Abstract
Workers can be exposed to fume, arising from welding activities, which contain toxic metals and metalloids. Occupational hygienists need to assess and ultimately minimize such exposure risks. The monitoring of the concentration of particles in workplace air is one assessment approach whereby fume, from representative welding activities, is sampled onto a filter and returned to a laboratory for analysis. Inductively coupled plasma-atomic emission spectrometry and inductively coupled plasma-mass spectrometry are generally employed as instrumental techniques of choice for the analysis of such filter samples. An inherent difficulty, however, with inductively coupled plasma-based analytical techniques is that they typically require a sample to be presented for analysis in the form of a solution. The efficiency of the required dissolution step relies heavily upon the skill and experience of the analyst involved. A useful tool in assessing the efficacy of this dissolution step would be the availability and subsequent analysis of welding fume reference materials with stated elemental concentrations and matrices that match as closely as possible the matrix composition of welding fume samples submitted to laboratories for analysis. This article describes work undertaken at the Health and Safety Laboratory to prepare and certify two new bulk welding fume reference materials that can be routinely used by analysts to assess the performance of the digestion procedures they employ in their laboratories.
INTRODUCTION
Welding is a universal endeavor. In Europe, it is estimated that there are approximately 750,000 workers engaged in welding,(Citation1) while in the United States the numbers employed are tabulated to be around 340,000.(Citation2) Workers can be exposed to fume arising from welding activities that contain toxic metals and metalloids. Occupational hygienists and those with responsibilities for workers’ health and safety need to assess and ultimately minimize such exposure risks.
The monitoring of the concentration of particles in workplace air is one assessment approach whereby fume from representative welding activities is sampled onto a filter and returned to a laboratory for analysis. Inductively coupled plasma-atomic emission spectrometry (ICP-AES) and inductively coupled plasma-mass spectrometry (ICP-MS) are generally employed as instrumental techniques of choice for the analysis of such filter samples. Performance-based analytical methods have long since been codified at national level such as US NIOSH Method 7300(Citation3) and OSHA ID-125G.(Citation4) More recently, building upon these methods, international standards such as ASTM D7439,(Citation5) ASTM D7035,(Citation6) ISO 15202,(Citation7,Citation8) and ISO 30011,(Citation9) have been promulgated, supported by additional method validation(Citation10) and collaborative trial studies.(Citation11,Citation12)
An inherent difficulty. however, with ICP-based analytical techniques is that they typically require a sample to be presented for analysis in the form of a solution, thus, in this case, requiring the dissolution of a welding fume filter sample in strong mineral acids. Although advances in dissolution procedures, in particular the increasing use of high-performance closed-vessel microwave-assisted digestion systems, coupled with the published guidance in the above-mentioned standards, have assisted the laboratory community. Nevertheless, this dissolution step does still rely heavily upon the skill and experience of the analyst involved. A reported study,(Citation13) drawing upon experiences of laboratories participating in a proficiency testing scheme has concluded that analytical bias can occur primarily as a result of errors in this key sample preparatory step.
A useful tool in assessing the efficacy of suitable dissolution procedures would be the availability, and subsequent analysis, of welding fume certified reference materials (CRM) with stated elemental concentrations and matrices that match as closely as possible the composition of welding fume samples submitted to laboratories for analysis. To date, as far is known, only one such welding fume CRM has been produced certified only for its chromium content.(Citation14) This fume on filter material is, however, prohibitively expensive for everyday use.
In summary, the aim of this article is to describe the work undertaken at the Health and Safety Laboratory (HSL) to prepare and certify two new bulk welding fume reference materials that can be used routinely by analysts to assess the performance of the digestion procedures they employ in their laboratories when undertaking welding fume analysis.
METHODS
Procurement of Candidate Fume Reference Materials
Welding fume was recovered from ventilation ducts above robotic laser beam welding stations employed at an automobile assembly plant. Two candidate materials were obtained: one fume material was derived from the spot welding of galvanized mild steel components (from here on defined as HSL MSWF-1) and the second fume material was derived from the spot welding of stainless steel components (from here on defined as HSL SSWF-1). Approximately 1.0 kg of each of the two materials was recovered and transported to HSL for processing. Welding fume upon generation consists of nm-sized particles which quickly condense to form agglomerates between 100 and 1000 nm.(Citation15,Citation16) By nature it is therefore a finely divided particulate powder which is relatively homogenous in nature and potentially does not require extensive preparatory steps to produce a suitable candidate CRM. A further advantage in obtaining fume above specific workstations at a car assembly line is the inherent purity of the material arising from the repeatability, from day to day, of the welding process and the fact that metallic particles from other metal finishing activities, as would be the case in a more typical welding shop, would generally be absent.
Preparation and Initial Characterization of Candidate Fume Reference Materials
At HSL, the materials were dispersed on plastic trays and air dried at a nominal 95°C before being sieved through a coarse 2-mm sieve to remove foreign debris. This sieved fraction was then passed through a finer 200 μm sieve to remove further debris such as (condensed) metal splash beads. Approximately 0.8 kg of each of the candidate materials was recovered at this stage. To ensure the best possible homogenization of these candidate materials, sample mixing was undertaken using both tubular and roller bottle mixers. The materials were subsequently decanted into glass sample bottles, capped and stored at a nominal 20°C. In total, 800 bottles of each candidate material containing a nominal 1 g of fume were prepared.
Initially qualitative XRD scans (PANalytical X’Pert Pro, Cambridge, UK) were undertaken to gain an insight into the fume morphology and to aid the selection of suitable digestion procedures for use in the subsequent certification exercise. Examination of XRD scans of HSL MSWF-1 material, against powder diffraction scans of reference compounds held in HSL databases (ICSD database v3.2 supplied by PANalytical and PDF database v4 supplied by The International Centre for Diffraction Data, Newtown Square, PA.), showed the presence of the following major crystalline phases best described as Fe3O4 and ZnO.(Citation17) Examination of more complex XRD scans of HSL SSWF-1 material showed the presence of the major crystalline phases best described as Fe3O4, Fe3Mn3O8, Mn3O4, and FeCr2O4.(Citation18) In summary, a spinel-type oxide can be considered the dominant crystalline phase which can be represented predominantly by the general formula AB2O4 (where A = Fe or Mn and B = Cr, Fe or Mn). It is thought that the minor nickel phase is also best represented also as a mixed spinel oxide. Findings from this study are consistent with previously reported XRD analysis.(Citation19)
Homogeneity and Stability Testing of Candidate Fume Reference Materials
It was decided that these two candidate materials should be certified for analytical use at a nominal sample aliquot size of 10 mg. This is a compromise value balancing the requirements in weighing out accurately small quantities of finely divided powder with the quantities often collected on workplace air filters (typically < 1 mg).
Meeting requirements set out in ISO Guide 35, ten bottles(Citation20) of each candidate material were chosen randomly following the sequence of bottling. A quantity of fume was removed from each bottle, air dried, and 10 (±1.0) mg sample aliquots, to the nearest 0.1 mg, taken for analysis. Each bottle was sampled in triplicate resulting in 30 test samples.
HSL SSWF-1 test samples were digested using a closed vessel microwave oven (Milestone Ethos, Analytix, Newcastle, UK) following a procedure involving the use of a nitric/hydrochloric/hydrofluoric acid mixture at 180°C as described in Annex G of ISO 15202-2.(Citation7) Experience has shown that this aggressive dissolution approach is required to dissolve spinel oxide material.(Citation10) HSL MSWF-1 test samples were successfully digested using a hotblock (SCP DigiPREP MS, QMX, Essex, UK) digestion procedure that involved the use of a nitric/hydrochloric acid mixture at 95°C as described in Annex H of ISO 15202-2.(Citation7)
The resultant test solutions obtained were analyzed by ICP-AES (Perkin Elmer 8300DV, Beaconsfield, UK) following procedures set out in ISO 15202-3.(Citation8) Measurements were performed under repeatability conditions, after sample randomization in one instrumental run sequence, and employing a single calibration prepared using certified multi-elemental solutions (Merck ICP Multi-Element Standards, Darmstadt, Germany) traceable to national standards. Calibration verification standards and spike digestion recovery samples were also prepared and analyzed.
Certification of Candidate Fume Reference Materials Via an Inter-laboratory Trial
Laboratories known to HSL, experienced in welding fume analysis or with expertise in the elemental analysis of metallurgical-based materials using ICP techniques, were invited to participate in a certification exercise. Thirteen laboratories subsequently participated in this exercise and their summary details are presented in .
TABLE I. Participants and the Methodologies They Employed in the Certification Exercise
Each laboratory received two randomly chosen bottles of each type of candidate fume material. Before analysis test aliquots had to be dried at 95°C overnight. Laboratories were requested to analyze five subsamples, nominal 10 (± 1.0) mg aliquots to the nearest 0.1 mg, from each of the two bottles. Advice regarding the selection of suitable digestion procedure(s) was supplied although participants were free to choose procedure(s) that they deemed appropriate for the sample matrix. All laboratories bar one used ICP-AES as their instrumental technique and sector field ICP-MS was used in this other facility.
Performance check samples were also supplied by HSL to be prepared and analyzed concurrently alongside the candidate fume samples. These samples consisted of 25 mm diameter membrane filters (Pall Life Sciences, Ann Arbor, Mich.) spiked with elements (nominal 10,000 ppm standards prepared in-house from high purity metal salts), which upon dissolution provided test solutions at elemental concentrations similar to those expected in digested fume samples. Blank membrane filters were also supplied so that process blanks could be evaluated. Laboratories also made use of their own internal quality control protocols, prescribing the use of independently prepared calibration verification standards, spike recovery samples, and spiked filter reference materials, in an endeavor to produce high-quality analytical data.
RESULTS AND CALCULATIONS
Data analysis was undertaken using statistical protocols outlined in ISO Guide 35(Citation20) using the freely available software package SoftCRM (SoftCRM v1.2.2, (downloadable at http://www.eie.gr/iopc/softcrm/index.html) the attributes or which are described in a published paper.(Citation21)
As an example and for brevity, only the Zinc (Zn) results for HSL MSWF-1 are reported in this article. Data for the other elements and subsequent similar calculations undertaken can be found in the accompanying certification reports.(Citation17,Citation18)
Homogeneity Testing Results
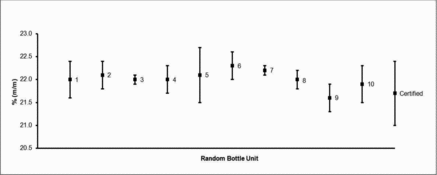
Results obtained at HSL for Zinc in HSL MSWF-1 are presented in .
An estimate of elemental specific inhomogeneity contributions ubb to be included in the total uncertainty budget was calculated according to procedures described in ISO Guide 35(Citation20) using Eqs. 1 and 2:
(1)
(2) where
MSamong = is the mean of squared deviations between bottles
MSwithin = is the mean of squared deviations within bottles
N = is the number of replicates per bottle analyzed
N = is the number of bottles selected for homogeneity study,
sbb equates to the between-bottle standard deviation, whereas ubb* denotes the maximum heterogeneity that can potentially be hidden by insufficient repeatability in the measurement method used. In summary, the larger of these two values has been used as ubb. EquationEq. 1(1) is not applied if MSwithin > MSamong. The calculated relative values of sbb, ubb* and ubb for Zn are reproduced in . Relative values of sbb, ubb*, and ubb for Fe and Mn in the mild steel fume candidate material ranged between 0.25 and 0.55%. Similarly, values for elements (Cr, Fe, Mn, and Ni) in the stainless steel candidate fume ranged between 0.31 and 1.15%.
TABLE II. Results of the Zinc Homogeneity Study Conducted on HSL MSWF-1
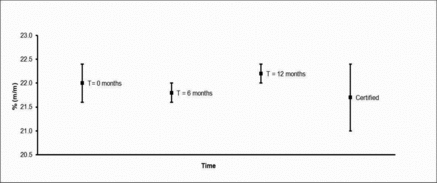
Ongoing Stability Testing
Over time, based upon experience in the repeat analysis of in-house welding fume quality control materials,(Citation10,Citation22) HSL considers these welding fume materials to remain stable if stored sealed at ambient temperatures. Therefore an uncertainty estimation of an elemental specific long-term stability contribution (ults) has not been deemed necessary. HSL, however, is conducting an ongoing long-term stability check study involving the reanalysis, in triplicate every 6 months, of material from selected bottles units used in the homogeneity study. Stability test results obtained to date for Zn in HSL MSWF-1 are presented in .
Technical Evaluation of Returned Results from Participants in the Certification Exercise
Prior to statistical examination of the data, returned participants’ results were technically evaluated on the basis of:
whether the required nominal 10 mg test aliquot was tested
data checks for possible transcription errors
whether recoveries from spiked membrane filter performance test samples were acceptable (where the acceptable performance requirement was results to be within ± 10% of spiked values determined at HSL)
whether the digestion parameters used were suitable, based upon HSL's experiences, for the fume matrix in question (in particular, factors such as digestion temperature and suitability and compatibility of acid mixture to dissolve matrix and to subsequently stabilize elements in solution were considered).
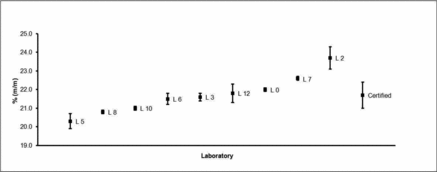
All 13 laboratories returned results for Zinc in HSL MSWF-1. Two laboratory data sets were rejected on technical grounds. In one laboratory, assays were performed on 100 mg aliquots rather than the 10 mg test aliquot size stated in the certification protocol. In the second laboratory, Zn recoveries from the spiked membrane filter performance test samples were biased low against the HSL measured spike values. Correspondingly, this laboratory reported a mean Zn result in HSL MSWF-1 that was also biased low in relation to results returned by the other laboratories. The 11 remaining laboratories reported spiked filter results that were within ± 5% of spiked values determined at HSL.
Statistical Evaluation of Returned Results from Participants in the Certification Exercise
The following statistical tests () were subsequently carried out on the remaining 11 laboratory data sets are discussed below.
Two further laboratories were removed as they were adjudged to be statistical outliers with mean reported Zinc values in HSL MSWF-1 which were on average 21% and 12% lower than the final calculated certified value. Outcomes of these statistical tests are reported in .
TABLE III. Statistical Tests Carried Out on Participants’ Data
TABLE IV. Results of Statistical Tests Carried Out on Accepted Participants’ Data
As no technical reasons could be identified for potentially removing the Zn data received from Laboratory 2 and its reported mean result was not flagged as a statistical outlier at a 99% confidence level, this data set was retained for further data processing. Accepted results are presented in .
Certification of the Candidate Fume Reference Materials
The unweighted mean of means of data sets from the resultant 9 laboratories were taken as the best estimate wchar for the Zn mass fraction to be certified in HSL MSWF-1. The standard deviation of this mean of means was taken to derive the uncertainty contributions uchar arising from this certification exercise:
(3) where
SDM = standard deviation of the mean of means of data sets
N = number of individual data sets
The combined uncertainties ucombined were calculated from the spread resulting from this certification exercise and the uncertainty contribution from possible inhomogeneity of the material:
(4) The calculated mass fractions wchar and absolute values of the various uncertainty components are reproduced in .
The expanded uncertainties U were obtained by multiplying the combined uncertainties ucombined by a coverage factor k:
(5) The value of the coverage factor k was chosen to give a level of confidence of approximately 95% for coverage of the interval ± U around the certified values. An appropriate k value was determined by calculating the effective degrees of freedom νeff of the linear combinations of uchar and ubb using the Welch-Satterthwaite formula.(Citation23) A factor of k = 2.5 was therefore chosen here for Zn (and similarly for all other analytes in both reference materials) to give a level of confidence of approximately 95%.
The Zn results subsequently used in the certification of HSL MSWF-1 and the resultant certified value and its calculated expanded uncertainty are presented graphically in . The certified elemental mass fractions and their corresponding expanded uncertainties, for both reference materials, rounded to an appropriate value, are shown in and I.
TABLE V. Mass Fractions and Uncertainty Components for Zinc in HSL MSWF-1
TABLE VI. Certified Mass Fractions and Expanded Uncertainties of Elements in HSL MSWF-1
DISCUSSION
Outcome of Homogeneity and Stability Studies
Results obtained confirm that welding fume is relatively homogenous in nature provided that debris such as metal splash (nugget effect) is sieved out. Historical experiences suggest that these materials should be stable over time. Nevertheless the materials are being monitored for stability at six monthly intervals and data obtained to date confirms this viewpoint.
Outcome of Certification Study
Thirteen laboratories volunteered in response to a request from HSL for participation in the certification trial. All laboratories, known to HSL, are well versed in the analysis of welding fume and/or test samples of a metallurgical origin using acid digestion procedures with an analytical finish using ICP techniques. Results from two laboratories, however, were not used. One laboratory, although providing good quality data, unfortunately undertook analysis using a nominal 100 mg test sample rather than the required 10 mg sample size advocated in the certification protocol. The second laboratory demonstrated a consistent low bias in analyzing both spiked membrane performance test filters and the candidate reference materials. The remaining 11 laboratories consistently demonstrated good performance on the spiked filter performance check samples and reported results that were typically within ± 5% of the HSL determined spike values.
Analysis on retained data sets from these 11 laboratories was undertaken using statistical protocols outlined in ISO Guide 35.(Citation20) Typically, on an element-by-element basis, between six and ten laboratory data sets passed these prescribed statistical tests and were subsequently used to derive the final certification values. It is generally accepted that a certified value should be based on no less than six sets of results.(Citation20)
Given the good recoveries obtained on the spiked filters, it can be assumed that instrumental conditions employed by these participants were in control (calibration, drift, spectral considerations). It was assumed that discarded data, which were invariably biased low against the final certified values, was either as a result of some technical issue with the digestion procedures employed or more likely, inherent limitations in selected procedures themselves for such fume matrices. Upon re-examination of the procedures used by these participants, it is possible to summarize that:
with respect to the mild steel welding fume material (HSL MSWF-1) or similar matrices, addition of HCl acid to the digestion could be beneficial in digesting bulk (mg quantities) materials containing appreciable concentrations of iron and
with respect to the stainless steel welding fume material (HSL SSWF-1) or similar, where such materials are of a refractory nature containing elements such as chromium, HSL advocates the use of an aggressive digestion procedure. Here the addition of HF acid to the digestion and the use of a sustained heating cycle at elevated temperatures are most beneficial. In summary, for the digestion of fume derived from stainless steel welding, the use of a closed-vessel microwave-assisted procedure, at a digestion temperature of 180°C for at least 15 min, is recommended as prescribed in standards such as ASTM D7035(Citation6) and ISO 15202-2.(Citation8)
TABLE VII. Certified Mass Fractions and Expanded Uncertainties of Elements in HSL SSWF-1
FUTURE WORK
In addition to ongoing material stability testing, HSL is proposing carrying out additional tests to further characterize these two reference materials.
The first test will assess the validity of this certification data if applied to testing smaller sample aliquots e.g., in the range 0.1–1 mg, that are more representative of sample masses collected on air filter samples from workplaces.
The second test will attempt to characterize more fully the elemental content of these materials so as to derive a more complete chemical v versus gravimetric mass closure. Currently, based upon the morphological information derived from XRD analysis, the mass, derived chemically, is calculated to be approximately 90–94% of the corresponding gravimetrically derived mass. It is obvious that some of this “missing” mass is due to the presence of elements such as silicon not measured in this certification study. Manufacturers of welding consumables are required to provide typical fume composition data as part of an overall product safety data sheet. These two materials could therefore also assist analytical laboratories tasked with undertaking such product testing activities.
Conclusion
For the first time, two bulk welding fume reference materials are now available and can be routinely used by analysts to check the performance of applying a dissolution step, as codified in standard workplace air methods such as ASTM D7035,(Citation6) ASTM D7439,(Citation5) ISO 15202-2,(Citation7,Citation8) NIOSH 7300,(Citation3) NIOSH 7303,(Citation24) and OSHA 125G.(Citation4) These materials can also be used in checking the application of dissolution methods described in more generic environmental measurement standards such as EN 13656(Citation25) and EPA 3052.(Citation26) Recommendations as to the selection of suitable digestion procedures are provided in the accompanying certification reports.(Citation17,Citation18) Other potential uses of these materials include: developing new in-house sample dissolution procedures, preparing matrix recovery quality control charts, or assisting in the training of new analysts.
The use of such materials will also assist laboratories fulfill their accreditation requirements under ISO 17025(Citation27) and will assist them in conforming to guidance(Citation28,Citation29) regarding the selection and use of suitable reference materials for this specific measurement sector.
ACKNOWLEDGMENTS
The authors would like to acknowledge the following who have contributed to the development of these reference materials: Dr. Martin Grosser (Müller-BBM); John Cuthbert and Karen Roberts (both from HSL), and the laboratories () who gave freely in participating in the certification exercise. Funding from the HSL Investment Research Programme is also gratefully acknowledged.
REFERENCES
- Taube, F.: Manganese in occupational arc welding fumes-Aspects on physiochemical properties, with focus on solubility. Ann. Occup. Hyg. 57(1):6–25 (2013).
- U.S. Department of Labor, Bureau of Labor Statistics (BLS): Occupational Outlook Handbook, 2012–13 Edition: Welders, Cutters, Solderers and Brazers Available at http://www.bls.goc/ooh/production/welders-cutters-solderers-and-brazers.htm (accessed January 7, 2014).
- National Institute for Occupational Safety and Health (NIOSH): Method 7300: Elements by ICP. In Manual of Analytical Methods, 4th ed. Cincinnati, Oh.: NIOSH, 2003.
- Occupational Safety and Health Administration (OSHA): ID-125G: Metal and Metalloid Particulates in Workplace Atmospheres (ICP Analysis). Salt Lake City, Ut.: OSHA, 2002.
- American Society for Testing and Materials (ASTM): D7439-08 Standard Test Method for Determination of Elements in Airborne Particulate Matter by Inductively Coupled Plasma-Mass Spectrometry. West Conshohocken, Pa: ASTM, 2007.
- American Society for Testing and Materials (ASTM): D7035-10 Standard Test Method for Determination of Elements in Airborne Particulate Matter by Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES). West Conshohocken, Pa.: ASTM, 2010.
- International Organization for Standardization (ISO): Workplace Air—Determination of Metals and Metalloids in Airborne Particulate Matter by Inductively Coupled Plasma Atomic Emission Spectrometry—Part 2: Sample Preparation (ISO 15202-2) [Standard] Geneva: ISO, 20112.
- International Organization for Standardization (ISO): Workplace Air—Determination of Metals and Metalloids in Airborne Particulate Matter by Inductively Coupled Plasma Atomic Emission Spectrometry—Part 3: Analysis (ISO 15202-3) [Standard] Geneva: ISO, 2004.
- International Organization for Standardization (ISO): Workplace Air—Determination of Metals and Metalloids in Airborne Particulate Matter by Inductively Coupled Plasma Mass Spectrometry (ISO 30011) [Standard] Geneva: ISO, 2010.
- Health and Safety Laboratory (HSL): ICP-AES Method for Metals in Air - Preliminary Tests on Bulk Reference Materials to Test the Effectiveness of the Proposed Sample Dissolution Procedures, IS/96/04. Buxton, UK: HSL, 1996.
- Butler, O.T., and A.M. Howe: Development of an international standard for the determination of metals and metalloids in workplace air using ICP-AES: Evaluation of sample dissolution procedures through an interlaboratory trial. J. Environ. Monit. 1:23–32 (1999).
- Ashley, K., S.A. Shulman, M.J. Brisson, and A.M. Howe: Interlaboratory evaluation of trace element determination in workplace air samples by inductively coupled plasma mass spectrometry. J. Environ. Monit. 14:360–367 (2012).
- Butler, O., and P. Stacey: Performance of laboratories analyzing welding fume on filter samples: Results from the WASP proficiency testing scheme. Ann. Occup. Hyg. 52(4):287–295 (2008).
- Institute for Reference Materials and Measurements: Certified Reference Material BCR 545 Welding Dust Loaded on a Filter. Geel, Belgium: Institute for Reference Materials and Measurements.
- Dasch, J., and J. D’Arcy: Physical and chemical characterization of airborne particles from welding operations in automotive plants, J. Occup. Environ. Hyg. 5: 444–454 (2008).
- Berlinger, B., N. Benker, S. Weinbruch et al.: Physicochemical characterization of different welding aerosols, Anal. Bioanal. Chem. 399:1773–1780 (2011).
- Health and Safety Laboratory (HSL): Certification Report: Reference Material HSL MSWF-1 Elements in Mild Steel Welding Fume, AS/2012/11, SL, 2013. . Available at http://www.hsl.gov.uk/products/welding-fume-reference-material.aspx (accessed January 7, 2014).
- Health and Safety Laboratory (HSL): Certification Report: Reference Material HSL SSWF-1 Elements in Stainless Steel Welding Fume, AS/2012/12, HSL 2013. . Available at http://www.hsl.gov.uk/products/welding-fume-reference-material.aspx (accessed January 7, 2014).
- Tanninen, V.P., H.K. Hyvarinen, A. Grekula, and P.L. Kalliomaki: X-ray Diffraction and X-ray Emission Spectroscopy in Chemical Compound Analysis of Welding Fumes. In Proceedings of the International Conference on Health Hazards and Biological Effects of Welding Fumes and Gases, Copenhagen, February 18–21, 1985, R.M. Stern, A. Berlin, A.C. Fletcher, and J. Järvisalo, (eds.). Amsterdam: Excerpta Medica, 1986. pp. 81–84.
- International Organization for Standardization (ISO): Guide 35 Reference Materials—General and Statistical Principles for Certification. Geneva, Switzerland: ISO, 2006.
- Bonas G., M. Zerou, T. Papaeoannou, and M. Lees: SoftCRM: A New Software for the Development of Certified Reference Materials (CRMs). Accred. Qual. Assur. 8:101–107 (2003).
- Health and Safety Laboratory (HSL): Investigation into the Accuracy and Completeness of Material Safety Data Sheet Information on the Chemical Composition of Welding Fume, IEAS/99/05. Harpur Hill, UK: HSL, 2000.
- National Institute of Standards and Technology (NIST): Engineering Statistical Handbook. . Available at http://www.itl.nist.gov/div898/handbook/prc/section3/prc31.htm (accessed January 7, 2014).
- National Institute for Occupational Safety and Health (NIOSH): Method 7303: Elements by ICP (Hotblock HCl/HNO3 Digestion). In Manual of Analytical Methods, 4th ed. Cincinnati, Oh.: NIOSH, 2003.
- European Standard: Characterization of Waste. Microwave Assisted Digestion with Hydrofluoric (HF), Nitric (HNO3) and Hydrochloric (HCl) Acid Mixture for Subsequent Determination of Elements in Waste (EN 13656) [Standard], . Brussels, Belgium, 2002.
- Environmental Protection Agency (EPA): SW 846 Test Methods for Evaluating Solid Waste. In Physical/Chemical Methods, 3rd ed., Update III. Method 3052 Microwave Assisted Digestion of Siliceous and Organically Based Matrices. Washington, DC: EPA, 1996.
- International Organisation for Standardization (ISO): General Requirements for the Competence of Testing and Calibration Laboratories (ISO 17025) [Standard] Geneva, Switzerland: ISO, 2005.
- American Industrial Hygiene Association (AIHA®): Laboratory Accreditation Program Communique on Using PAT Samples for Method Verification. Falls Church, Va.: AIHA, 2011. . Available at http://www.aihaaccreditedlabs.org/programfees-guidelines-forms/Documents/Communique_Samples%20for%20Method%20Verification_Validation.pdf (accessed January 7, 2014).
- United Kingdom Accreditation Service: TP57 Edition 2, Guidance and Policy on the Selection and Use of Reference Materials. Feltham, U.K.: UKAS, 2011. . Available at http://www.ukas.com/library/Technical-Information/Pubs-Technical-Articles/Pubs-List/TPS%2057_March2011.pdf (accessed January 7, 2014).
