTIR analysis.
Table 1. Fourier transform infrared spectroscopy analysis.
Antibacterial assessment
To analyze the bacterial population (total colony forming units) of all the treated samples, a standard quantitative test method (AATCC-100 Test method) was employed (Gupta Citation2016). The antibacterial activity of the controlled and treated fabric samples was tested against Bacillus cereus and Pseudomonas aeruginosa. For the inoculation of the bacterium on the finished cotton samples, the samples were put in different flasks. Each flask was inoculated with 1 ml bacterium using a pipette and incubated in a shaking incubator at 37°C for 24 hours.
A series of 10 test tubes were taken having 9 ml distilled water. For the inoculation of the bacterium on the finished cotton samples, the samples were put in different flasks. Each flask was inoculated with 1 ml bacterium using a pipette and incubated in a shaking incubator at 37°C for 24 hours. Then, 1 ml of inoculated broth was taken from a conical flask and transferred aseptically to the first test tube to other. Serial dilution was done till its reduced dilution was 10−10 dilutions. Only serial dilution 10−10 was used to determine bacterial count. From each test tube, one ml of diluted culture was taken and transferred into petri plates having NA medium. After that, the diluted culture was spread with help of a spreader. The plates were incubated for 24 hours at 37°C. After 24 hours, the bacterial colonies were counted and total colony forming units were calculated by using following formula:
(Rani Citation2016).
Results and discussion
Standardization of variables for the application of antibacterial finish
As presented in , pH and temperature were optimized to assess the highest antibacterial activity of cotton fabric treated with Ghamra and Apamarga leaf extracts against Bacillus cereus and Pseudomonas aeruginosa. The different pH ranges viz., 4.5, 5.5 and 6.0 were taken on review basis and assessed for application of finish on cotton fabric using pad-dry-cure method keeping other variables constant. It is evident from the data that highest antibacterial activities of Ghamra and Apamarga leaf extracts treated fabric were found at pH 5.5 with a bacterial count 2.7 × 1010 and 3.0 × 1010 against Bacillus cereus and at bacterial count of 2.9 × 1010 and 3.1 × 1010 against Pseudomonas aeruginosa. To optimize the treatment temperature for Ghamra and Apamarga leaf extracts to be fully absorbed by cotton fabric, different temperature ranges viz. 30°C, 45°C, 60°C were measured. The cotton fabric samples were treated using optimum extracts concentration and optimum pH values while keeping other variables constant. It is clear from the results that the highest antibacterial activities of Ghamra and Apamarga leaf extracts treated fabric samples were found at 60°C with a bacterial count of 3.8 × 1010 and 4 × 1010 against Bacillus cereus and bacterial count of 3.9 × 1010 and 4.1 × 1010 against Pseudomonas aeruginosa.
Table 2. Optimization of treatment pH on the basis of antibacterial activity against Bacillus cereus and Pseudomonas aeruginosa.
Table 3. Optimization of treatment temperature on the basis of antibacterial activity against Bacillus cereus and Pseudomonas aeruginosa.
Application of Ghamra and Apamarga leaf extracts on cotton fabric
Application of Ghamra and Apamarga leaf extracts finish on cotton fabric using optimized variables was done by pad-dry-cure method as described under methodology section. For application, material-to-liquor ratio was taken as 1:20 and citric acid was used as cross linking agent. Concentration of extracts was taken as 20 g/l.
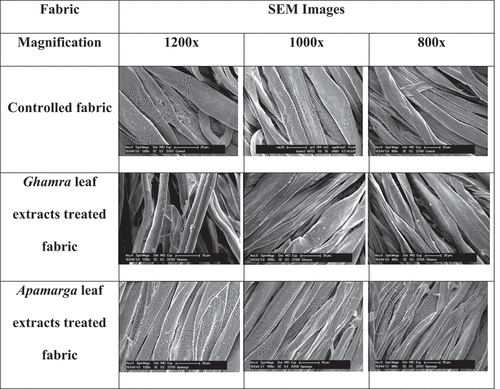
Scanning electron microscopic analysis of finished fabric
SEM images of controlled and finished fabric samples are presented in to illustrate the surface morphologies. The SEM photographs revealed that the fibers of controlled fabric exhibited neat plain spun structures and smooth surfaces. There is a noticeable fiber surface without matrix adherence in the image of controlled fabric. It was apparent from photographs of treated fabric samples that the fiber pull out in the woven structure and the small micro-fibrils ejecting from the main fiber was due to the acidic reaction of extract with the fiber. The fiber surface was seemed to be covered with granular structures. These granules were deposits of extracts on the fiber surface. The deposit of such granules was found more in fabric sample treated with Apamarga leaf extract as compared to Ghamra leaf extract treated fabric sample.
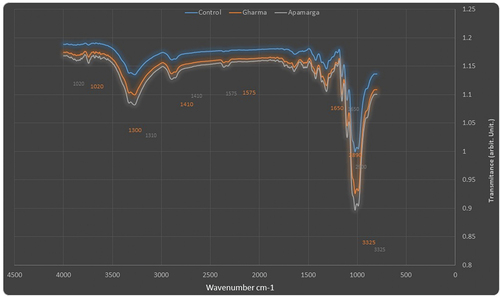
Fourier transform infrared spectroscopy analysis of finished fabric
FTIR analysis was performed for understanding the functional groups. FTIR analysis of controlled and finished fabric is presented in . The spectrum showed broad peaks at 3325 cm−1, indicating the stretching vibration of phenolic – OH group. Small, but sharp peaks at 2870 cm−1 (controlled), 2890 cm−1 (Ghamra leaf extract treated) and 2900 cm−1 (Apamarga leaf extract treated) assigned the presence of C-H stretching in alkanes. The peaks at 1650 cm−1 assigned the presence of C=C group present in the saponin. The peaks at 1575 cm−1 indicated the presence N-O group. The characteristic peak of C-H bending was visible at 1410 cm−1. The peaks at 1290 cm−1 (controlled), 1300 cm−1 (Ghamra leaf extracts treated) and 1310 cm−1 (Apamarga leaf extracts treated) indicated the stretching vibration of C-O group. The peaks at 1050 cm−1 (controlled), 1020 cm−1 (Ghamra and Apamarga leaf extracts treated) assigned to the stretching vibration of C-N group.
Bacterial resistance of Ghamra and Apamarga leaf extracts finished fabric against growth of Bacillus cereus and Pseudomonas aeruginosa
The antibacterial activity of Ghamra and Apamarga leaf extracts finish against growth of Bacillus cereus and Pseudomonas aeruginosa on finished samples was counted quantitatively by AATCC-100 test method. The antibacterial activity of finished gray cotton samples inoculated with Bacillus cereus and Pseudomonas aeruginosa was compared to their controlled samples, i.e., desized and scoured samples by calculating the percentage reduction in bacterial count. The observations were taken soon after 24 hours of inoculation and also after 48, 72 and 96 hours of inoculation with dilution factor 1010.
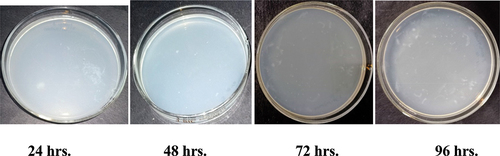
The antibacterial activity of Ghamra and Apamarga leaf extracts treated cotton samples against the growth of Bacillus cereus and Pseudomonas aeruginosa on ensuing hours of inoculation have been represented in . The bacterial resistance was determined by comparing the bacterial colony counts and calculating their percentage reduction in finished samples with controlled samples, i.e., desized and scoured. The samples with lesser bacterial count and higher per cent reduction were considered to be more resistant against the growth of Bacillus cereus and Pseudomonas aeruginosa. Confluent lawn growth was found on controlled sample ().
Figure 3. Antibacterial activity of Ghamra and Apamarga leaf extracts finished fabric against growth of Bacillus cereus.
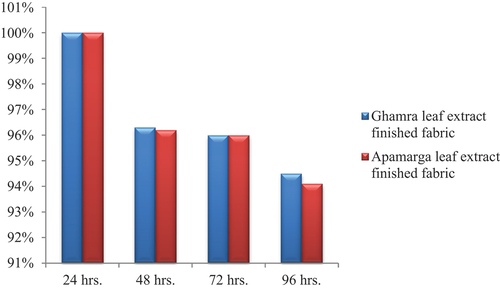
Figure 4. Antibacterial activity of Ghamra and Apamarga leaf extracts finished fabric against growth of Pseudomonas aeruginosa.
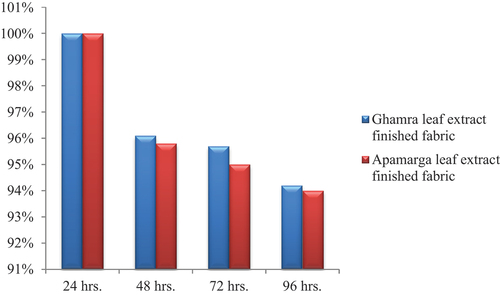
Figure 5a. Antibacterial activity of controlled fabric against growth of Bacillus cereus and Pseudomonas aeruginosa.

After 24 hours of inoculation, it was observed that there was 100% reduction in bacterial growth with 0 × 1010 CFUs for 1010 dilution factor in fabric treated with 20 g/l concentration of Ghamra leaf extract against both bacteria, i.e., Bacillus cereus and Pseudomonas aeruginosa. Similarly, when same cotton fabric was finished with 20 g/l concentration of Apamarga leaf extract, there was 100% reduction in bacterial count with 0 × 1010 CFUs for 1010 dilution factor against both bacterial strains, i.e., Bacillus cereus and Pseudomonas aeruginosa.
After 48 hours, the per cent reduction was recorded as 96.3% with 3.7 × 1010 CFUs against Bacillus cereus and 96.1% reduction with 3.9 × 1010 CFUs against Pseudomonas aeruginosa for 1010 dilution factor on cotton fabric sample finished with Ghamra leaf extract. While, when same cotton fabric was finished with a concentration of 20 g/l Apamarga leaf extract, the per cent reduction was recorded as 96.2% with 3.8 × 1010 CFUs against Bacillus cereus and 95.8% with 4.2 × 1010 CFUs against Pseudomonas aeruginosa for 1010 dilution factor.
Similarly, after 72 hours and 96 hours of inoculation, the bacterial reduction values of the same cotton fabric samples decreased to 96 and 94.5% with 4 × 1010 and 5.5 × 1010 CFUs against Bacillus cereus. While, against Pseudomonas aeruginosa, the bacterial reduction values decreased to 95.7 and 94.2% with 4.3 × 1010 and 5.8 × 1010 CFUs, respectively for 1010 dilution factor on cotton fabric sample finished with a concentration of 20 g/l Ghamra leaf extract. On the other hand, for the desized and scoured cotton samples finished with Apamarga leaf extract, the bacterial reduction values of the same cotton fabric samples against Bacillus cereus decreased to 96 and 94.1% with 4 × 1010 and 5.9 × 1010 CFUs. While, against Pseudomonas aeruginosa, the per cent reduction was found to be 95 and 94% with 5 × 1010 and 6 × 1010 CFUs for 1010 dilution after 72 hours and 96 hours of inoculation, respectively.
Conclusively, it can be deduced from the results presented in ), ), ) and ) that the antibacterial finish applied using both extracts, i.e., Ghamra and Apamarga leaf extracts with 20 g/l concentration by pad dry cure method with 1010 dilution factor was highly effective after 24 hours of inoculation against both the pathogenic bacteria, i.e., Bacillus cereus and Pseudomonas aeruginosa. After 48 hours, percentage reduction gradually decreased. After 96 hours it decreased to 94.5 and 94.1% against Bacillus cereus and 94.2 and 94% against Pseudomonas aeruginosa in cotton fabric sample finished with Ghamra and Apamarga leaf extracts, respectively.
Figure 6a. Antibacterial activity of apamarga leaf extract finished fabric against growth of Bacillus cereus.
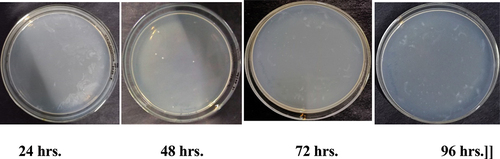
Figure 6b. Antibacterial activity of Apamarg leaf extract finished fabric against growth of Pseudomonas aeruginosa.
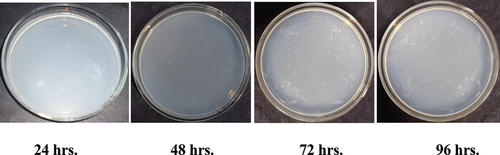
Figure 5b. Antibacterial activity of Ghamraa leaf extract finished fabric against growth of Bacillus cereus.
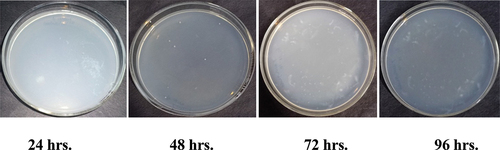
Figure 5c. Antibacterial activity of Ghamra leaf extract finished fabric against growth of Pseudomonas aeruginosa.
Therefore, samples treated with Ghamra and Apamarga leaf extracts showed very good resistance against the growth of Bacillus cereus and Pseudomonas aeruginosa.
Also, the efficacy of Ghamra and Apamarga leaf extracts was determined after five wash cycles (24 hours, 48 hours, 72 hours, and 96 hours of inoculation for 1010 dilution factor). The results showed that after five wash cycles, the bacterial per cent reduction of Ghamra and Apamarga leaf extracts treated fabric samples was 98.2 and 98.0% against Bacillus cereus and 98.0 and 97.9% against Pseudomonas aeruginosa, respectively, after 24 hours of incubation. After an incubation period of 96 hours, the per cent reduction constantly decreased.
Effect of antibacterial finish on ultra violet protection property of cotton fabric
Ultra-violet protection factor (UPF) is the scientific term used to indicate the amount of ultraviolet (both UVA and UVB) protection provided to skin by fabric. UVA rays can cause premature skin aging, while UVB rays are responsible for tanning and sunburn. The higher the UPF value, the greater is the fabric’s protection level. For determination of UPF of controlled and finished samples, the samples were sent to CIRCOT, MUMBAI, where UPF was calculated by using AATC-183:2004 test method. The higher the UPF value, the greater is the fabric’s protection level. It is evident from that the UPF value of controlled fabric sample was 32.14, indicating that the controlled fabric sample showed very good protection factor. When cotton fabric was treated with Ghamra and Apamarga leaf extract using pad dry cure method, the UPF value increased to 44.51 and 45.37 respectively, exhibiting excellent protection category. Hence, it was apparent from the table that the fabrics treated with Ghamra and Apamarga leaf extracts displayed higher UPF value when compared to controlled fabric.
Table 4. Effect of Ghamra and apamarga leaf extracts treatment on ultra violet protection property of cotton fabric.
Finished/Dyed fabrics protect more than unfinished/undyed ones and their protection levels rise with the increase in extract concentration. In general, light colors reflect solar radiation more efficiently than dark ones, but part of the radiation penetrates more easily through the fabric thanks to multiple scattering.
Conclusion
It was clear from the results that both Ghamra and Apamarga leaf extracts treated fabric showed good antibacterial activity against both bacterial strains. The extracts were observed to be effective with (100%) reduction after 24 hours of inoculation against both bacterial strains, respectively. After 48 hours, the per cent reduction gradually decreased and after 96 hours it decreased to (94.5% and 94.1% against Bacillus cereus) and (94.2% and 94% against Pseudomonas aeruginosa) in cotton fabric samples treated with Ghamra and Apamarga leaf extracts, respectively. The fabrics treated with Ghamra and Apamarga leaf extracts provided excellent ultraviolet protection as exhibited by higher UPF values when compared to controlled (untreated) fabric which provided very good protection category. Microscopic observation of Ghamra and Apamarga leaf extracts treated cotton fabric using SEM indicated the presence of deposit of extracts on the fabric. FTIR analysis of scoured and Ghamra and Apamarga leaf extracts treated cotton fabric showed the presence of different functional groups such as primary amine, alkyl aryl ether, methane, nitro compound, alkene/amide and alcohol/amine. Peaks of these functional groups after treatment shifted to new positions which were considered responsible for change in properties. Also the treated fabric resulted in good antibacterial efficacy against washing.
Since Ghamra and Apamarga weed plants are widely distributed throughout the world, the use of nontoxic, herbal and eco-friendly extract to develop antibacterial finish for textile application is high. Also, these are unwanted weed plants. Hence, synthesis of antibacterial agent from such waste weed plants is a novel idea. Also, the raw material is purely obtained from natural resources, it is eco-friendly having social, economic and environmental benefit.
Highlights
Ghamra and Apamarga are unwanted weed plants. They have no importance rather pose problems while planting. Hence, synthesis of antibacterial agent from such waste weed plants is a novel idea.
The raw material used for imparting antibacterial finish being purely natural, it is eco-friendly having social, economic and environmental benefit.
The antibacterial renewable treatment on cotton fabric not only provided resistance against the growth of bacteria (Bacillus cereus and Pseudomonas aeruginosa) but also enhanced the UPF value. This will help in manufacturing of antibacterial, ultraviolet protection and easy care textiles required for medical and field activities.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ashish, P. V., and N. S. Annasaheb. 2015. “Antimicrobial and Antifungal Activity of Tridax Procumbens Linn.Whole Plant Ethanolic Extract on Different Pathogens and Its Phytochemical Screening.” World Journal of Pharmaceutical Research 4 (10): 784–13.
- Balbhadra, D. S., D. A. Rajnala, and S. Balbhadra. 2020. “Review on the Ancient Drug Apamara-(Achyranthes aspera).” World Journal of Pharmaceutical Research 9 (7): 512–519.
- Beck, S., H. Mathison, T. Todorov, E. A. Calderón-Juárez, and O. R. Kopp. 2018. “A Review of Medicinal Uses and Pharmacological Activities of Tridax procumbens (L.).” Journal of Plant Studies 7 (1): 19–35. https://doi.org/10.5539/jps.v7n1p19.
- Bijauliya, R. K., P. K. Singh, D. Y. Khatoon, S. Chaudhari, Y. Singh, S. Kumar, and M. K. Bhardwaj. 2022. “A Descriptive Review on Phytochemistry of Tridax Procumbens.” Journal of Pharmaceutical Negative Results 13 (8): 2646–2653.
- Christudas, S., T. M. Kulathivel, and P. Agastian. 2012. “Phytochemical and Antibacterial Studies of Leaves of Tridax Procumbens L.” Asian Pacific Journal of Tropical Biomedicine 2 (1): 159–161. https://doi.org/10.1016/S2221-1691(12)60149-X.
- Cui, H. X., L. S. Zhang, H. G. Yan, K. Yuan, and S. H. Jin. 2020. “Constituents of flavonoids from Tridax procumbens L. And antioxidant activity.” Pharmacognosy Magazine 16 (67): 201–205. https://doi.org/10.4103/pm.pm_229_19.
- Dewick, P. M. 2009. Medicinal Natural Products: A Biosynthetic Approach. 3rd edn ed. Chichester, UK: John Wiley & Sons, Ltd.
- Edwin, S., E. Edwin Jarald, L. Deb, A. Jain, H. Kinger, K. R. Dutt, and A. Amal Raj. 2008. “Wound Healing and Antioxidant Activity of Achyranthes Aspera.” Pharmaceutical Biology 46 (12): 824–28. https://doi.org/10.1080/13880200802366645.
- Gupta, V. 2016. UV protective and antibacterial finish on cotton using plant extracts. Doctoral Thesis, Department of Textile and Apparel Designing, CCS Haryana Agricultural University, Hisar.
- He, X., X. Wang, J. Fang, Y. Chang, N. Ning, H. Guo, L. Huang, and X. Huang. 2017. “The Genus Achyranthes: A Review on Traditional Uses, Phytochemistry and Pharmacological Activities.” Journal of Ethnopharmacology 203 (35): 260–278. https://doi.org/10.1016/j.jep.2017.03.035.
- Ikewuchi, C. C., J. C. Ikewuchi, and M. O. Ifeanacho. 2015. “Phytochemical Composition of Tridax Procumbens Linn. Leaves: Potential as a Functional Feed Supplements.” Food and Nutrition Sciences 6 (11): 992–1004. https://doi.org/10.4236/fns.2015.611103.
- Jakhar, S., and P. Dahiya. 2017. “Antimicrobial, Antioxidant and Phytochemical Potential of Alternanthera Pungens HB&K.” Journal of Pharmaceutical Sciences and Research 9 (8): 1305–1311.
- Khurshid, M. F., M. Ayyoob, M. Asad, and S. N. H. Shah. 2015. “Assessment of Eco-Friendly Natural Antimicrobial Textile Finish Extracted from Aloe Vera and Neem Plants.” Fibres & Textiles in Eastern Europe 23 (6(114)): 120–124. https://doi.org/10.5604/12303666.1172176.
- Krishnaswamy, V. G., and A. Christina. 2015. “Antibacterial Activity of Different Parts of Tridax Procumbens Against Human Pathogens.” International Journal of Current Research and Academic Review 3 (6): 211–218.
- Kumar, S. S., R. John, and G. Lakshmi-Narayanan. 2015. “Antimicrobial activity of Tridax procumbens leaf.” International Journal of Pharma Sciences and Research 6 (3): 517–518.
- Lakshmi, T., A. Roy, and A. R. S. Merlin. 2020. “Antibacterial Activity of Achyranthes Aspera Extract Against Oral Pathogens- an In Vitro Study.” Plant Cell Biotechnology and Molecular Biology 21 (25&26): 37–40.
- Mankilik, M. M., I. Y. Longdet, and C. D. Luka. 2021. “Evaluation of Achyranthes Aspera Shoot Extract as an Alternative Therapy for Malaria.” The Journal of Basic and Applied Zoology 82 (14): 1–9. https://doi.org/10.1186/s41936-021-00211-4.
- Mir, A. S., M. A. Dar, S. Mir, S. M. Ahmad, and G. Chitale. 2016. “Analysis of Phytochemistry and Antimicrobial Activity of Tridax Procumbens Linn.” Chemical sciences journal 7 (2): 1–4. https://doi.org/10.4172/2150-3494.1000132.
- Mir, S. A., Z. Jan, S. Mir, A. M. Dar, and G. Chitale. 2017. “A concise review on biological activity of Tridax procumbens Linn.” Organic Chemistry Current Research 6 (1): 1–4.
- Mishra, D. 2018. “Antibacterial Activity of Alkaloids Present in Plant Achyranthes Aspera.” The Pharma Innovation Journal 7 (6): 147–153.
- Mundada, S., and R. Shivhare. 2010. “Pharmacology of Tridax procumbens a Weed: Review.” International Journal of PharmTech Research 2 (2): 1391–1394.
- Nagpal, A. 2017. Application of weed plants extracts on cotton and silk for microbial resistance. Doctoral Thesis, Department of Textile and Apparel Designing, CCS Haryana Agricultural University, Hisar.
- Ndhlala, A. R., H. M. Ghebrehiwot, B. Ncube, A. O. Aremu, J. Gruz, M. Šubrtová, K. Doležal, C. P. du Plooy, H. A. Abdelgadir, and J. Van Staden. 2015. “Antimicrobial, Anthelmintic Activities and Characterisation of Functional Phenolic Acids of Achyranthes Aspera Linn.: A Medicinal Plant Used for the Treatment of Wounds and Ringworm in East Africa.” Frontiers in Pharmacology 6 (274): 1–8. https://doi.org/10.3389/fphar.2015.00274.
- Patel, M. H., and P. B. Desai. 2014. “Grafting of Medical Textile Using Neem Leaf Extract for Production of Antimicrobial Textile.” Research Journal of Recent Sciences 3 (IVC–2014): 24–29.
- Patil, U., and M. C. Sharma. 2013. “Studies on Antibacterial Effect of Apamarga (Achyranthes Aspera) on Multi Drug Resistant Clinical Isolates.” International Journal of Research in Ayurveda and Pharmacy 4 (2): 262–265. https://doi.org/10.7897/2277-4343.04236.
- Rajkumari, J., C. M. Magdalane, B. Siddhardha, J. Madhavan, G. Ramalingam, N. A. Al- Dhabi, M. V. Arasu, A. K. M. Ghilan, V. Duraipandiayan, and K. Kaviyarasu. 2019. “Synthesis of Titanium Oxide Nanoparticles Using Aloe Barbadensis Mill and Evaluation of Its Antibiofilm Potential Against Pseudomonas aeruginosa PAO1.” Journal of Photochemistry and Photobiology, B: Biology 201:1–9. https://doi.org/10.1016/j.jphotobiol.2019.111667.
- Rani, S. 2016. Effect of extract of peach leaves on cotton for microbial resistance. Master’s Thesis, Department of Textile and Apparel Designing, CCS Haryana Agricultural University, Hisar.
- Saraf, A. A., and A. C. Samant. 2015. “High- Performance Thin Layer Chromatography Method for Identification and Quantification of Oleanolic Acid in the Roots of Achyranthes Aspera Linn.” International Journal of Green Pharmacy 9 (4): 70–74.
- Sathianarayanan, M. P., N. V. Bhat, S. S. Kokate, and V. E. Walunj. 2010. “Antibacterial Finish for Cotton Fabric from Herbal Products.” Indian Journal of Fibre and Textile Research 35 (1): 50–58.
- Sathianarayanan, M. P., B. M. Chaudhari, and N. V. Bhat. 2011. “Development of Durable Antibacterial Agent from Ban-Ajwaiin Seed (Thymus serpyllum) for Cotton Fabric.” Indian Journl of Fibre and Textile Research 36 (3): 234–241.
- Sharma, D., A. A. Lavania, and A. Sharma. 2009. “In vitro Comparative Screening of Antibacterial and Antifungal Activities of Some Common Plants and Weeds Extracts.” Asian Journal of Experimental Sciences 23 (1): 169–172.
- Shrivastava, A. 2010. “Use of Aloe Vera for Furnishing Antimicrobial Finish on Cotton Fabrics.” Indian Journal of Textile Association 7 (8): 68–77.
- Sinan, K. I., G. Zengin, D. Zheleva-Dimitrova, O. K. Etienne, M. F. Mahomoodally, A. Bouyahya, D. Lobine, et al. 2020. “Qualitative Phytochemical Fingerprint and Network Pharmacology Investigation of Achyranthes Aspera Linn. Extracts.” Molecules 25 (8): 1–19. https://doi.org/10.3390/molecules25081973.
- Singh, M., and R. Bist. 2017. “Antibacterial Behavior of Common Winter Season Weeds on Human Pathogens in Doon Valley, Uttarakhand, India.” International Journal of Science and Research 6 (1): 296–299.
- Singh, P., K. Jain, S. Khare, and P. Shrivastav. 2017. “Evaluation of Phytochemical and Antioxidant Activity of Tridax Procumbens Extract.” Pharmaceutical and Biosciences Journal 5 (6): 41–47. https://doi.org/10.20510/ukjpb/5/i6/166569.
- Srivastav, S., P. Singh, G. Mishra, K. K. Jha, and R. L. Khosa. 2011. “Achyranthes Aspera- an Important Medicinal Plant: A Review.” Journal of Natural Product and Plant Resources 1 (1): 1–14.
- Thapa, R., S. Sikha, and D. S. Arora. 2022. “A Review of Acyranthes Aspera.” Annals of Forest Research 65 (1): 10601–10624.
- Tiwari, Y., N. Morya, S. P. Singh, and S. P. Singh. 2023. “A Concise Review on Versatile Medicinal Plant Achyranthes aspera: Traditional Use, Phytochemistry and Pharmacological Activities.” International Journal of Pharma Professiona’s Research 14 (1): 152–162.
- Udayaprakash, N. K., S. Bhuvaneswari, R. Aravind, V. Kaviyarasan, and H. Sekarbabu. 2011. “A Comparative Studies on Antibacterial Activity of Common Weeds.” International Journal of Pharma and Bio Sciences 2 (1): 677–683.
- Vastrad, J. V., and S. A. Byadgi. 2018. “Eco-Friendly Antimicrobial Finishing of Cotton Fabric Using Plant Extracts.” International Journal of Current Microbiology and Applied Sciences 7 (2): 284–92. https://doi.org/10.20546/ijcmas.2018.702.037.
- Wittenberg, R., and M. J. W. Cock. 2001. Invasive Alien Species: A Toolkit of Best Prevention and Management Practices. Oxon, London: CABI Publication.