ABSTRACT
To examine the impact of in situ water maceration in macerating ponds on the degumming process and fiber quality of freshly kenaf and jute whole plants. Different kenaf/jute fresh samples (whole plant, bast fresh hides) were subjected to distinct maceration treatments: padded film + cover film, cover film only, and no film. Water temperature, degumming bacterial colony counts, and fiber quality were assessed. Results revealed that composting pools with padded film + cover film maintained the highest water temperature. For colony count, padded film + cover film treatment displayed the highest colony count for kenaf, and no film treatment had the highest for jute. Kenaf whole fresh rods treated with padded film + cover film and cover film alone demonstrated the highest fiber strengths at 489.01 N and 494.85 N, respectively. Jute whole fresh rods macerated in padded film + cover film achieved the greatest strength at 392.32 N. Comparisons of fresh fiber strength between whole fresh rod and bastshowed a maximum difference of 150.69 N (kenaf) and 152.88 N (jute). Comprehensive analysis concluded, that the most effective degumming method involved using the whole fresh rods of red hemp/jute in maceration ponds equipped with padded film + cover film.
摘要
考察浸渍池原位水浸对鲜红麻和黄麻全株脱胶过程和纤维品质的影响. 对不同的红麻/黄麻新鲜样品(整株、韧皮新鲜皮)进行了不同的浸渍处理: 加垫膜+覆盖膜、只覆盖膜和不覆盖膜。对水温、脱胶菌落数和纤维质量进行了评估. 结果表明,垫膜+覆盖膜堆肥池的水温最高. 在菌落计数方面,棉膜+覆膜处理对红麻的菌落计数最高,无膜处理对黄麻的菌落计数也最高. 用填充膜+覆盖膜和单独覆盖膜处理的红麻全新鲜棒的纤维强度最高,分别为489.01N和494.85N. 浸渍在加垫膜+覆盖膜中的黄麻全新鲜棒的强度最大,为392.32N. 全新鲜棒与韧皮部新鲜纤维强度的比较显示,最大差异为150.69N(红麻)和152.88N(黄麻). 综合分析得出,最有效的脱胶方法是将红麻/黄麻的整根新鲜棒放入配有衬垫膜+覆盖膜的浸渍池中.
Introduction
The hemp crop is one of the main fiber crops grown and utilized by human beings, and is widely used in production and life (An, Chen, and Jin Citation2020; W. Wang and Cai Citation2008). Hemp is a crop group including agricultural products, such as kenaf (An et al. Citation2018), jute (L. Zhang et al. Citation2021), ramie (An et al. Citation2015), flax (An et al. Citation2023), industrial hemp (Tang et al. Citation2018), and sisal (Z. Yang et al. Citation2023), and is an important cash crop. Kenaf (Hibiscus cannabinus L.) is an annual bast fiber crop of the genus Hibiscus in the family Mallowaceae, also known as kenaf and hibiscus hemp, which has the characteristics of fast growth, strong resistance and wide adaptability (An et al. Citation2022). Kenaf fiber has water absorption, fast water dispersion and antibacterial and antimicrobial effects. Kenaf fiber is recognized as one of the new fiber raw materials that can replace wood (Sapuan et al. Citation2018). Kenaf bast fiber, which has strong tensile strength, soft fiber as well as fiber moisture absorption and water dispersal fast, etc., is an important basic raw material for textile and manufacturing industry, traditionally used in cotton and hemp blending, manufacture of sacks, linen, linen carpets and ropes, and recently developed red hemp mulch, lightweight composite panels, activated charcoal, fodder, and so on, as well as used for soil and water conservation, cultivation substrates, etc (Chen and Yang Citation2014; J. Liu et al. Citation2018; Papadopoulou et al. Citation2014).
Jute (Corchorus capsularis L.) is an annual herbaceous plant of the genus Jute in the family Tiliaceae, and is also known as green hemp and loosestrife. Jute has the advantages of high fiber yield, excellent performance, low price, easy to grow, ecological and environmental protection, and is one of the important natural bast fiber crops in the world (Ning, Li, and Ling Citation2020; L. Zhang et al. Citation2021). In addition to being used to produce low-grade paper products such as textile sacks, linen cloth, hemp rope, curtain cloth, and so on, jute fiber can also be used to manufacture natural plant fiber fabrics with good breathability, moisture absorption and natural antibacterial, antistatic and anti-ultraviolet radiation (S. Zhang and Wu Citation2009), and can be well blended with cotton, wool, viscose and other fibers (Fang et al. Citation2009; Jin et al. Citation2008). It has a wide range of uses, being an important raw material for textiles and paper, and is widely used in construction, panels and automobile interiors (Cheng Citation2017).
The annual production and consumption of kenaf and jute are the second highest among plant fibers after cotton (Xiong Citation2017). Since the fibers of the hemp crop are deeply embedded in the bast, to separate the fibers it is necessary to destroy their gums without compromising the quality of the fibers themselves (J. Zhang et al. Citation2005). Therefore, before the use of red hemp fiber must be degummed, the quality of degumming is a key factor affecting the subsequent fiber spinning and fiber dyeing (Ramaswamy et al. Citation1994; Y. Yang Citation2005). Degumming can be carried out by traditional maceration, biological degumming, oxidative degumming, chemical cooking degumming, and physical combined degumming. Traditional water composting degumming is the harvested red hemp/jute bast or whole culms bundled and soaked in natural waters such as ponds, ditches, lakes, etc., using natural microorganisms for fermentation so as to achieve the removal of non-cellulose and the extraction of kenaf/jute cellulose (Duan Citation2018; H. Li Citation2010).
Bio-degumming refers to the process of obtaining natural fibers that meet the subsequent processing requirements by mainly biodegradation (e.g., bacterial fermentation, fungal fermentation, or enzyme catalysis), that is, removing more than 50% of the gums that should be removed in the fiber raw material, appropriately supplemented by mechanical-physical action or a little chemical action (Z. Liu Citation2009; Yu et al. Citation2023; Zheng Citation2007). Oxidative degumming is the degumming of kenaf with sodium hydroxide and strong oxidizing agents (Yan, Gu, and Yu Citation2011). Chemical cooking degumming is the process of removing gums and extracting cellulose from raw materials like kenaf and jute by alkali cooking and other processes (Ohtani, Mazumder, and Sameshima Citation2001). Physical combined degumming is a combined flash explosion-ultrasonic degumming of kenaf or jute (X. Zhang Citation2017).
So far, biological degumming is still imperfect, and there are problems such as mismatch of enzyme-producing enzyme systems of degumming strains and insufficient degumming capacity of gums, resulting in incomplete degumming of hemp and unstable quality of degumming. Due to the constraints of the red jute degumming mechanism as well as the cost of enzymes, the research and application of hemp biological degumming by directly using mono-enzymes or complex enzymes also need to be further improved (Duan Citation2018; Q. Yang et al. Citation2022). Oxidative degumming, chemical cooking degumming, and physical joint degumming are all experimental studies that have not been promoted to the growers to apply, and the chemical degumming method is complicated with high energy consumption, pollution, and damage to the fiber (He, Yu, and Liu Citation2008; D. Wang et al. Citation2023). Traditional water degumming is still the most commonly used method for degumming kenaf or jute, but degumming in natural water not only pollutes the water surface but is also affected by the temperature, water quality, and technical proficiency, resulting in varying fiber quality (Duan Citation2018; D. Li Citation2023; Ma et al. Citation2009). Based on this, this study pioneered the excavation of an in-situ macerating ponds in a field where kenaf and jute were harvested, and then degumming with water retting. Various treatments were applied to the macerating ponds and experiments were conducted using fresh kenaf/jute plants under different water retting conditions. The objective of this study was to find out the optimum method of retting hemp that minimizes energy consumption, reduces pollution and makes the retting hemp process simpler and more efficient, thereby increasing the utility of the retting hemp technology. Based on this, this study provides the first valuable insights in kenaf/jute cultivation management and resource utilization, and contributes significantly to the promotion of sustainable development of kenaf/jute industry.
Materials and methods
Test material
The late-maturing hybrid combination variety “H368” selected by the Hemp Research Institute of the Chinese Academy of Agricultural Sciences was used for kenaf, and the conventional variety “MY-118” selected by the Xiaoshan Cotton and Hemp Research Institute of Zhejiang Province was used for jute ().
Experimental design
Experiment in the local harvested kenaf ground around the digging macerating pool, macerating pool length 400 cm, width 200 cm, height 50 cm, set up three treatments of macerating pool: (1) macerating pool bottom pad with plastic film and macerating after the top is covered with plastic film; (2) macerating pool after the top is covered with plastic film; (3) macerating pool bottom is not cushioned with plastic film and the top is not covered with plastic film (the traditional method of composting; ); each macerated hemp pools were put into 100 kenaf fresh whole poles, 100 kenaf fresh skins, and 100 jute fresh whole poles and 100 jute fresh skins, with 3 replications per treatment. Each macerating pool was hung into the mercury thermometer in order to be read. 15 September began to macerate hemp, 15 days after the washing of hemp ().
Figure 2. Kenaf macerating pool: macerating pool bottom pad with plastic film and macerating after the top is covered with plastic film (left picture); (2) macerating pool after the top is covered with plastic film (intermediate picture); (3) macerating pool bottom is not cushioned with plastic film and the top is not covered with plastic film (right picture).

Figure 3. Jute macerating pool: (1) macerating pool bottom pad with plastic film and macerating after the top is covered with plastic film (left picture); (2) macerating pool after the top is covered with plastic film (intermediate picture); (3) macerating pool bottom is not cushioned with plastic film and the top is not covered with plastic film (right picture).

Test investigation and detection
Temperature reading: After soaking hemp every day at 8:00, 14:00, and 20:00, respectively, to record the temperature of the macerating pool water, every two days at 2:00 a.m. to record the temperature of the macerating pool water.
Bacterial colony detection: mark the diagonal ends and the middle three places of the macerating pool, take 100 ml of macerating water samples from the three markers each time, and take samples every two days, and separate and detect the number of colonies of its degumming bacteria through enrichment culture (X. Zhang et al. Citation2015). In this, the reagent bottles were cleaned, put in water, and boiled for 20 minutes (counting from the beginning of boiling) to sterilize before sampling. The sampling time was 16:00–17:00.
Fiber quality testing: the composting pool of fresh whole kenaf, fresh kenaf, fresh skin, and jute fresh whole pole and jute fresh skin washed fine hemp fiber samples were taken for line density, strength, and residual glue rate testing (Leng, Xiao, and Nie Citation2003). Line density was detected by a micro imager test (X. Yang et al. Citation2015), strength was tested by fiber strength meter, in which the clamping distance was 0.1 mm (approximate zero spacing), and residual glue rate was detected with reference to the method of quantitative analysis of the chemical composition of ramie (GBT Citation5889–1986 Citation1986).
The data were preliminarily organized and graphed using Excel 2010, analyzed by ANOVA using SAS 9.1.3, and tested for significance using Duncan’s new complex polarity method.
Results and analysis
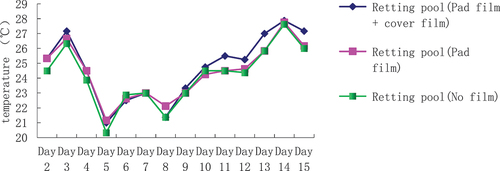
The effect of different measures of composting pond on the temperature of kenaf composting water
The analysis of the temperature of kenaf maceration water in different treatment maceration ponds (), the results show that the water temperature of maceration pond (mat film + cover film) and maceration pond (cover film) is always in the top of maceration pond (no film), indicating that the water temperature of maceration pond (mat film + cover film) and maceration pond (cover film) is higher than that of maceration pond (no film), and the highest water temperature is that of the maceration pond (mat film + cover film) followed by the water temperature of the maceration pond (mat film + cover film) and the highest water temperature is that of the maceration pond (mat film + cover film), followed by the water temperature of the maceration pond (mat film + cover film), followed by the water temperature of the maceration pond (no film). The highest water temperature was in the macerated pool (padded film + covered film), followed by the macerated pool (covered film), but the difference between them was not very obvious, with a mean temperature difference of between 0.2 and 0.3°C.
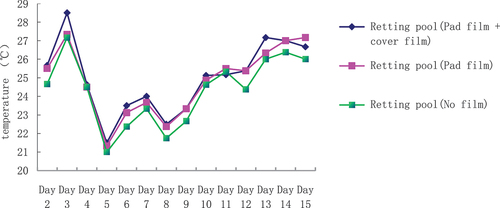
The effect of different measures of composting pool on jute maceration water temperature
Different treatments of macerating pond jute maceration water temperature analysis () show that the same macerating pond (pad film + cover film) and macerating pond (cover film) water temperature are higher than the macerating pond (no film). Water temperature, the highest water temperature for the macerating pond (pad film + cover film), followed by the macerating pond (cover film). The difference between them is obvious: the difference in average temperature of 0.11°C. The water temperature of the macerating pond (without film) is the lowest, followed by the macerating pond (pad film + cover film) and the macerating pond (cover film). The lowest water temperature was in the composting pond (without film), with a difference of 0.6–0.7°C compared with the composting pond (mat film + cover film) and the composting pond (cover film).
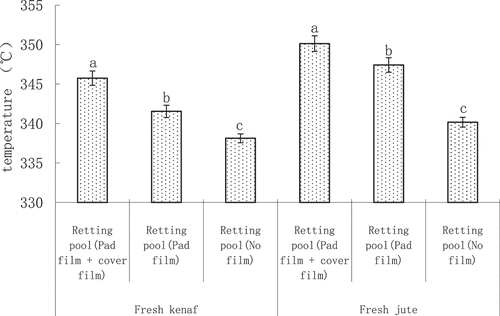
The effect of different measures of maceration pool on the kenaf and jute maceration pool water temperature
From the kenaf fresh body and jute fresh body of the non-treatment maceration pool water pool temperature analysis (), the results show that there is a significant difference between the kenaf fresh body and jute fresh body of the non-treatment maceration pool water pool temperature. From the kenaf fresh body, the highest water pool temperature of maceration pool (pad film + cover film) was 345.75°C, and the smallest water pool temperature of maceration pool (no film) was 338.13°C. That is, composting pond (padded film + covered film) > composting pond (covered film) > composting pond (no film). From the jute fresh body, the overall maceration pool water temperature is higher than the kenaf fresh body maceration pool water temperature, similarly, the highest water temperature for the maceration pool (pad film + cover film), for 350.13°C, the water temperature of the smallest water temperature of the smallest water temperature, for 340.17°C. The order of size was also (mat film + cover film) > maceration pond (cover film) > maceration pond (no film).
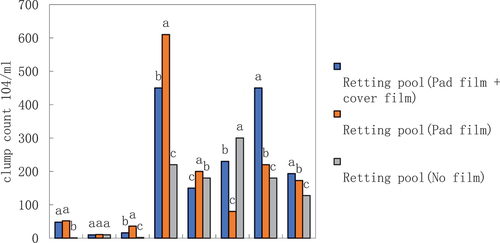
The effect of different measures of maceration pool on the kenaf maceration water colony number
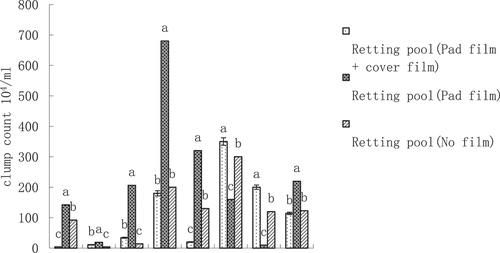
From the three kenaf maceration pools of maceration water colony number (), except for the 4th day, there are significant differences, in the first 6 days, the colony number were less than 53 × 104/ml in the first 8 days, the colony number of the maceration pools without film cover were less than the colony number of the maceration pools with film cover. On the 8th day, the colony counts of macerating ponds (padded + covered with film), macerating ponds (covered with film) and macerating ponds (without film) soared sharply, with values of 450.18 × 104/ml, 610.15 × 104/ml, and 220.14 × 104/ml, respectively, followed by a fall on the 10th day, with the macerating ponds (padded + covered with film) and the macerating ponds (covered with film) falling back to higher values of 66.65% and 67.65%, respectively, 66.65% and 67.20%, respectively, which were more than half, while the composting pond (without film) had a smaller fall of 18.20%. It is interesting to note that the colony counts of the composting ponds (padded + covered with film) rose again on the 12th and 14th day, and finally rose to the level of the 8th day. The colony counts of the macerating ponds (cover film) on days 12 and 14 then fell and then rose, while the colony counts of the macerating ponds (no film) on days 12 and 14 followed the opposite trend, reversing and rising to the peak of the whole macerating period, which was 300.21 × 104/ml. In the first 10 days, the colony counts of the three macerating ponds, from the largest to the smallest, were cover film>pad film + cover film>no film. Analyzing the mean values, the composting pool (mat film + cover film) had the highest colony number of 193.54 × 104/ml, followed by the composting pool (cover film) with 172.85 × 104/ml, and the composting pool (no film) had the lowest number of 127.67 × 104/ml.
The effect of different measures of composting pool on jute maceration water colony number
From the three jute macerating ponds, there were significant differences in the colony counts of macerated water (), similarly, on the 8th day, the colony counts of the macerating ponds (padded film + covered film), macerating ponds (covered film), and macerating ponds (no film) were all soaring, with the values of 180.14 × 104/ml, 680.32 × 104/ml, and 200.14 × 104/ml, respectively, followed by a fall in colony counts on the 10th day, with the largest fall of 88.91%, followed by the largest fall of 88.91%. On the 10th day, all of them fell back, with the largest fall in macerating pond (padded film + covered film), 88.91%, followed by macerating pond (covered film), 52.94%, and the smaller fall in macerating pond (no film), 35.04%. The trend of colony counts in macerating ponds (padded + covered film) was different from that of red sisal ponds (padded + covered film), which rose directly to the peak of the whole macerating period on day 12 at 350.08 × 104/ml, and then declined to a slightly higher level than that on day 8 on day 14 at 200.04 × 104/ml. Similarly, the colony counts of the macerating ponds (covered film) on days 12 and 14 were similar to those of red sisal ponds (padded + covered film). Similarly, the colonization number of the macerating pond (without film) on days 12 and 14 was different from that of the red sisal pond (with film cover), with a significant drop to only 10 × 104/ml on day 14, while the colonization number of the macerating pond (without film) on days 12 and 14 was the same as that of the red sisal pond (without film), with a reversal of the trend back to the peak of the entire period of the maceration, with a level of 300.25 × 104/ml. The mean value was different from the red sisal pond, with the macerating pond (with film cover) having the highest number of colonies, at 21.04 × 104/ml colonies were the highest at 219.69 × 104/ml, and the least at 114.20 × 104/ml and 112.94 × 104/ml for the macerating pond (padded + covered with film) and the macerating pond (without film).
The effect of different measures of maceration pool on the fiber quality of kenaf fresh body
From different kenaf fresh body, different maceration pool and its interactions on the line density, strength, residual glue rate (), there is a significant or non-significant effect. From the analysis of different kenaf fresh body, only the strength of the existence of a significant effect, line density and residual glue rate are not significant. From the analysis of different measures of maceration hemp pool, line density, strength, residual glue rate are significant effects. From the analysis of their interactions, there is a significant effect of strength, residual glue rate, line density does not have a significant effect.
Table 1. Interactions of various factors on kenaf fiber quality.
From the analysis of the treatments of kenaf fresh whole pole and kenaf fresh skin (), there was a significant effect except for the residual glue rate of kenaf fresh skin, which was not significant. From the line density of kenaf fresh whole pole, the line density of macerated pool (no film) treatment was the largest, 276.12 m/g. Macerated pool (pad film + cover film) and macerated pool (cover film) treatments were the smallest, and there was no significance. From the strength of fresh whole stalks of kenaf, macerated pool (mat film + cover film) and macerated pool (cover film) treatments had the greatest strength of 489.01 N, 494.85 N, respectively, and the difference was not significant, and macerated pool (no film) had the least with 457.78 N. From the residual gelatinization of fresh whole stalks of kenaf, macerated pool (mat film + cover film) treatment had the least residual gelatinization of 10.46%, followed by (padded film + covered film) treatment was the smallest with 10.46%, followed by macerating pond (covered film) treatment with 11.53% and macerating pond (no film) treatment with 13.47%.
Table 2. Effect of different treatment retting pool on fiber quality of fresh kenaf.
In terms of linear density of fresh skin of red jute, the same treatment of macerating pond (no film) had the highest linear density of 274.38 m/g. The treatments of macerating pond (padded film + covered film) and macerating pond (covered film) had the lowest linear density and there was no significance in this case. In terms of strength of fresh skin of red jute, it was maximum in macerated pool (no film) treatment with 364.26 N and minimum in macerated pool (padded film + covered film) and macerated pool (covered film) treatments. In terms of residual glue rate of fresh skin of kenaf, there was no significant difference among the treatments.
Overall analysis, fresh whole rod composted fibers than peeled off the fresh skin composted fiber strength values are large, in the same kenaf composting pool degumming of the whole fresh rod and fresh skin degumming out of the maximum difference of 150.69 N, in the same jute composting pool degumming of the whole fresh rod and fresh skin degumming out of the jute fiber strength maximum difference of 152.88 N, and the fresh whole rod and fresh skin of the strength of the negative correlation. Fresh whole rod and fresh skin composted fiber density is positively correlated. Fresh whole rods and fresh hides composted fiber residue rate is not correlated.
The effect of different measures of maceration pool on the fiber quality of jute fresh body
From the effects of different jute fresh bodies, different macerating ponds, and their interactions on line density, strength, and residual glue rate (), there were significant differences. From the line density of jute fresh whole stalks (), similarly, maceration pool (no film) treatment had the highest line density of 527.73 m/g, followed by maceration pool (padded film + covered film) treatment of 483.17 m/g, and maceration pool (covered film) treatment had the smallest of 394.13 m/g. From the potency of jute fresh whole stalks, the maceration pool (padded film + covered film) treatment had the highest of 502.02 m/g, and the strength of the macerating pond (film cover) treatment was also the smallest. From the view of the residual gum rate of jute fresh whole stalks, macerated pool (covered film) treatment was the largest, macerated pool (padded film + covered film) and macerated pool (no film) treatments were the smallest, 12.70% and 12.44%, respectively, and the difference between them was not significant.
Table 3. Interactions of various factors on jute fiber quality.
Table 4. Effects of different retting pools on fiber quality of jute fresh body.
From the linear density of jute fresh skin, maceration pond (covered film) treatment had the maximum linear density of 557.61 m/g, followed by maceration pond (no film) treatment, and maceration pond (padded film + covered film) treatment had the minimum of 447.15 m/g. From the strong force of jute fresh skin, maceration pond (padded film + covered film) treatment had the maximum of 392.32 N, followed by maceration pond (no film) treatment. In terms of residual glue percentage of fresh jute hides, the minimum was 12.55% in the macerated pool (no film) treatment, followed by 14.81% in the macerated pool (film covered) treatment, and the maximum was 17.58% in the macerated pool (padded film + film covered) treatment.
In the overall analysis, similarly, the fiber strength of fresh whole poles composted was higher than that of peeled fresh hides composted, while the strength of fresh poles and hides was positively correlated. There was no correlation between the linear density and residual glue rate of fibers composted from fresh whole poles and fresh hulls.
Discussion and conclusion
Whether it is a kenaf composting pool or a jute composting pool, the temperature difference between them is within 1°C. The difference in water temperature is not very large, which may be the reason that the temperature in Zhejiang after September falls.
Three kenaf composting pools of the average colony size order: composting pool (mat film + cover film) > composting pool (cover film) > composting pool (no film), the three kenaf fresh whole pole degumming hemp fiber residual gelatinization rate corresponds to the size of the composting pool (mat film + cover film) < composting pool (cover film) < composting pool (no film) for 10.46%, 11.53%, and 13.47%, consistent with the theory of logic, and residual gum rate is relatively low. The study showed that the residual gum rate of ramie inoculated with the degumming microorganism LY11 strain was 11.48% after water soaking. It was comparable to the residual gum rate of the whole plant of kenaf treated with mat film + cover film (Wu et al. Citation2021). Three kenaf fresh skin degumming of hemp fiber correspond to residual gum rates of 11.99%, 11.97%, and 11.76%; the difference between them is not significant (; ).
Table 5. Strength comparison with similar degummed fibers.
Figure 10. Comparison of kenaf and jute fiber quality with different treatments.
The average colony number of the jute maceration pool corresponds to the jute fresh whole rod and fresh skin degumming out of the hemp fiber residue rate; there is no law. Three jute macerating pools of the average colony size of the order of macerating pool (cover film) > macerating pool (no film) > macerating pool (mat film + cover film) three jute fresh whole poles degummed hemp fiber residue rate corresponding to the size of the macerating pool (no film) and fresh skin degummed hemp fiber residue rate, there is no regularity. The corresponding residual gelatinization rates of the three jute fresh whole stalks were macerated pool (no film) < macerated pool (mat film + cover film) < macerated pool (cover film) while the corresponding residual gelatinization rates of the three jute fresh hulls degummed from the jute fibers were 12.55, 17.58, and 14.81% (; ). The reason for this lack of regularity needs to be further investigated in future experiments.
Kenaf or jute fresh whole rod composted fiber than peeled off the fresh skin composted fiber strength values are larger; it should be a series of fresh skin peeled out of the fiber damage causes. The fiber strengths of fresh whole-stem composted kenaf were 489.01 N, 494.85 N, and 457.78 N, respectively (; ), which were all 28.95%–39.39% greater than the fiber strength of 355 N obtained by degumming with biofungus by Duan (Citation2018). Compared with similar degelled fibers (), the fiber strength of kenaf fresh whole stalk and fresh bark retting was 65.97% and 31.88% larger than that of dry bark retting, and the fiber strength of jute fresh whole stalk and fresh skin retting was 77.68% and 37.05% larger than that of dry bark retting.
In addition, different degumming processes can also have an important effect on fiber quality. Różańska et al. degummed flax and hemp straw in three different ways, osmotic degumming, dew retting and water retting (). The morphological structure of the fibers was examined using SEM and it was found that the fibers obtained using the water retting method had the highest tenacity (Różańska, Romanowska, and Rojewski Citation2023). The effect of extraction methods on the physical and mechanical properties of the William banana peduncle fibers were examined. And SEM mapping was used (). The results showed that, water retting, gave a high yield (42.55%) compared to fibers extracted by rolling (23.40%) (Anafack et al. Citation2023).
Figure 11. SEM images (a) view of flax and hemp fibers; (b) SEM images of raw jute and extracted jute fibers.

Combined with the strength and residual glue rate, all three measures of macerating ponds can be used for popularization and application, and the best effect is to use the whole fresh poles of kenaf or jute in macerating ponds (padded film and covered film) for degumming.
Author contributions
Conceptualization, Changli Chen, Xia An, Qingqing Ji; Data curation, Xia An; Formal analysis, Changli Chen; Funding acquisition, Xia An; Investigation, Xiahong Luo; Methodology, Xia An and Qingqing Ji; Project administration, Xia An; Resources, Xia An; Software, Xia An and Tingting Liu; Supervision, Xia An; Validation, Xia An, Lina Zou and Tingting Liu; Visualization, Xia An; Writing-original draft, Changli Chen and Xia An; Writing-review & editing, Xia An and Qingqing Ji.
Highlights
本试验采用的就地围池沤麻方法能有效解决对天然水造成污染的问题。
采用本沤麻技术能节本、增效及易推广。
通过本试验沤出的红麻和黄麻纤维品质好。
本试验为红麻和黄麻种植户提供技术保障,促进红麻和黄麻产业的可持续发展具有重要意义。
The pollution of natural water can be solved effectively by the method of in situ pool retting.
Using this retting technique can save cost, increase efficiency and popularize easily.
The quality of kenaf and jute fiber produced by this test was good.
The quality of kenaf and jute fiber produced by this test was good. This experiment is of great significance to provide technical support for kenaf and jute growers and promote the sustainable development of kenaf and jute industry.
Composted fibers from whole rods of kenaf or jute exhibit greater strength than peeled off fresh skin.
Kenaf macerating ponds with padded film + cover film had the highest degumming bacterial; for jute, top film alone did so.
The best effect of degumming is performed with whole fresh poles of kenaf or jute in padded film + cover film treatment.
Highlights.doc
Download MS Word (24.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15440478.2024.2377269.
Additional information
Funding
References
- An, X., J. Chen, and G. Jin. 2020. “Transcriptome Profiling of Kenaf (Hibiscus Cannabinus L.) Under Plumbic Stress Conditions Implies the Involvement of NAC Transcription Factors Regulating Reactive Oxygen Species-Dependent Programmed Cell Death.” PeerJ 8:e8733. https://doi.org/10.7717/peerj.8733.
- An, X., J. Chen, T. Liu, W. Li, X. Luo, and L. Zou. 2022. “Transcriptomic and Metabolic Profiling of Kenaf Stems Under Salinity Stress.” Plants 11 (11): 1448. https://doi.org/10.3390/plants11111448.
- An, X., J. Chen, J. Zhang, Y. Liao, L. Dai, B. Wang, L. Liu, and D. Peng. 2015. “Transcriptome Profiling and Identification of Transcription Factors in Ramie (Boehmeria Nivea L. Gaud) in Response to PEG Treatment, Using Illumina Paired-End Sequencing Technology.” International Journal of Molecular Sciences 16 (2): 3493–15. https://doi.org/10.3390/ijms16023493.
- An, X., G. Jin, J. Zhang, X. Luo, C. Chen, W. Li, G. Ma, et al. 2018. “Protein Responses in Kenaf Plants Exposed to Drought Conditions Determined Using iTRAQ Technology.” FEBS Open Bio 8 (10): 1572–1583. https://doi.org/10.1002/2211-5463.12507.
- An, X., L. Qin, J. Hui, G. Dong, D. Tian, X. Luo, C. Chen, et al. 2023. “Bioinformatics Analysis of WRKY Family Genes in Flax (Linum usitatissimum).” Life (Basel) 13 (6): 1258. https://doi.org/10.3390/life13061258.
- Anafack, S. M., O. Harzallah, D. E. Nkemaja, P. W. M. Huisken, A. Decker, R. N. S. Tagne, J.-Y. Drean, et al. 2023. “Effects of Extraction Techniques on Textile Properties of William Banana Peduncle Fibers.” Industrial Crops and Products 201:116912. https://doi.org/10.1016/j.indcrop.2023.116912.
- Chen, S., and H. Yang. 2014. “Research on the Comprehensive Utilization and Strategic Study of Hemp.” Hunan University Press.
- Cheng, Y. 2017. “Research on the Application Technology of Hemp Fiber/Phenolic Resin Composite Materials in Automotive Interiors.” Master’s thesis, Nanjing University of Science and Technology.
- Duan, S. 2018. “Microbial Diversity and Protein Differential Expression of Degummed Hemp Bark.” Master’s thesis, Chinese Academy of Agricultural Sciences.
- Fang, P. P., J. M. Qi, J. G. Su, G. Q. Zhang, and L. H. Lin. 2009. “Status and Prospects on Jute and Kenaf Production in the World.” Zhongguo Maye Kexue (Plant Fiber Sciences in China) 31 (3): 215–219.
- He, X., J. Yu, and L. Liu. 2008. “Effects of Different Biological Enzyme Treatments on Jute Fiber.” 73–75. Textile Technology Progress.
- Jin, G., F. Fu, Q. Zou, and X. Luo. 2008. “Main Factors and Countermeasures Restricting the Development of Jute Fiber Production.” China Jute Science 1:48–53. https://doi.org/10.3969/j.issn.1671-3532.2008.01.012.
- Leng, J., A. Xiao, and J. Nie. 2003. “Research on Quality Evaluation of Ramie Fibers.” Chinese Textile Inspection 5:31–34. https://doiorg/CNKI:SUN:ZHXJ.0.2003-05-019.
- Li, D. 2023. “In-Depth Degumming Process and Mechanism of Red Hemp Bark.” Master’s thesis, Zhongyuan Institute of Technology.
- Li, H. 2010. “Research Status and Development Trend of Degumming of Jute Fibers.” 218. Technology Wind.
- Li, W., C. Chen, X. Luo, T. Liu, X. An, G. Jin, and G. Zhu. 2021. “Genetic Diversity Analysis of Phenotypic Traits in Jute Resources in Zhejiang Province.” Molecular Plant Breeding 16:19, 23 http://kns.cnki.net/kcms/detail/46.1068.s.20210830.1408.006.html.
- Liu, J., J. Yang, Y. Yu, Z. Xu, and X. Zhang. 2018. “Preparation and Performance of Non-Woven Mulching Film of Red Jute.” Journal of Textile Science & Engineering 142–148. https://doi.org/10.3969/j.issn.1008-5580.2018.01.027.
- Liu, Z. 2009. “Progress in Extraction and Engineering Research of Jute Fiber.” China Jute Science 31:93–97. https://doi.org/10.3969/j.issn.1671-3532.2009.z1.012.
- Ma, H., Z. Wang, T. Yu, and X. Wang. 2009. “Research Progress on Biological Degumming of Jute Fibers.” Hunan Agricultural Science 11–14. https://doi.org/10.3969/j.issn.1006-060X.2009.11.004.
- National Standards Bureau. 1986. “GBT 5889-1986 Method for Quantitative Analysis of Chemical Composition of Ramie.”
- Ning, J., Z. Li, and X. Ling. 2020. “Research Progress on Properties and Modification Technology of Jute Fibers.” Journal of Textile Science & Engineering 37 (1): 88–96.
- Ohtani, Y., B. B. Mazumder, and K. Sameshima. 2001. “Influence of the Chemical Composition of Kenaf Bast and Core on the Alkaline Pulping Response.” Journal of Wood Science 47 (1): 30–35. https://doi.org/10.1007/BF00776642.
- Papadopoulou, E., D. Bikiaris, K. Chryssafis, M. Wladyka-Przybylak, D. Wesolek, J. Mankowski, J. Kolodziej, et al. 2014. “Value-Added Industrial Products from Bast Fiber Crops.” Industrial Crops and Products 68:116–125. https://doi.org/10.1016/j.indcrop.2014.10.028.
- Ramaswamy, G. N., and C. R. Boyd. 1994. “Kenaf As a Textile Fiber: Processing, Fiber Quality and Product Development.” Mississippi State University Bulletin 10 (11): 31–33.
- Różańska, W., B. Romanowska, and S. Rojewski. 2023. “The Quantity and Quality of Flax and Hemp Fibers Obtained Using the Osmotic, Water-, and Dew-Retting Processes.” Materials 16 (23): 7436. https://doi.org/10.3390/ma16237436.
- Sapuan, S. M., M. R. Ishak, J. Sahari, and M. Sanyang. 2018. Kenaf Fibers and Composites. Taylor and Francis. CRC Press.
- Tang, K., A. Fracasso, P. C. Struik, X. Y. Yin, and S. Amaducci. 2018. “Water- and Nitrogen-Use Efficiencies of Hemp (Cannabis Sativa L.) Based on Whole-Canopy Measurements and Modeling.” Frontiers in Plant Science 9:951. https://doi.org/10.3389/fpls.2018.00951.
- Wang, D., J. Kang, D. Ma, L. Han, J. Li, X. Tan, and Y. He. 2023. “Research Progress on Degumming of Ramie.” Progress in Textile Technology 4:1–5. https://doi.org/10.19507/j.cnki.1673-0356.2023.04.011.
- Wang, W., and Z. Cai. 2008. “Research on Chemical Degumming Process of Jute Fiber.” Dyeing and Finishing Agents 25 (9): 21–23.
- Wu, Y., Y. Liu, T. Shu, H. Wang, P. Li, Y. Yang, and L. Yu. 2021. “Analysis on the Dynamic Process of Bacillus Ramie Degumming.” Biotechnology Bulletin 37 (12): 22–28. https://doi.org/10.13560/j.cnki.biotech.bull.1985.2020-1395.
- Xiong, H. 2017. “Research on the Sustainable Development Strategy of Modern Agricultural Industry in China - Hemp Volume.”
- Yan, T., W. Gu, and C. Yu. 2011. “Research on Kenaf Degumming with Oxidation Process.” Advanced Materials Research 332-334:1109–1113. https://doi.org/10.4028/www.scientific.net/AMR.332-334.1109.
- Yang, Q., L. Cheng, X. Feng, K. Zheng, Z. Peng, and S. Duan. 2022. “Research Progress on Biological Degumming Technology of Jute Fibers in Recent Three Years.” China Jute Science 44 (4): 245–252. https://kns.cnki.net/kcms2/article/abstract?v=Xlf5kQqXAOmcHBVhd8VXJhb76qCoC2WNGuC5hVQ-itPePEriEpNmAwHzPw4k1Fvy4_XkQH1_q7ma6EnsGX9ZWDOkJv-mvLWrm8wQCp83REHWKWhXSIaRq-r3zJBmrfNIRubgnMjo19FMzMDMLyf5xtTK-jrK41WR519H_6xCw7c=&uniplatform=NZKPT.
- Yang, X., A. Xiao, J. Leng, Y. Cheng, L. Liao, and X. Sun. 2015. “Research on Rapid Detection Method of Red Jute Fiber Line Density.” Detection Technology 3:68–71. https://doiorg/CNKI:SUN:ZHXJ.0.2015-03-031.
- Yang, Y. 2005. “Current Situation and Countermeasures of Jute Fiber Degumming.” Shandong Textile Technology 1 (46): 51–54.
- Yang, Z., Q. Yang, Q. Liu, X. Li, L. Wang, Y. Zhang, Z. Ke, et al. 2023. “A Chromosome-Level Genome Assembly of Agave Hybrid NO.11648 Provides Insights into the CAM Photosynthesis.” Horticulture Research 11 (2): uhad269. https://doi.org/10.1093/hr/uhad269.
- Yu, C., Y. Zheng, Y. Sun, R. Li, J. Wang, and D. Wang. 2023. “Research Progress on Degumming of Jute Fibers.” Heilongjiang Textile 16–19. http://kns-cnki-net-s.webvpn.zjda.net:8118/kcms2/article/abstract?v=Xlf5kQqXAOmW43qDDc3X3sn0xFyGJI3vED8VpObOsdJKTDM6U6iDWhLHGUNjcCd-6oJhQ14q5ALaPZsl1yo0JGkIh5koL6QSAhj2GljNmP5iPbUYXqVpIIHU76y8n_o-eMKuq0A8PcXICCkYWgAMRxcyEUdwzvdaxlStxnZ5V-ctdq2Q0bPtwszGO9p32NQD&uniplatform=NZKPT&language=CHS.
- Zhang, J., H. Zhang, and H. Zhang. 2005. Comprehensive Utilization Technology of Hemp, 309–312. Great Wall Publishing House.
- Zhang, L., X. Ma, X. Zhang, Y. Xu, A. K. Ibrahim, J. Yao, H. Huang, et al. 2021. “Reference Genomes of the Two Cultivated Jute Species.” Plant Biotechnology Journal 19 (11): 2235–2248. https://doi.org/10.1111/pbi.13652.
- Zhang, S., and L. Wu. 2009. “Research on Biochemical Joint Degumming of Jute Fibers.” International Textile Journal 37 (3): 14–16.
- Zhang, X. 2017. “A Method for Joint Degumming of Jute or Ramie.” Jiangxi, CN106283210A.
- Zhang, X., Y. Yuan, D. Kong, C. Yu, G. Jin, J. Fu, and R. Ding. 2015. “Analysis of Bacterial Diversity in Retting Tanks of Flax and Jute.” China Jute Science 37 (1): 14–20. https://doi.org/10.3969/j.issn.1671-3532.2015.01.004.
- Zheng, L. J. 2007. A Study on the Degumming and Modification of Kenaf Bast Fiber by Bio-Enzyme Degradation. Shanghai: Donghua University.