?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Pineapple leaf fiber is an environment-friendly natural fiber that does not harm any natural substance. To produce commercially available and attractive textile materials, pineapple leaf fibers were bleached with hydrogen peroxide and colored with natural dyes. Four natural dyes (turmeric powder, ebony fruit, lac, and sappan wood) were selected as natural sources for the extraction of natural colorants with and without salt, alum, and lime as mordants. The hydrogen peroxide concentration of 10 %owf, liquor ratio of 1:100, contact time of 30 min, and bleaching temperature of 70 °C were found to be the optimum bleaching parameters. The uses of different types of mordant on natural dyes give a variety of shades. The positive value of color axes (a* and b*) was an affirmation of mixture of red and yellow colors of mordanted-dyed PALF. PALF displayed a satisfactory dyeing performance for ebony fruit extracted dye with adequate fastness properties (from good to excellent) for light, wash, and perspiration, whether unmordanted or mordanted. Meta-mordanting increased the colorfastness properties of the samples. Results show excellent coloring ability for pineapple fiber dyeing, which can be a potential substitute for synthetic fiber as a sustainable approach to clean production.
摘要
菠萝叶纤维是一种环保的天然纤维,对任何天然物质都没有危害. 为了生产商业上可买到的有吸引力的纺织材料,菠萝叶纤维用过氧化氢漂白,并用天然染料染色. 对漂白纤维进行了白度指数和拉伸性能等分析研究. 选择四种天然染料(姜黄粉、乌木、紫胶和蓝宝石)作为天然来源,提取天然着色剂,并添加和不添加盐、明矾和石灰作为媒染剂. 根据CIELAB(L*、a*和b*)、K/S值和色牢度特性研究了每种染色菠萝叶纤维的颜色. 过氧化氢浓度为10%owf,溶液比例为1:100,接触时间为30 min,漂白温度为70°C是最佳的漂白参数. 在天然染料上使用不同类型的媒染剂会产生各种色调. 色轴(a*和b*)的正值是对媒染PALF的红黄混合色的肯定. PALF对乌木果实提取染料表现出令人满意的染色性能,具有足够的耐光、耐洗和耐汗牢度(从好到好),无论是未经染色还是经媒染. 后媒染提高了样品的色牢度. 结果表明,菠萝纤维染色具有良好的着色能力,可作为合成纤维的一种可持续的清洁生产方法.
Introduction
The textile industry demands large amounts of raw materials, including both plastics based on fossil raw materials and natural fibers such as cotton, linen, and jute, which also require a high level of resources. Innovative fibers are needed to reduce the use of nonrenewable raw materials in the textile sector. Nowadays, textile products that use natural fibers are more in demand because they offer various benefits, such as renewable properties, excellent biodegradability, and easy manufacturing that do not negatively affect the environment. Pineapple (Ananas comosus L.) is the third most important tropical fruit in world production after the banana and citrus (Asim et al. Citation2015; Botella and Smith Citation2008). Commercially, pineapple fruits have significant value, and its leaves are considered as waste materials of fruit that are used to produce natural fibers.
Normally, after the harvesting season, pineapple leaves can be found plentifully in waste areas. Therefore, pineapple leaf waste increased along with demand for its fruits. Most discarded pineapple leaves are burned and fermented to provide organic fertilizer, as in the conventional method (Todkar and Patil Citation2019). However, enormous amounts of particulate matter (PM) with high concentrations have appeared from burning that may cause cardiovascular diseases in humans exposed to them (Ferreira et al. Citation2014). Moreover, a significant amount of carbon dioxide (CO2) released from the pyrolysis of organic compounds certainly contributes to atmospheric pollution and climate change (Phan et al. Citation2023; Shen, Ma, and Ge Citation2017). This is a well-recognized issue that necessitates a better approach.
Recently, pineapple leaf has become a candidate to develop into a natural fiber because cellulose content in pineapple leaf fiber (PALF) is quite high compared to other natural fiber sources, reaching 85% and is only lower than hemp cellulose content at 91% (Ravindran, Sreekala, and Thomas Citation2019; Rejo, Rizky, and Rajagukguk Citation2018). PALF is more delicate in texture than any other vegetable fiber (Alam et al. Citation2022). The PALF possesses a silky luster, a pure white color, is finer than jute, and possesses good antibacterial properties. The fiber sources obtained from the pineapple leaf plant can be processed into variable value-added products such as cloth and textile products (Amin et al. Citation2021). There are several functions that pineapple leaf fiber (PALF) can take responsibility for, such as a green acoustic absorber (Putra, Prasetiyo, and Selamat Citation2020), heavy metal adsorbent (Daochalermwong et al. Citation2020; Gogoi, Chakraborty, and Saikia Citation2018; Lim et al. Citation2020), lithium-ion battery anode (Pimphor and Roddecha Citation2021), thermal insulation material (Rajeshkumar et al. Citation2020), biodegradable plastic (Ramli, Md Fadzullah, and Mustafa Citation2017), and rubber reinforcement (Surajarusarn et al. Citation2021; Yantaboot and Amornsakchai Citation2017). However, only a few researchers have studied the potential use of PALF in conventional apparel and technical textiles, and the utilization of natural dyes in PALF dyeing has not been fully explored.
Natural dyes utilizing development as textile dyes in recent years have increased. This is related to environmental sustainability issues and stringent environmental laws causing a revival of natural dyes. Besides, natural dyes are the principal sources of eco-friendly dyes. Natural dyes are derived from naturally occurring sources such as plants, insects, and minerals without or with a minimum of chemical processing (Chengaiah et al. Citation2010). Natural dyes have advantages and disadvantages. A good dye on textile materials will become unattractive to consumers if the textile material is faded in color (Harsito et al. Citation2021). Natural dyes usually have weak colorfastness when applied to fibers. It is common to improve their colorfastness with the application of mordant in the natural dyeing process. The mordants form a complex with the dye molecule, making it water-insoluble and resulting in higher fastness (Ghaheh et al. Citation2014). Salts of chromium (Cr), cobalt (Co), copper (Cu), iron (Fe), manganese (Mn), and tin (Sn) are conventional chemical mordants used in enhancing fastness properties of natural dyed textiles (Adeel et al. Citation2019). Global Organic Textile Standard (GOT), Ecological and Toxicological Association of Dye and Textile Manufacturers (ETAD) discouraged the use of toxic metal salts like Cu, Cr, Mn, and Sn as mordants in the dyeing of textiles but instead encouraged the use of eco-friendly metal salts (aluminum, iron) and bio-mordants (Jabar, Adedayo, and Odusote Citation2021; Jabar, Ogunmokun, and Taleat Citation2020; Jabar, Ogunsade, and Odusote Citation2023). Since ancient times, the most common metallic mordant that has been used in the water-based extraction of natural dyes is alum, also known as aluminum potassium sulfate (Haar, Schrader, and Gatewood Citation2013; Haja Citation2019; Mozaffari and Maleki Citation2018). Lime, an inorganic material composed primarily of calcium hydroxide, has been reported to be used as a metallic mordant for natural fiber dyeing (Feiz and Norouzi Citation2014; Kusumastuti, Fardhyanti, and Anis Citation2020; Kusumawati et al. Citation2017; Venil et al. Citation2016). Sodium chloride salt has been used as mordant and an exhausting agent for the dyeing of natural fibers (Azeem et al. Citation2019; Jabar, Adedayo, and Odusote Citation2021; Jalil et al. Citation2018; Rani Citation2023).
Thailand has an abundance of dye-yielding plant species in different parts of the country. Traditionally, the rural folks of different regions dyed their materials from leaves, roots, and bark of the plants, mostly by boiling to get the desired color. Curcuma longa L., is an important spice used as a cosmetic, and a coloring agent has also been used as a Thai traditional medicine. Turmeric is mainly valued for its principal coloring constituent curcumin, which imparts the yellow color on textile fibers and food (Hosen et al. Citation2021; Ma et al. Citation2020; Rahman et al. Citation2023). Diospyros mollis Griff., known as the ebony tree, is a shrub tree and is distributed mainly in Southeast Asian countries. In Thai traditional medicine, this plant has been used for purging intestinal parasites, emaciation, nausea, vomiting, and wasting due to chronic illnesses (Bunfueang et al. Citation2019; Pham and Bechtold Citation2023; Suwama et al. Citation2018). In addition, the extract of the ebony fruit has long been used as a traditional black dye for silks and an anthelmintic medicine. Lac dye is a red-colored natural dye, which is present in the body fluid of the lac insect, Coccus laccae (Laccifer lacca Kerr), found growing on the twigs of certain tree native to Southeast Asia. The use of lac dye in the dyeing of silk and leather seems to have been known for a long time (Banerjee, Kotnala, and Maulik Citation2018; Divya et al. Citation2011; Do et al. Citation2023). Caesalpinia sappan L. is widely distributed in Thailand, Indonesia, Vietnam, Burma, India, and south and southwest China (Flora of China Editorial Committee Citation1988). Its heartwood has long been used as a Thai traditional medicine for the treatment of infectious diseases such as an anti-inflammatory, antioxidant, and for antimicrobial activities (Nirmal et al. Citation2015; Zhao et al. Citation2013). It is reported that sappan wood is rich in tannin and can be produced for use in historical paintings and textiles (Mulyanto et al. Citation2016; Sholikhah and Widowati Citation2023). This research aims to evaluate the dyeing ability of four different natural dyes that are easily found in Thailand: turmeric, ebony fruit, lac, and sappan wood. The study investigates the effect of mordant existence on the dyeing and colorfastness properties after washing treatment, exposure of perspiration and light.
Materials and methods
Materials
Pineapple leaf fiber (PALF), extracted by mechanical process, were obtained from Ban Kha community enterprise (Ratchaburi province, Thailand). Hydrogen peroxide 30% (H2O2, CAS #7722-84-1), salt (NaCl, CAS #7647-14-5), Alum (KAl(SO₄)₂.12 H₂O, CAS #7784-24-9), lime (Ca(OH)2, CAS #1305-62-0) were supplied by Merck (Germany). The turmeric powders, ebony fruit, lac, and sappan wood were purchased from Healthy Hills Farm Company Limited, Thailand.
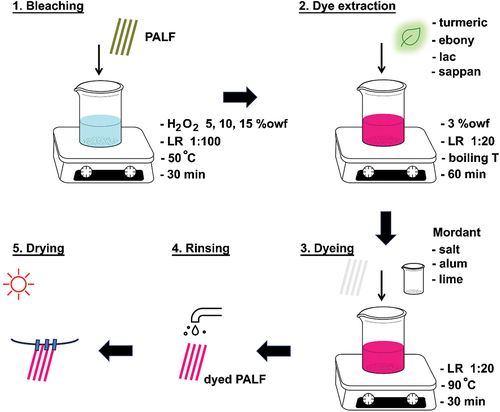
Bleaching of PALF
Pineapple leaves were cleaned and retted to soften the fibers and the inner part of the fibers were removed. The leaves were soaked in a water-filled container overnight and then dried in an oven at 70°C. The extraction process was carried out using a mechanical extraction method to remove the waxy layer. A rubber mallet was used for crushing the waxy layer on pineapple leaves. Then, a scrapping tool was used to completely scratch the waxy layer leaving its fibers exposed beneath the pineapple leaf. Combing was performed after the oven-drying process to separate the fibers into a single fiber.
Hydrogen peroxide was used as a scouring and bleaching agent. The hydrogen peroxide concentration of 5, 10, and 15% owf (on-weight-fiber) with a liquor ratio of 1:100. The PALF was bleached at 50°C for 30 min. After bleaching, treated samples were rinsed with cold water and then dried at room temperature.
Extraction of natural dyes
The turmeric powders, ebony fruit, sappan wood, and lac concentration of 3% owf with a liquor ratio of 1:20 were used for the extraction. The extracting procedure was done for 60 min at the boiling temperature. Finally, the solution temperature was reduced to 25°C and it was filtered with filter paper.
Dyeing procedure
All dyeing was carried out using a bath ratio of 1:20 at 90°C for 30 min by the exhaustion method. Dyeing was done with a concentration of 3% owf for each dye extracted by the methods mentioned above. Finally, the dyed PALF samples were removed, rinsed with running tap water in order to remove non-absorbed dyes and then dried at room temperature. The dyeing experiments following the temperature/time dyeing conditions are shown in .
Mordanting
The mordanting process can be achieved by simultaneously mordanting and dyeing. Sodium chloride, aluminum sulfate, and calcium hydroxide with a concentration of 1% owf were used as mordants. The PALF samples were immersed in a dye bath solution containing both mordant and dye at a liquor ratio of 1:20 to investigate the effect of mordant on the color properties of the dyed PALF with natural colorant extracts. PALF samples were introduced into the mordant and dye solution at 90°C for 30 min. After dyeing, the dyed PALF samples were removed, rinsed with running tap water, and then dried at room temperature. The schematic diagram of dyeing procedure is shown in .
Characterization of bleached PALF
Colorimetric data
The color coordinates ,
, and
of the bleached PALF were measured by a Hunter Lab-UltraScan VIS spectrophotometer and illuminant D65, with a 10° standard observer. All the PALF samples were wrapped around cardboard to get the PALF sheet and tested in four different spots to get average numbers.
corresponds to the brightness,
refers to the red-green coordinate (+ve = red, -ve = green), and
is related to the yellow blue coordinate (+ve = yellow, - ve = blue).
Moreover, the total color difference () between the unbleached and bleached PALF was calculated using the following equation (EquationEquation (1)
(1)
(1) ) (Liu et al. Citation2020):
where ,
, and
are the color coordinates of the unbleached PALF, and
,
, and
are the color coordinates of the bleached PALF.
Whiteness index and yellowness index
The whiteness index () values were determined based on Hunter whiteness index equation (EquationEquation (2)
(2)
(2) ) (Ganz Citation1979; Samanta Citation2022).
The yellowness index () according to ASTM E313/1973 can be expressed as EquationEquation (3)
(3)
(3) (Hirschler Citation2012):
where ,
,
are the measured tristimulus values of the specimen and coefficients
,
are illuminant- and observer-specific constants.
Weight loss
The percentage of weight loss occurred in the PALF due to bleaching which was evaluated by oven dry-weight basis. Usually, it is calculated from the following equation which shows the difference between unbleached and bleached PALF sample weight. The fiber weight before and after bleaching was measured five times to get the average weight change.
The weight loss of fiber affects the quality of the fiber. The percentage of weight loss was calculated using EquationEquation (4)(4)
(4) :
where is weight of the fiber before bleaching and
is weight of the fiber after bleaching.
Linear density
The linear density (tex) of unbleached and bleached sample PALF was determined based on the ASTM D1577:2007 standard test method. Ten test specimens, each composed of a bundle of 50 individual fibers of 50 mm length, were weighed, and the linear density was calculated using EquationEquation (5)(5)
(5) :
where is the linear density in dtex,
is mass of fiber bundle in mg,
is number of fibers per bundle, and
is length of fibers.
Tensile properties
Tensile properties (breaking force, breaking tenacity, and elongation) of unbleached and bleached sample PALF were determined in a constant-rate-of-extension (CRE) type tensile testing machine, based on standard test method ASTM D3822:2007. Twenty test specimens were tested with 50 mm of gauge length and 10 mm/min of rate of extension.
The breaking tenacity of individual specimens was evaluated based on the linear density of each fiber sample, using EquationEquation (6)(6)
(6) :
where is the breaking tenacity in cN/tex,
is the breaking force in cN, and
is the linear density in tex.
Characterization of dyed PALF
Colorimetric data
The reflectance of the PALF samples was measured at and color strength (
values) of the dyed PALF was determined using the Kubelka-Munk equation (EquationEquation (7)
(7)
(7) ) (Kubelka and Munk Citation1931):
where is the absorption coefficient,
is the scattering coefficient, and
is the reflectance value of the dyed PALF at the wavelength of maximum absorbance.
The effect of the various mordant to the color strength was determined using the percentage of the color strength SUM () based on DIN 55,986, where the
represents the ratio of (
) data between natural dyed PALF with mordant and natural dyed PALF without mordant at all visual wavelength (400–700 nm) according to the equation (EquationEquation (8)
(8)
(8) ).
where >100 is dyed PALF with mordant has more strength in color than dyed PALF without mordant,
<100 is dyed PALF with mordant has less strength in color than dyed PALF without mordant, and
= 100 is dyed PALF with and without mordant have the same color strength.
Colorfastness properties
The light fastness properties of dyed PALF samples were exposed to daylight according to the AATCC 16:2004 test method. Color changes (fading) were assessed using the blue scale. The tests for colorfastness to perspiration are based on the solution prepared by simulating the acidic and alkaline perspiration according to the ISO 105 E04:2013 test method. To evaluate wash fastness, the dyed PALF samples were evaluated according to the AATCC 61:2013 test method. The change in the color of dyed fabrics and the staining of the adjacent white fabric were evaluated by gray-scale standards.
FTIR Analysis
Bleached and differently dyed PALF samples were cut into small particle sizes of about 1 mm and analyzed using Perkin Elmer Spectrum Two FTIR Spectrometer. The spectra were recorded from 4000 to 500 cm−1 wavenumber with 16 scans and a spectral resolution of 1 cm−1.
Results and discussion
Characterization of bleached PALF
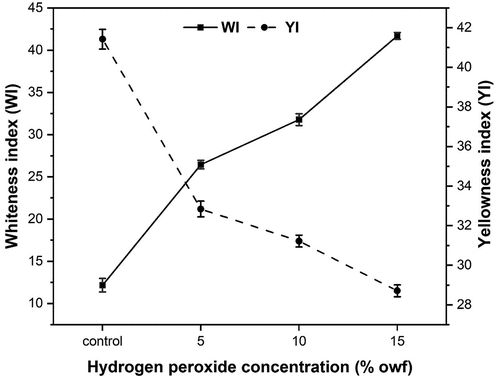
Whiteness index and yellowness index
Finishing processes like the dyeing and printing on cellulosic fibers, yarns, and fabrics necessitate some pretreatments, in other words, scouring and bleaching, to remove natural and added impurities with the aim of improving their absorbency and satisfactory whiteness to receive the color (Dickinson Citation1986). Hydrogen peroxide is an effective oxidizing agent thoroughly bleached the fabric and removed from the fabric after washing.
As can be seen in , the PALF in the natural condition, prior to the bleaching process, has a natural more yellowish color and a rigid appearance. This phenomenon can be attributed to the nature of each plant species, but also to the plant maturity and the method in which the fibers were extracted (Medina Citation1959). Therefore, due to the significantly yellowing shade of the PALF samples, it was necessary to the bleaching process to achieve a visually satisfactory degree of whiteness, resulting in whiter fibers than the unbleached fibers.
In order to increase the efficiency of the bleaching process, increasing the concentration of hydrogen peroxide and analyzing the effects on the color coordinates and whiteness index of the PALF were studied. From , it was observed that an increasing concentration of hydrogen peroxide increased the whiteness index of PALF, whereas the yellowness index decreased. In bleaching processes, hydrogen peroxide removes the pigment colorant from natural fibers by oxidative reaction. Thus, as the concentration of hydrogen peroxide increases, the rate of bleaching increases, and correspondingly, whiteness increases, and yellowness is reduced.
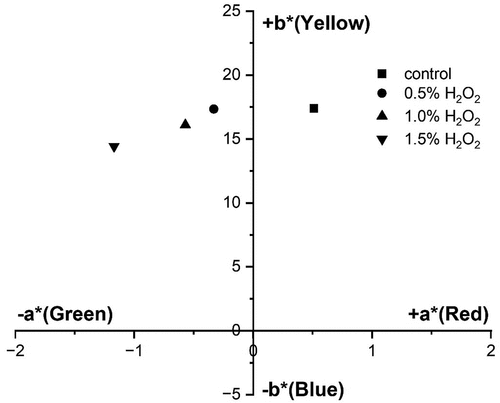
Color coordinates
From , the brightness () of the bleached PALF samples has generally shown an increase as the hydrogen peroxide concentration increases. From
-
plot (), it is evident that the unbleached PALF sample falls in the red-yellow coordinate of CIE
color space. After bleaching with hydrogen peroxide, samples were shifted toward the green-yellow region of the color space. When the hydrogen peroxide concentration increased, the lower
* value of samples shifted color more toward the blue region of CIE
color space, which corresponds to the decrease in the yellowness index.
Table 1. The effect of H2O2 concentration (%owf) on color coordinates of bleached PALF.
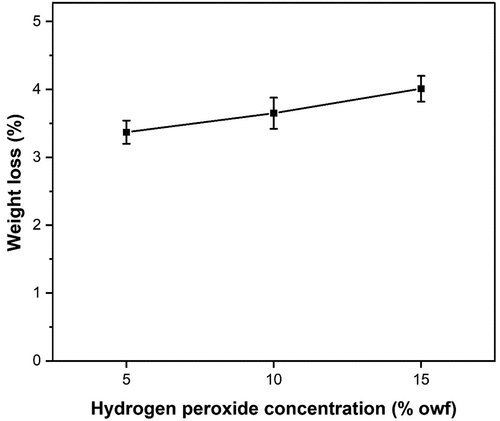
Weight loss
Weight loss is a quantitative indicator of the effectiveness of bleaching. Bleaching is expected to reduce the impurity content, which leads to the weight reduction of the raw fibers. As can be seen from , the weight loss percentage of the bleached PALF samples has generally shown an increase as the hydrogen peroxide concentration increases. This can be explained by the effect of the removal of noncellulosic matter content (more specifically oils, waxes, and fats) from the fiber surface.
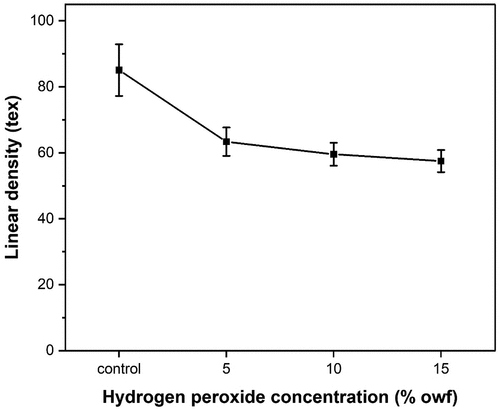
Linear density
Linear density is a property related to fiber fineness and intrinsic mass. Because most natural fibers have an irregular diameter along their length, the definition of linear density is the most useful and reliable method of describing the fiber’s fineness and coarseness (Harwood and Harwood Citation2012; Sief, Shahat, and Arafa Citation2022). In this work, this property is particularly useful for studying the effects of bleaching on the changes in the diameter of fibers and also providing a quantitative indication of bleaching efficiency.
PALF consists of noncellulosic materials which render the fiber to be brittle and coarser and varies between 77 to 93 tex; therefore, it has to be pretreated in order to make the fibers soft and fine. The results from demonstrate that the linear density of bleached PALF is less than that of unbleached PALF, which indicates that bleached PALF is finer than unbleached PALF. Moreover, the linear density of the bleached PALF has generally shown a decrease as the hydrogen peroxide concentration increases. This considerable decrease in linear density for PALF indicates that the bleaching process allowed for better individualization of thick fiber bundles into elementary fibers and fiber bundles with lower linear density.
Tensile properties
The mechanical properties are one of the most important properties of textile fibers, especially because they affect their behavior in processing as well as the characteristics of the final product, which are determinants for the evaluation of the textile application viability (Morton and Hearle Citation1997). Bleaching processes, which modify the structural characteristics of fibers, also alter their mechanical properties, generally reducing fiber strength. Thus, for the bleaching process to be successful, it is desirable that the improvement in whiteness of the fiber be equal to or greater than its loss in strength, which is represented by the ratio of breaking force (cN) to linear density (tex), also called breaking tenacity (cN/tex).
shows the effect of the amount of hydrogen peroxide in the reaction medium on the mechanical properties of the bleached PALF. The obtained results reveal that increasing the concentration of hydrogen peroxide is accompanied by a significant gradual decrement in the percent breaking force, breaking tenacity, and elongation at break compared with the unbleached PALF. These behaviors reflect the extent of removal of the noncellulosic materials from the PALF structure as the concentration of hydrogen peroxide in the bleaching medium increases. These noncellulosic materials are very important in the structure of cellulose fibers, and certain amounts of noncellulosic materials must remain together with the cellulose fibers to bind them and maintain reasonable tensile properties. It is clear that low concentrations of hydrogen peroxide in the bleaching medium (5%owf) lead to little removal of noncellulosics and accordingly good mechanical properties, but the whiteness index is not satisfactory. On the contrary, high concentrations of hydrogen peroxide in bleaching medium (15%owf) lead to high removal of noncellulosics and, accordingly, very bad mechanical properties and a high whiteness index. This result suggests that the optimum concentration of hydrogen peroxide, which leads to an acceptable whiteness index and retains a good deal of the PALF’s tensile strength, is 10%owf.
Table 2. The effect of H2O2 concentration (%owf) on mechanical properties of bleached PALF.
Characterization of dyed PALF
Color strength and color coordinates
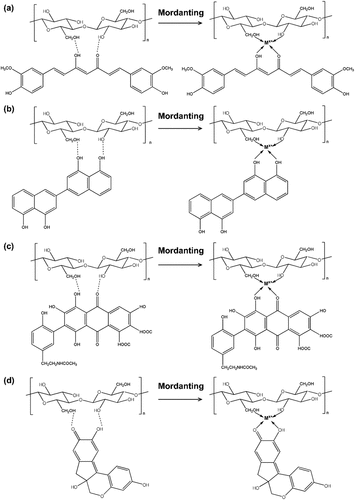
Natural dyes mainly consist of phenolic compounds which play an important role in plant growth and reproducibility. Phenolic compounds based on their different chemical structures, are divided into groups corresponding to flavonoids, quinones, curcuminoids, and tannins. The chemical structures of the coloring agents in the plant extracts are shown in .
Figure 7. The chemical structures of the coloring agents in the plant extracts (a) turmeric, (b) diospyrol, (c) laccaic acid a and B, and (d) brazilin and brazilein.
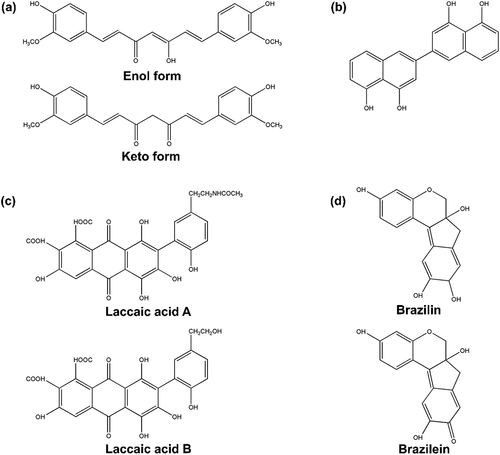
The structure of curcumin, the main component of turmeric, is shown in . Curcumin belongs to the carotenoid compounds which are completely different in chemical structure from anthocyanins (Han and Yang Citation2005). Ebony fruit is a rich source of diospyrol, which is a polyhydroxybinaphthyl compound, as shown in (Chitichotpanya et al. Citation2023). Lac dye or laccaic acid has two major compositions: laccaic acid A and B whose structures are shown in (Kongkachuichay, Shitangkoon, and Chinwongamorn Citation2002). The major coloring component of sappan wood is Brazilian but Brazilian, which is the oxidized product of Brazilin, is also present in (Ohama and Tumpat Citation2014).
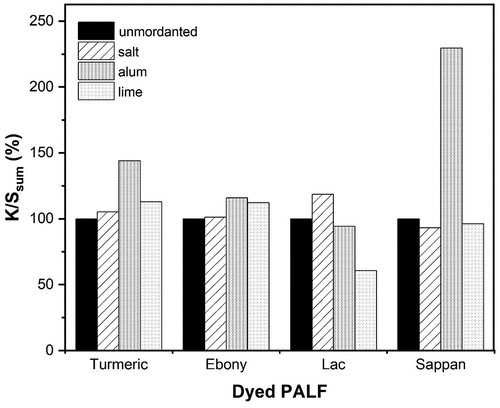
The effects of mordant on color strength are shown in . As can be observed from the data, unmordanted PALF dyed with turmeric and ebony extract displayed a lower dye uptake than mordanted PALF dyed with the same natural colors. The color strength was increased in the following order: alum > lime > salt.
The affinity of the dye to the fiber depends on several parameters such as dye/fiber interactions including the polarity and the nature of their functional groups. PALF is composed of insoluble cellulose made of glucoses which have free hydroxyl groups. Hydrogen bonds and physical bonding occurs between the dyestuff and the cellulose fiber, leading to the coloring of the fiber.
In addition to hydrogen bonds and physical bonding, the dye affinity was also dependent to the metal mordant due to the formation of a metal complex between dye, metal, and fiber. As a result, an insoluble complex including a dye molecule, a metal ion, and a functional group of fibers forms. Therefore, by applying metal mordant, the uptake of colorant and the washing fastness is increased (Baliarsingh et al. Citation2012).
However, when dyed with lac extract, the lime-mordanted PALF had a significantly lower color strength than the unmordanted PALF. The result may be explained by the lac’s coagulation-flocculation with lime as a coagulant, which reduced dye molecules are transferred to the fiber (Malakootian and Fatehizadeh Citation2010). Notably, when dyeing with lac extract, the alum-mordanted PALF greatly increases the color strength compared to the unmordanted PALF.
The colorimetric coordinates such as ,
and
of the dyed PALF samples are shown in . Lightness values shows that mordants changed
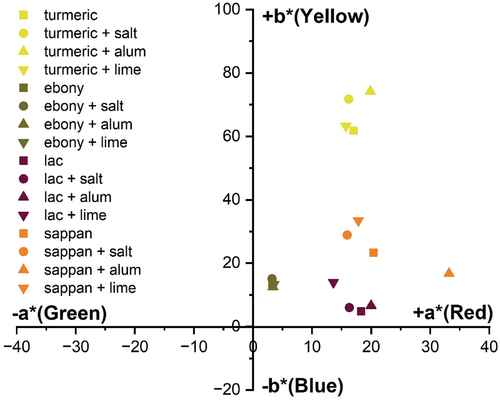
values significantly depending on the type of mordant used. Apart from samples dyed using alum as a mordant, the mordanted dyed PALF samples had a higher lightness (L*) value than those dyed without the use of a mordant. Additionally, all samples had a yellowish and reddish color depth, as shown by the values of
and
(). This suggests that the electronic transition caused by alum mordanting occurs at a maximal wavelength. The result shows that alum mordanting indicates the occurrence of an electronic transition at a longer wavelength. It is evident that each mordant used has considerable effects on the electronic transition and the final colors due to the presence of chromophores and auxochromes (Kusumawati, Samik, and Muslim Citation2020).
Table 3. Color coordinate values of dyed PALF with extracts of turmeric powders, ebony fruit, lac, and sappan wood.
Colorfastness
The majority of natural dyes have an affinity for adsorption on cellulosic materials. In the process of dyeing, dye molecules are retained by the fiber via van der Waal forces or hydrogen bonding (Shahid, Islam, and Mohammad Citation2013). Mordanting is a part of the fiber treatment process that also determines the success rate of dyeing. The stability of the reaction between the dye and fibers is determined by mordanting. Besides being closely related to color stability, the success of mordanting will also determine the intensity and levelness of the final color. Metal mordant is one type of mordant that has been widely used in natural textile dyeing. In dyeing, mordants act as an intermediary for the reaction between fibers and natural dyes that have the same negative charge tendency. The same charge tendency causes the minimum reaction that occurs between fibers and natural dyes. This condition results in a low intensity of colors. The presence of a positively charged mordant that acts as an intermediate reaction between the two is crucial for solving this issue. The bindings that are formed between the fibers, mordants, and natural dyes will play an important role in determining the change in color intensity in the fiber caused by washing, sunlight, and sweat.
Metal meta-mordanting improved the affinity of PALF for natural dye through formation of PALF-metal-dye coordination complex. The mechanisms of interaction between PALF, mordants, and dye extracted from turmeric powder, ebony fruit, lac, and sappan wood are shown in .
Figure 10. Schematic representation of interaction of PALF, metal, and dye; (a) turmeric, (b) ebony, (c) lac, and (d) sappan (when Mx+ = mordant Al3+, Ca2+, Na+).
shows the colorfastness results of the PALF dyed with different natural dyes. A negligible, no color change (good) was observed for light fastness properties of the unmordanted PALF dyed with ebony fruit. These appreciable properties of the unmordanted dyed PALF might be as a result of hydrogen bonding, van der Waals force and mechanical adhesion existing between PALF and dye molecules according to previous works (Chitichotpanya et al. Citation2023). It should be noted that the PALF samples dyed with turmeric, lac and sappan had low light fastness. Turmeric, lac, and sappan undergo severe photooxidation leading to the degradation of the chromophores. The light fastness of dyed PALF was improved by using the mordants. Furthermore, the formation of the metal complex is believed to dissipate the absorbed energy within the linked molecules by resonance; hence, it protects the dye structure from photolytic degradation (Vankar Citation2000).
Table 4. Fastness properties of dyed PALF with extracts of turmeric powders, ebony fruit, lac, and sappan wood.
As expected, mordanted-dyed PALF demonstrated high washing fastness despite the type of natural dyes, while dyed PALF without using mordants gained a lower value of fastness. The fundamental interactions between dye and fiber are hydrogen bonds and physical bonds. By using a mordant, dye molecules form complexes with the fabric, increasing washing fastness. The complex forms through the coordination of metal ions with specific groups of dyes, such as two hydroxyl groups or a hydroxyl group and a carbonyl group in neighboring positions (Zarkogianni et al. Citation2010).
The perspiration fastness test shows good results for no color change and negligible color staining in both acidic and alkaline media, except for mordanted PALF dyed with sappan, which shows a loss of color, and moreover negligible color staining in both acidic and alkaline media from the sappan. The loss of color and variation of grade value results can be related to the ionization of the natural dye during the alkaline test since most natural dyes have hydroxyl groups that ionize under alkaline conditions. Some of the samples were tested in acidic conditions, which can make them fade (Jothi Citation2008). However, it was clearly noted that the graded value results can be acceptable, and the dye extract can be useful for textile coloring.
The color strength and colorfastness properties indicate that alum mordant gave the best dyeing performance for natural dyed PALF. These results confirm that metal (Al) from alum mordant makes strong chemical bonds with cellulosic substrate and dye since color strength values increase in comparison to color strength values of the PALF samples mordanted in the same way. These results correlate well with other research (Haja Citation2019; Kovačević et al. Citation2021) which has shown that optimization of metal mordant concentrations and usage of lower concentrations can improve colorfastness to wash, light, and perspiration.
FTIR Analysis
FTIR is one of the spectroscopic methods widely used to characterize the functional groups and describe the interactions of molecules. shows FTIR spectra of the bleach, dyed, and mordanted-dyed PALF within the range of 400–500 cm−1. From . A, the spectra of the bleach PALF revealed strong absorption bands at 1032, 1106, 1157, 1427, 1640, 1731, 2900, and 3335 cm−1. The region at 3335 cm−1 indicates bands of O-H functional groups in cellulose, the main constituent of PALF. Transmission bands at 2917 and 1640 cm−1 are identified to the C-H stretching and absorbed water, respectively (Gaba et al. Citation2021). The band at 1427 cm−1 corresponds to O-H bending and other bands indicated at 1159, 1104, and 1032 cm−1 correspond to the C-O-C stretch of the −1,4-glycosidic linkage (Ketabchi et al. Citation2016). The FTIR spectra of turmeric dyed PALF have been presented in . The typical absorption peaks at 1031, 1644, 2919, and 3364 cm−1, are related to the stretching of C2H3, R2-C=O, C-H, and O-H, respectively (Ghoreishian et al. Citation2013). The FTIR spectra of turmeric dyed PALF with various mordants show for the R2-C=O and O-H bands a shift toward the lower wavenumber. This revealed that in the complex formation, the ions interacted with the carbonyl and hydroxyl groups of dye.
Figure 11. FTIR spectra of dyed PALF; (a) turmeric, (b) ebony, (c) lac, and (d) sappan (when A: bleached, B: dyed, C: salt mordanted-dyed, D: alum mordanted-dyed, E: lime mordanted-dyed).
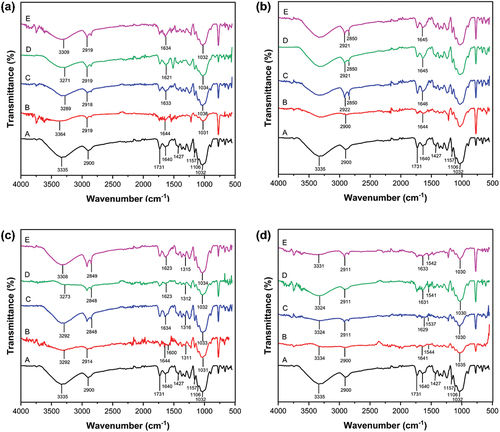
shows that the composition of ebony fruit dyed PALF appearing at peak 1644, and 2900 cm−1, are related to the stretching of C=C aromatic group, and O-H, respectively. O-H stretching vibration was a characteristic of polyphenols (Bharadwaj et al. Citation2021). It is seen in that there is an alteration of intensity and shifting of peaks in the case of O-H stretching frequency of ebony fruit dyed PALF with various mordants at 2850 cm−1, which indicates the involvement of hydroxyl groups in the interaction between fiber, mordant, and dye molecules.
From , a broad peak observed between 3000 cm−1 and 3400 cm−1 could be assigned to the N-H stretching, phenolic O-H or O-H of the carboxylic acid. The transmission peak observed at 2914 cm−1 was assigned to the C-H stretching of aliphatic or aromatic groups. The peak at 1600 cm−1 was referred to the C=C aromatic group. The peaks between 1000 cm−1 and 1320 cm−1 were indicated to the C-O stretching of primary alcoholic group (-CH2-OH) and COOH group, respectively (Khan et al. Citation2012; Nacowong and Saikrasun Citation2016). The aromatic C-H bending appeared between 675 cm−1 and 1000 cm−1 peak. The C=O group was observed at 1644 cm−1. The FTIR spectra of the lac dyed PALF with various mordants show a weak broad band for the C=O group shift toward a lower wavenumber.
As shown in , the FTIR spectra of sappan wood dyed PALF show peaks at 3334, 1641, 1544, and 1032 cm−1 responsible for the -OH stretching, C=O stretching, C=C, and C-O ester present in the components of this dye (Bukhari et al. Citation2023). The weak peaks for the -OH and C=O stretching were found in mordanted-dyed PALF samples.
The dyed PALF with salt, alum, and lime mordants show low-intensity peaks and a shift toward the lower wavenumber for the O-H bands, which is attributed due to the formation of complex between metal ions and PALF. The main difference between the samples is observable at 500–600 cm−1, which is related to sulfate groups of the mordants. It seems that functional groups can form coordination bonds with metallic ions, which appear at frequencies lower than 600 cm−1, which is difficult to record.
Conclusion
Pineapple leaf fibers were bleached and dyed using natural dyes to generate commercialized and colored textile materials. Bleaching with hydrogen peroxide provided the best results with a 10%owf concentration, which offered a good whiteness index and an acceptable tensile strength. The extract of turmeric powder, ebony fruit, lac, and sappan wood can be used as a natural dye for pineapple fibers. A variety of shades can be created by using different types of mordants and their combinations with meta-mordanting dyeing techniques. Mordanting with salt, alum, and lime during simultaneous dyeing increased the color strength of the PALF. The only exceptions were for the mordanted PALF and dyed with lac extract exhibited significantly lower color strength than the unmordanted PALF. PALF demonstrated a satisfactory affinity for ebony fruit extracted dye with adequate fastness properties (from good to excellent) for light, wash, and perspiration, whether unmordanted or mordanted. Moreover, mordanting during simultaneous dyeing increased the fastness properties of the samples. When compared with NODS (Natural Organic Dye Standard) criteria, the extracted dyestuffs from turmeric powder, ebony fruit, and sappan with salt, alum, and lime as mordants are suitable for PALF dyeing. There is potential for the utmost utilization of natural dyes for dyeing pineapple fibers, which will protect the environment and earth from pollution and ecological imbalances in other ways.
Highlights
Pineapple leaf fibers were bleached with hydrogen peroxide and colored with natural dyes i.e. turmeric, ebony fruit, lac, and sappan wood.
Eco-friendly and easily found metal salts i.e. salt, alum, and lime were used as metallic mordant.
Meta- mordanting increased the color strength and colorfastness properties of the PALF.
Acknowledgments
The authors would like to thank Srinakharinwirot University for providing financial support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adeel, S., K. M. Zia, M. Abdullah, F. U. Rehman, M. Salam, and M. Zuber. 2019. “Ultrasonic Assisted Improved Extraction and Dyeing of Mordanted Silk Fabric Using Neem Bark as Source of Natural Colorant.” Natural Product Research 33 (14): 2060–22. https://doi.org/10.1080/14786419.2018.1484466.
- Alam, A., Z. Ahmed, N. Morshed, P. Talukder, and T. Rahman. 2022. “Analysis of Physio-Mechanical Properties of Pineapple Leaf Fiber.” International Journal of Life Science Research Archive 3 (2): 113–116. https://doi.org/10.53771/ijlsra.2022.3.2.0127.
- Amin, A. N. M., W. S. Ruznan, S. A. Suhaimi, J. M. Yusuf, M. I. A. Kadir, and M. A. M. Nor. 2021. “Washfastness Properties of Dyed Pineapple Leaf Fibre Using Different Dyeing Techniques.” Journal of Academia 9 (1): 66–72.
- Asim, M., K. Abdan, M. Jawaid, M. Nasir, Z. Dashtizadeh, M. R. Ishak, and M. E. Hoque. 2015. “A Review on Pineapple Leaves Fibre and Its Composites.” International Journal of Polymer Science 2015:1–16. https://doi.org/10.1155/2015/950567.
- Azeem, M., N. Iqbal, R. A. Mir, S. Adeel, F. Batool, A. A. Khan, and S. Gul. 2019. “Harnessing Natural Colorants from Algal Species for Fabric Dyeing: A Sustainable Eco-Friendly Approach for Textile Processing.” Journal of Applied Phycology 31 (6): 3941–3948. https://doi.org/10.1007/s10811-019-01848-z.
- Baliarsingh, S., A. K. Panda, J. Jena, T. Das, and N. B. Das. 2012. “Exploring Sustainable Technique on Natural Dye Extraction from Native Plants for Textile: Identification of Colourants, Colourimetric Analysis of Dyed Yarns and Their Antimicrobial Evaluation.” Journal of Cleaner Production 37:257–264. https://doi.org/10.1016/j.jclepro.2012.07.022.
- Banerjee, A. N., O. P. Kotnala, and S. R. Maulik. 2018. “Dyeing of Eri silk with natural dyes in presence of natural mordants.” Indian Journal of Traditional Knowledge 17 (2): 396–399.
- Bharadwaj, K. K., B. Rabha, S. Pati, B. K. Choudhury, T. Sarkar, S. K. Gogoi, N. Kakati, D. Baishya, Z. A. Kari, and H. A. Edinur. 2021. “Green Synthesis of Silver Nanoparticles Using Diospyros Malabarica Fruit Extract and Assessments of Their Antimicrobial, Anticancer and Catalytic Reduction of 4-Nitrophenol (4-NP).” Nanomaterials: Overview and Historical Perspectives 11 (8): 1999. https://doi.org/10.3390/nano11081999.
- Botella, J. R., and M. Smith. 2008. “Genomics of Pineapple, Crowning the King of Tropical Fruits.” In Genomics of Tropical Crop Plants, edited by P. H. Moore and R. Ming, 441–451. New York: Springer.
- Bukhari, M. N., M. A. Wani, M. Fatima, J. S. S. Bukhari, M. Shabbir, L. J. Rather, and F. Mohammad. 2023. “Dyeing of Wool with Sappan Wood Natural Dye Using Metal Salts for Enhancement in Color and Fastness Properties.” Journal of Natural Fibers 20 (2): 2208890. https://doi.org/10.1080/15440478.2023.2208890.
- Bunfueang, P., S. Samosorn, J. B. Bremner, W. Sajomsang, P. Gonil, A. Saisara, and M. Chairat. 2019. “Additive Effects on Cotton Dyeing with Dye Extract from Achiote Seeds.” Indian Journal of Fibre & Textile Research 44 (4): 466–474. https://doi.org/10.56042/ijftr.v44i4.19562.
- Chengaiah, B., K. M. Rao, K. M. Kumar, M. Alagusundaram, and C. M. Chetty. 2010. “Medicinal Importance of Natural Dyes-A Review.” International Journal of PharmTech Research 2 (1): 144–154.
- Chitichotpanya, P., N. Vuthiganond, P. Chutasen, and T. Inprasit. 2023. “Green Production of Simultaneous Coloration and Functional Finishing on Hemp Textiles Through Dyeing with Diospyros Mollis Griff. Extract.” Journal of Metals, Materials and Minerals 33 (2): 108–119. https://doi.org/10.55713/jmmm.v33i2.1697.
- Daochalermwong, A., N. Chanka, K. Songsrirote, P. Dittanet, C. Niamnuy, and A. Seubsai. 2020. “Removal of Heavy Metal Ions Using Modified Celluloses Prepared from Pineapple Leaf Fiber.” American Chemical Society Omega 5 (10): 5285–5296. https://doi.org/10.1021/acsomega.9b04326.
- Dickinson, K. 1986. “Preparation.” In Cellulose, edited by C. Preston, 55–105. London: Dyers’ Company Publications Trust.
- Divya, R., P. Singh, B. Baboo, and K. M. Prasad. 2011. “Evaluation of Coloring Efficacy of Lac Dye in Comminuted Meat Product.” Journal of Food Science and Technology 48 (3): 378–381. https://doi.org/10.1007/s13197-011-0308-1.
- Do, K. L., M. Su, A. Mushtaq, and F. Zhao. 2023. “Dyeing of Silk with Natural Lac Dye from Laccifer Lacca Kerr. and Evaluation of Antibacterial and UV-Protective Properties.” Fibers and Polymers 24 (8): 2773–2783. https://doi.org/10.1007/s12221-023-00254-0.
- Feiz, M., and H. Norouzi. 2014. “Dyeing Studies of Wool Fibers with Madder (Rubia Tinctorum) and Effect of Different Mordants and Mordanting Procedures on Color Characteristics of Dyed Samples.” Fibers and Polymers 15 (12): 2504–2514. https://doi.org/10.1007/s12221-014-2504-x.
- Ferreira, L. E., B. V. Muniz, T. O. Bittar, L. A. Berto, S. R. Figueroba, F. C. Groppo, and A. C. Pereira. 2014. “Effect of Particles of Ashes Produced from Sugarcane Burning on the Respiratory System of Rats.” Environmental Research 135:304–310. https://doi.org/10.1016/j.envres.2014.07.030.
- Flora of China Editorial Committee. 1988. In Flora of China, 105. Beijing: Science Press.
- Gaba, E. W., B. O. Asimeng, E. E. Kaufmann, S. K. Katu, E. J. Foster, and E. K. Tiburu. 2021. “Mechanical and Structural Characterization of Pineapple Leaf Fiber.” Fibers 9 (8): 51. https://doi.org/10.3390/fib9080051.
- Ganz, E. 1979. “Whiteness Formulas: A Selection.” Applied Optics 18 (7): 1073–1078. https://doi.org/10.1364/AO.18.001073.
- Ghaheh, F. S., S. M. Mortazavi, F. Alihosseini, A. Fassihi, A. S. Nateri, and D. Abedi. 2014. “Assessment of Antibacterial Activity of Wool Fabrics Dyed with Natural Dyes.” Journal of Cleaner Production 72:139–145. https://doi.org/10.1016/j.jclepro.2014.02.050.
- Ghoreishian, S. M., L. Maleknia, H. Mirzapour, and M. Norouzi. 2013. “Antibacterial Properties and Color Fastness of Silk Fabric Dyed with Turmeric Extract.” Fibers and Polymers 14 (2): 201–207. https://doi.org/10.1007/s12221-013-0201-9.
- Gogoi, S., S. Chakraborty, and M. D. Saikia. 2018. “Surface Modified Pineapple Crown Leaf for Adsorption of Cr(vi) and Cr(iii) Ions from Aqueous Solution.” Journal of Environmental Chemical Engineering 6 (2): 2492–2501. https://doi.org/10.1016/j.jece.2018.03.040.
- Haar, S., E. Schrader, and <. T. A. I. T.<. N. A. I.<. N. Gatewood 2013 B. M. 2013. “B Comparison of Aluminum Mordants on the Colorfastness of Natural Dyes on Cotton.” Clothing and Textiles Research Journal 31 (2): 97–108. https://doi.org/10.1177/0887302X13480846.
- Haja, A. 2019. “Dyeing of Cotton Fabric with Natural Dyes Improved by Mordants and Plasma Treatment.” Progress in Color, Colorants and Coatings 12 (3): 191–201.
- Han, S., and Y. Yang. 2005. “Antimicrobial Activity of Wool Fabric Treated with Curcumin. 2005.” Dyes and Pigments 64 (2): 157–161. https://doi.org/10.1016/j.dyepig.2004.05.008.
- Harsito, C., A. R. Prabowo, S. D. Prasetyo, and Z. Arifin. 2021. “Enhancement Stability and Colorfastness of Natural Dye: A Review.” Open Engineering 11 (1): 548–555. https://doi.org/10.1515/eng-2021-0055.
- Harwood, J., and R. Harwood. 2012. “Testing of Natural Textile Fibres.” In Handbook of Natural Fibres: Types, Properties and Factors Affecting Breeding and Cultivation, edited by R. M. Kozłowski, 345–390. Cambridge: Woodhead Publishing.
- Hirschler, R. 2012. “Whiteness, Yellowness, and Browning in Food Colorimetry: A Critical Review.” In Color in Food, edited by J. L. Caivano and M. D. P. Buera, 93–103. Boca Raton: CRC Press.
- Hosen, M. D., M. F. Rabbi, M. A. Raihan, and M. A. A. Mamun. 2021. “Effect of Turmeric Dye and Biomordants on Knitted Cotton Fabric Coloration: A Promising Alternative to Metallic Mordanting.” Cleaner Engineering and Technology 3:100124. https://doi.org/10.1016/j.clet.2021.100124.
- Jabar, J. M., T. E. Adedayo, and Y. A. Odusote. 2021. “Green, Eco-Friendly and Sustainable Alternative in Dyeing Cotton Fabric Using Aqueous Extract Mucuna Slonaei F Dye: Effects of Metal Salts Pre-Mordanting on Color Strength and Fastness Properties.” Current Research in Green and Sustainable Chemistry 4:100151. https://doi.org/10.1016/j.crgsc.2021.100151.
- Jabar, J. M., A. I. Ogunmokun, and T. A. A. Taleat. 2020. “Color and Fastness Properties of Mordanted Bridelia Ferruginea B Dyed Cellulosic Fabric.” Fashion and Textile 7 (1): 1–13. https://doi.org/10.1186/s40691-019-0195-z.
- Jabar, J. M., A. F. Ogunsade, and Y. A. Odusote. 2023. “Utilization of Nigerian Mango (Mangifera Indica L) Leaves Dye Extract for Silk Fabric Coloration- Influence of Extraction Technique, Mordant and Mordanting Type on the Fabric Color Attributes.” Industrial Crops & Products 193:116235. https://doi.org/10.1016/j.indcrop.2022.116235.
- Jalil, A. J., S. Mahmood, A. H. A. Rashid, S. H. Nasir, S. A. Ibrahim, and M. R. Ahmad. 2018. “Extraction of Eco-Friendly Natural Dyes from Pina Leaves and Their Application on Wool Fabrics.” International Journal of Engineering & Technology 7 (4.14): 382–386. https://doi.org/10.14419/ijet.v7i4.14.27689.
- Jothi, D. 2008. “Extraction of Natural Dyes from African Marigold Flower (Tagetes Ereecta L.) for Textile Coloration.” AUTEX Research Journal 8 (2): 49–53. https://doi.org/10.1515/aut-2008-080204.
- Ketabchi, M. R., M. Khalid, C. T. Ratnam, S. Manickam, R. Walvekar, and E. Hoque. 2016. “Sonosynthesis of Cellulose Nanoparticles (CNP) from Kenaf Fiber: Effects of Processing Parameters.” Fibers and Polymers 17 (9): 1352–1358. https://doi.org/10.1007/s12221-016-5813-4.
- Khan, M. I., M. Shahid, S. A. Khan, M. Yusuf, F. Mohammad, and M. A. Khan. 2012. “Studies on Application of Lac Natural Dye on Wool Using Eco-Friendly Metal Mordants.” Colourage 59 (3): 42–51.
- Kongkachuichay, P., A. Shitangkoon, and N. Chinwongamorn. 2002. “Studies on Dyeing of Silk Yarn with Lac Dye: Effects of Mordants and Dyeing Conditions.” Science Asia 28 (2): 161–166. https://doi.org/10.2306/scienceasia1513-1874.2002.28.161.
- Kovačević, Z., A. Sutlović, A. Matin, and S. Bischof. 2021. “Natural Dyeing of Cellulose and Protein Fibers with the Flower Extract of Spartium Junceum L. Plant.” Materials 14:4091.
- Kubelka, P., and F. Munk. 1931. “An Article on Optics of Paint Layers.” Fuer Tekn Physik 12 (593–601): 593–609.
- Kusumastuti, A., D. S. Fardhyanti, and S. Anis. 2020. “Brachiaria Mutica Dyes Powder for Textile Application: Dyeing Quality of Cotton Fabrics.” Journal of Physics Conference Series 1444 (1): 012010. https://doi.org/10.1088/1742-6596/1444/1/012010.
- Kusumawati, N., A. B. S. Samik, and S. Muslim. 2020. “New Natural Dyes Development: Caesalpinia Sappan L.-Curcuma Longa Blended Dyes.” Rasayan Journal of Chemistry 13 (2): 992–999. https://doi.org/10.31788/RJC.2020.1325410.
- Kusumawati, N., A. B. Santoso, M. M. Sianita, and S. Muslim. 2017. “Extraction, Characterization, and Application of Natural Dyes from the Fresh Mangosteen (Garcinia Mangostana L.) Peel.” International Journal on Advanced Science, Engineering and Information Technology 7 (3): 878–884. https://doi.org/10.18517/ijaseit.7.3.1014.
- Lim, Z. E., Q. B. Thai, D. K. Le, T. P. Luu, P. T. T. Nguyen, N. H. N. Do, P. K. Le, N. Phan-Thien, X. Y. Goh, and H. M. Doung. 2020. “Functionalized Pineapple Aerogels for Ethylene Gas Adsorption and Nickel (II) Ion Removal Applications.” Journal of Environmental Chemical Engineering 8 (6): 104524. https://doi.org/10.1016/j.jece.2020.104524.
- Liu, T., J. Wang, F. Chi, Z. Tan, and L. Liu. 2020. “Development and Characterization of Novel Active Chitosan Films Containing Fennel and Peppermint Essential Oils.” Coatings 10 (10): 936. https://doi.org/10.3390/coatings10100936.
- Malakootian, M., and A. Fatehizadeh. 2010. “Color Removal from Water by Coagulation/Caustic Soda and Lime.” Iranian Journal of Environmental Health Science and Engineering 7 (3): 267–272.
- Ma, X., Y. Wei, S. Wang, X. Zuo, and B. Shen. 2020. “Sustainable Ultrasound-Assisted Ultralow Liquor Ratio Dyeing of Cotton Fabric with Natural Turmeric Dye.” Textile Research Journal 90 (5–6): 685–694. https://doi.org/10.1177/0040517519878793.
- Medina, J. C. 1959. “Plantas Fibrosas da Flora Mundial.” In Fiber Plants, edited by Instituto Agronômico (Seção de Plantas Fibrosas), 787–792. Campinas: Instituto Agronômico.
- Morton, W. E., and J. W. S. Hearle. 1997. Physical Properties of Textile Fibres. 3rd ed. Manchester: The Textile Institute.
- Mozaffari, E., and B. Maleki. 2018. “Alum Mineral and the Importance for Textile Dyeing.” Current Trends in Fashion Technology & Textile Engineering 3 (4): 85–87. https://doi.org/10.19080/CTFTTE.2018.03.555619.
- Mulyanto, S., S. Suyitno, R. A. Rachmanto, L. L. G. Hidayat, A. H. Wibowo, and S. Hadi. 2016. “Synthesis and Characterization of Natural Red Dye from Caesalpinia Sappan Linn.” AIP Conference Proceedings 1717 (1): 040032.
- Nacowong, P., and S. Saikrasun. 2016. “Thermo‑Oxidative and Weathering Degradation Affecting Coloration Performance of Lac Dye.” Fashion and Textiles 3 (1): 18. https://doi.org/10.1186/s40691-016-0070-0.
- Nirmal, N. P., M. S. Rajput, R. G. S. V. Prasad, and M. Ahmad. 2015. “Brazilin from Caesalpinia Sappan Heartwood and Its Pharmacological Activities: A Review.” Asian Pacific Journal of Tropical Medicine 8 (6): 421–430. https://doi.org/10.1016/j.apjtm.2015.05.014.
- Ohama, P., and N. Tumpat. 2014. “Textile Dyeing with Natural Dye from Sappan Tree (Caesalpinia Sappan Linn.) Extract.” International Journal of Fashion and Textile Engineering 8 (5): 432–434.
- Pham, T., and T. Bechtold. 2023. “Natural Dyes in Eastern Asia (Vietnam and Neighboring Countries).” In Handbook of Natural Colorants, edited by C. Stevens, T. Bechtold, A. Manian, and T. Pham, 75–87. UK: John Wiley & Sons Ltd.
- Phan, H. N. Q., J. H. Leu, K. T. Tran, V. N. D. Nguyen, and T. T. Nguyen. 2023. “Rapid Fabrication of Pineapple Leaf Fibers from Discarded Leaves by Using Electrolysis of Brine.” Textiles 3 (1): 1–10. https://doi.org/10.3390/textiles3010001.
- Pimphor, K., and S. Roddecha. 2021. “The Optimization and Comparison of the Electrochemical Performances for the Polyaniline and Melamine Doping Onto Activated Porous Carbon Material Derived from Pineapple Leaf Fiber as Anode Material for Lithium-Ion Batteries.” Materials Today: Proceedings 47:3610–3616. https://doi.org/10.1016/j.matpr.2021.04.259.
- Putra, A., I. Prasetiyo, and Z. Selamat. 2020. “Green Acoustic Absorber from Pineapple Leaf Fibers.” In Pineapple Leaf Fibers: Processing, Properties and Applications, edited by M. Jawaid, M. Asim, P. M. Tahir, and M. Nasir, 143–165. Singapore: Springer.
- Rahman, M. M., M. Kim, K. Youm, S. Kumar, J. Koh, and K. H. Hong. 2023. “Sustainable One-Bath Natural Dyeing of Cotton Fabric Using Turmeric Root Extract and Chitosan Biomordant.” Journal of Cleaner Production 382:135303. https://doi.org/10.1016/j.jclepro.2022.135303.
- Rajeshkumar, G., S. Ramakrishnan, T. Pugalenthi, and P. Ravikumar. 2020. “Performance of Surface Modified Pineapple Leaf Fiber and Its Applications.” In Pineapple Leaf Fibers: Processing, Properties and Applications, edited by M. Jawaid, M. Asim, P. M. Tahir, and M. Nasir, 309–321. Singapore: Springer.
- Ramli, S., S. Md Fadzullah, and Z. Mustafa. 2017. “The Effect of Alkaline Treatment and Fiber Length on Pineapple Leaf Fiber Reinforced Poly Lactic Acid Biocomposites.” Jurnal Teknologi 79 (5–2): 111–115. https://doi.org/10.11113/jt.v79.11293.
- Rani, C. P. 2023. Natural Dyes for Colouring Fabrics. In Innovation Trends in Biological Science, edited by B. Singh, M. M. Barwant, S. Tripathi, V. C. Karande, V. K. Singh, and R. Shrivastava, 119–129. Uttar Pradesh: Blue Rose Publishers.
- Ravindran, L., M. S. Sreekala, and S. Thomas. 2019. “Novel Processing Parameters for the Extraction of Cellulose Nanofibres (CNF) from Environmentally Benign Pineapple Leaf Fibers (PALF): Structure-Property Relationships.” International Journal of Biological Macromolecules 131:858–870. https://doi.org/10.1016/j.ijbiomac.2019.03.134.
- Rejo, A., T. A. Rizky, and D. G. Rajagukguk. 2018. “Study of Natural Dyes and Pineapple Leaf Fibres Growing Locations within Plant Stems on Dyeing Intensity.” E3S Web of Conferences 68:01030. https://doi.org/10.1051/e3sconf/20186801030.
- Samanta, P. 2022. “Basic Principles of Colour Measurement and Colour Matching of Textiles and Apparels.” In Colorimetry, edited by A. K. Samanta, 1–25. London: Intechopen.
- Shahid, M., S. Islam, and F. Mohammad. 2013. “Recent Advancements in Natural Dye Applications: A Review.” Journal of Cleaner Production 53:310–331. https://doi.org/10.1016/j.jclepro.2013.03.031.
- Shen, Y., D. Ma, and X. Ge. 2017. “CO2-looping in Biomass Pyrolysis or Gasification.” Sustainable Energy & Fuels 1 (8): 1700–1729. https://doi.org/10.1039/C7SE00279C.
- Sholikhah, R., and S. N. Widowati. 2023. “The Impact of the Use of Different Mordants Types on the Ecoprint Dyeing Using Secang Wood (Caesalpinia Sappan Linn) Dye on Primisima Fabric.” AIP Conference Proceedings 2677 (1): 100003.
- Sief, M. G., S. A. Shahat, and H. M. Arafa. 2022. “Impact of Fiber Maturity Levels on Fineness, Maturity, Strength and Elongation Measurements in Egyptian Cotton.” IOSR Journal of Polymer and Textile Engineering 9 (4): 13–23.
- Surajarusarn, B., S. Thaiwattananon, S. Thanawan, K. Mougin, and T. Amornsakchai. 2021. “Realising the Potential of Pineapple Leaf Fiber as Green and High-Performance Reinforcement for Natural Rubber Composite with Liquid Functionalized Rubber.” Fibers and Polymers 22 (9): 2543–2551. https://doi.org/10.1007/s12221-021-1018-6.
- Suwama, T., K. Watanabe, O. Monthakantirat, P. Luecha, H. Noguchi, K. Watanabe, and K. Umehara. 2018. “Naphthalene Glycosides in the Thai Medicinal Plant Diospyros Mollis.” Journal of Natural Medicines 72 (1): 220–229. https://doi.org/10.1007/s11418-017-1134-1.
- Todkar, S. S., and S. A. Patil. 2019. “Review on Mechanical Properties Evaluation of Pineapple Leaf Fibre (PALF) Reinforced Polymer Composites.” Composite Part B Engineering 174:106927. https://doi.org/10.1016/j.compositesb.2019.106927.
- Vankar, P. S. 2000. “Chemistry of Natural Dyes.” Resonance 5 (10): 73–80. https://doi.org/10.1007/BF02836844.
- Venil, C. K., N. Z. Yusof, C. A. Aruldass, and W. A. Ahmad. 2016. “Application of violet pigment from Chromobacterium violaceum UTM5 in textile dyeing.” Biologia (Pakistan) 71 (2): 121–127. https://doi.org/10.1515/biolog-2016-0031.
- Yantaboot, K., and T. Amornsakchai. 2017. “Effect of Mastication Time on the Low Strain Properties of Short Pineapple Leaf Fiber Reinforced Natural Rubber Composites.” Polymer Testing 57:31–37. https://doi.org/10.1016/j.polymertesting.2016.11.006.
- Zarkogianni, M., E. Mikropoulou, E. Varella, and E. Tsatsaroni. 2010. “Colour and Fastness of Natural Dyes: Revival of Traditional Dyeing Techniques.” Coloration Technology 127 (1): 18–27. https://doi.org/10.1111/j.1478-4408.2010.00273.x.
- Zhao, M. B., J. Li, S. P. Shi, C. Q. Cai, P. F. Tu, L. Tang, K. W. Zeng, and Y. Jiang. 2013. “Two New Phenolic Compounds from the Heartwood of Caesalpinia Sappan L.” Molecules 19 (1): 1–8. https://doi.org/10.3390/molecules19010001.