Abstract
Occupational exposure limits (OELs) serve as health-based benchmarks against which measured or estimated workplace exposures can be compared. In the years since the introduction of OELs to public health practice, both developed and developing countries have established processes for deriving, setting, and using OELs to protect workers exposed to hazardous chemicals. These processes vary widely, however, and have thus resulted in a confusing international landscape for identifying and applying such limits in workplaces. The occupational hygienist will encounter significant overlap in coverage among organizations for many chemicals, while other important chemicals have OELs developed by few, if any, organizations. Where multiple organizations have published an OEL, the derived value often varies considerably—reflecting differences in both risk policy and risk assessment methodology as well as access to available pertinent data. This article explores the underlying reasons for variability in OELs, and recommends the harmonization of risk-based methods used by OEL-deriving organizations. A framework is also proposed for the identification and systematic evaluation of OEL resources, which occupational hygienists can use to support risk characterization and risk management decisions in situations where multiple potentially relevant OELs exist.
INTRODUCTION
Occupational exposure limits (OELs) are important tools for the interpretation of workplace exposures within a health risk context.(Citation1) Although the term “Occupational Exposure Limit” was adopted by the International Labour Organisation (ILO) in 1977 to encompass all chemical exposure guidelines for workplace air,(Citation2) the need for quantitative benchmarks for occupational exposures was identified much earlier. Early work on OELs for airborne workplace chemicals occurred in Germany in the 1880s, when the pioneering animal experiments of Gruber and Lehmann were used to identify safe exposure levels for carbon monoxide, ammonia, and hydrogen chloride.(Citation3) Since the publication of the first table of acute exposure limits by Kobert in 1912,(Citation3) many organizations around the world have been added to the global capacity for establishing OELs ().(Citation4) The first list of maximum allowable concentrations (MACs)—eventually known as Threshold Limit Values (TLVs)—was published by the American Conference of Governmental Industrial Hygienists (ACGIH) in the 1940s.(Citation3) By the 1970s, many countries were developing or adopting their own values, such as the U.S. Occupational Safety and Health Administration Permissible Exposure Limits (OSHA-PELs) and the German Maximale Arbeitsplatz-Konzentration (MAKs).(Citation5) The lack of OELs for many commercially important chemicals spurred the creation of non-governmental OEL-setting organizations, such as the Workplace Environmental Exposure Level (WEEL) Committee initially established under the auspices of the American Industrial Hygiene Association (AIHA), to help meet this need. International initiatives aimed at resource sharing and harmonization have also emerged, including collaboration among Nordic countries, and joint publication of criteria documents between these countries and NIOSH.(Citation6) The European Scientific Experts Group (now the Scientific Committee on Occupational Exposure Limits [SCOEL]) was created in 1990, and has been proposing OELs for adoption by European Commission (EC) Member States since 1991.(Citation7)
Table 1 The Early History of Institutional Occupational Exposure Limit (OEL) Development
Since the first introduction of OELs over a century ago, the processes for developing, setting, and using the occupational exposure guidelines have enjoyed widespread global uptake.(Citation3,Citation8,Citation9) However, the proliferation of international OEL-setting bodies, faced with the challenges of evaluating and interpreting complex scientific data on potential health impact of occupational exposures, has yielded a confusing landscape of OELs. As a result, occupational hygienists can be confronted with multiple relevant—but often conflicting—OELs for a particular situation, leading to difficulties in selecting the most appropriate value for health protection purposes. In addition, duplication of effort can result in missed opportunities to develop OELs for new agents.
The aim of this article is to highlight the aspects of the OEL-setting process contributing to differences in guideline values, with the goal of assisting occupational hygienists in making more informed decisions when selecting between several potentially relevant OELs. Although this article discusses various issues that might be of relevance during the OEL-derivation process, the aim is not to instruct occupational hygienists to calculate OELs; therefore, readers seeking detailed discussions of the science behind OEL derivation should consult two additional article published in this issue. (Citation10,Citation11) The key points of emphasis in this article include:
exposure limit guidance is absent for most chemicals, and existing OELs often vary quantitatively among organizations from around the world;
the basis for differences in OELs for the same chemical reflects a mix of differences in risk policy and risk science methodology, which are discussed in detail;
harmonization of the approaches used to develop OELs can contribute to increased consistency in OEL derivation by organizations around the world; and
a systematic framework can aid the occupational hygienist in documenting and selecting OELs when multiple relevant values are encountered, encouraging the most effective use of current OEL resources.
These points are elaborated upon in the subsequent sections.
AVAILABILITY OF TRADITIONAL INTERNATIONAL OEL RESOURCES
OELs are derived by various organizations around the world, including those listed in . Because these global OEL efforts are in general not directly coordinated among organizations, a confusing landscape of traditional OELs has emerged. Existing values span only a small percentage of all chemical compounds, with different organizations often deriving different values for the same substance. Evaluation of the current status of OEL availability can be framed in the context of several considerations, including: (1) the relationship between traditional OELs and other alternative exposure guidance benchmarks, using a hierarchy of OEL concept; (2) the extent to which existing OELs cover the universe of chemicals of interest in occupational exposure settings; and (3) an evaluation of the reasons for variability in OELs provided by different organizations.
Hierarchy of OELs
Traditional OELs are developed by many international bodies; these values vary as to whether they are legally binding and with respect to the consideration given to feasibility of implementation. A brief summary of several well recognized OELs from different organizations and their attributes—including analytical, economic, and engineering feasibility, and whether or not they are health based—has been highlighted by Waters et al.(Citation1) Those that are adopted as legally binding under an appropriate rulemaking authority include various state or provincial level OELs, OSHA PELs, and OELs promulgated by various countries around the world.(Citation12,Citation13) If the European Commission, based on scientific advice received from SCOEL, develops a Binding Occupational Exposure Limit Value (BOELV), member states must establish their own binding OEL at or below the BOELV.(Citation14) Many examples of non-binding or recommended OELs exist, such as the NIOSH Recommended Exposure Limits (RELs), ACGIH TLVs, Occupational Alliance for Risk Science (OARS) Workplace Environmental Exposure Levels (WEELs; formerly developed under the purview of AIHA), and SCOEL Indicative Occupational Exposure Limit Values (IOELVs). This distinction between binding and non-binding limits can become blurred, however, as some regulatory authorities adopt non-binding OELs under existing rulemaking authority. For example, the ACGIH TLVs are adopted as de facto legally binding standards in many Canadian provinces,(Citation3,Citation15) various European countries,(Citation2) and many other countries around the world.(16-18) Moreover, distinctions can be made between “health-based” OELs and those that are “regulatory-adjusted,”(Citation8) with the latter involving consideration of technical and economic feasibility. Feasibility considerations might not be limited to binding values, as some OELs—such as the NIOSH RELs—may be a hybrid of both health-based and technical considerations. In some cases, organizations have clearly delineated between the two, such as with the German MAKs based on health effects and Technische Richtkonzentrationen (TRKs), the latter of which are based primarily on technological feasibility.(Citation19) However, other organizations might not clearly identify when OELs are hybrids of health-based and regulatory adjusted values; the opacity in these hybrids could create difficulties in the implementation of risk management decisions.
These traditional OELs can be viewed as a component of a larger body of occupational risk-based exposure benchmarks. Alternative methods exist that can provide a useful approach for occupational hygienists to consider when an OEL is not available or cannot be derived for a chemical of concern. These alternatives comprise a hierarchy of OELs (). The hierarchy concept provides a means to develop occupational risk benchmarks similar to OELs where traditional OELs are not available. Consistent with the concept of problem formulation (ensuring that the risk assessment approach meets the needs of the scenario being evaluated),(Citation20,Citation21) the alternative techniques in the hierarchy may be adequate for preliminary assessments, screening processes, or specific risk assessment protocols. In general, as one moves down the hierarchy, the available methods can accommodate less data, although the reduced resource needs may be achieved at the expense of increased uncertainty in the assessment. In some cases, there may be adequate data to set a formal traditional OEL. The lower rungs of the hierarchy are designed to allow development of benchmarks for making risk decisions and are often precautionary in nature. The hierarchy and more in-depth descriptions of the alternative occupational exposure benchmarks are presented elsewhere.(Citation22) Examples of these OEL alternatives include working provisional OELs, values derived for internal use within a company or by trade associations or vendors, which serve to fill an information gap in the absence of an OEL from a recognized body.(Citation23) Prescriptive process-based levels are those that are developed using a prescribed derivation approach: the Derived No Effect Levels (DNELs) required under the European Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Regulation fit in this category, as DNELs are required for all compounds manufactured, used, or imported in the EU in volumes exceeding 10 tons, independent of the data availability.(Citation24) DNELs should be considered with some caution, because there is no peer review or public consultation involved in their establishment. Furthermore, although they should be derived according to extensive regulatory guidance, there are no established competency requirements for those who carry out the derivation. Where inadequate data exist to derive OELs or the alternative benchmarks, qualitative strategies—such as Hazard Banding(25-30)—can be applied. A similar concept is the Threshold of Toxicological Concern (TTC), a semi-quantitative approach that can be used to identify levels where exposure to a compound would be expected to have little toxicological concern derived based on observed distributions of potency for large numbers of chemicals.(Citation23,Citation31) Refinements to the TTC approach have included assigning different potency cut points based on a chemical's structural features, such as those embodied in the Cramer Class.(Citation31,Citation32) Such tools have been proposed for use in occupational risk assessments within the pharmaceutical industry.(Citation33) Despite the availability of these alternative methods for deriving quantitative benchmarks for assessing occupational risk, there remains a strong emphasis on new OEL derivation, which requires substantial data and resources, as the preferred approach.
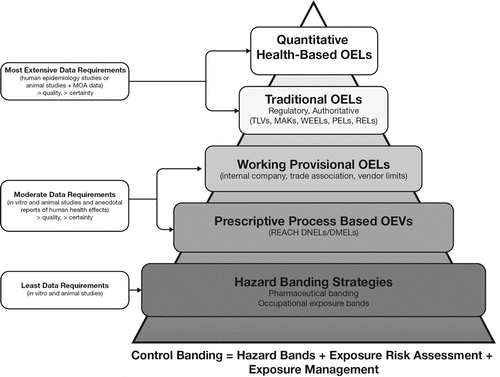
Other exposure limits might not be considered as part of the hierarchy of OELs, but could still be useful tools for occupational hygienists. These tools might have levels of data requirements and scientific validity that are consistent with the traditional OELs, but differ from OELs in the exposure scenarios to which they apply. Exposure limits intended for shorter duration, such as Immediately Dangerous to Life or Health (IDLH) values and Acute Exposure Guidelines (AEGLs) are not OELs in the sense described earlier in this article, but are sometimes erroneously treated as such. A recent publication on NIOSH IDLH values has stated that “it is important to note that IDLH values are concentrations that may cause adverse effects, and thus, they are not intended to be used as surrogates for [OELs]. OELs … are intended to protect workers from adverse health effects associated with repeated chemical exposure … for a working lifetime. The IDLH values should not be used as comparative indices of toxicity or to infer a ‘safe’ level for exposures to chemicals under routine occupational exposure conditions.”(Citation34) These tools, however, might be useful for application in non-routine occupational exposure scenarios. Another tool that might be helpful for occupational hygienists faced with an absence of OELs is an environmental health exposure guideline. Environmental exposure guidelines have parallel derivation processes to OELs, but tend to be more conservative as they are typically derived for continuous exposures for a 70-year duration, and are also applied to subpopulations that might be more sensitive than healthy workers (e.g., children, pregnant women, and elderly). The potential application of tools such as IDLH values, AEGLs, and general population exposure guidelines in absence of OELs will be further discussed in the section entitled “Framework for the Selection of Appropriate OELs.”
The Patchwork Landscape of OELs
The extent to which commercial chemicals have traditional OELs is graphically represented in , which demonstrates that OELs only exist for a small fraction of the universe of chemicals. Brandys and Brandys(Citation35) published a list of OELs from around the world, which includes over 5,000 different chemicals, and a separate study of 18 organizations identified OELs for 1341 compounds.(Citation36) Although these OELs encompass a wide variety of chemicals—particularly those that are most common in the occupational environment—a vast number of chemicals still do not have OELs. The Chemical Abstracts Service Registry recently registered its 75 millionth substance, with 5 million substances added during the past year. The rate of innovation in the area of chemicals is rapid and broad, including aspects such as the development of nanomaterials. While all of these chemicals are not commercially produced, it is clear that the potential for numerous and varied chemical exposures in workplaces is substantial.(Citation37) Given that chemicals considered “in commerce” in the U.S. are numbered at approximately 84,000,(Citation38) that Canada's Domestic Substances List includes 23,000 chemicals, (Citation39) and notifications for over 107,000 different substances have been received under REACH,(Citation40) the majority of chemicals in use currently have no OEL. In addition, many of the existing lists of OELs include substances that were added many years ago, and are no longer commercially important; thus, the number of relevant OELs is even smaller than the total number included.
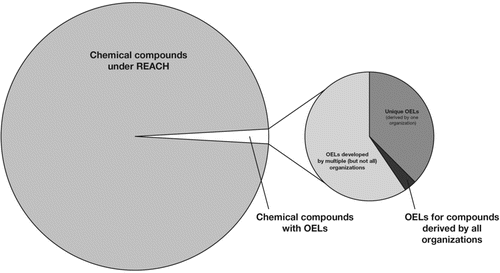
Even when traditional OELs exist for a particular compound, it is possible that not all OEL-setting bodies have that chemical in its lists of OELs. In a comparative study of values from 18 organizations, most of the OEL-setting bodies addressed less than half of the 1,341 substances that comprised the total list of compounds covered by the organizations in the study.(Citation36) More than one-third (460) of the substances in the study were mentioned by only one organization (Finland was exceptional in that it had 189 unique OELs). Less than 2% of the substances (25) were mentioned by all 18 organizations. The reason that the selection of substances is not more harmonized might be explained in part by differences in industry base among countries. This patchwork nature of the OEL landscape might result in occupational hygienists’ need to consult OELs from diverse organizations, which can become especially complicated when various organizations have derived different values for the same chemical. In addition, the need for a comprehensive search for OELs results from the lack of an easily accessible compendium of OELs for all agencies and organizations.
When multiple organizations have established a traditional OEL, these values often vary, as demonstrated for n-hexane in . Based on a review of 8 different organizations, the values of 14 different OELs (benzo[a]pyrene, carbon tetrachloride, p-dichlorobenzene, dichlorofluoromethane [FC-21], enflurane, 2-ethoxyethanol, ethylene dibromide, halothane, 2-hexanone, hydrazine, nickel subsulfide [as Ni], phenyl glycidyl ether, tetranitromethane, and vinyl cyclohexene dioxide) varied at least 100-fold, with some differences as high as 200-fold (for ethylene dibromide and tetranitromethane).(Citation41) The variation in occupational risk assessment practice is not limited to the evaluation of inhalation exposures. In an exploratory investigation of seven different organizations, a total of 480 chemicals were carrying at least one skin notation (SN). Only approximately 3% of these chemicals were considered by all of the evaluated organizations as a skin exposure hazard, whereas 47% were only assigned a SN from a single organization.(Citation42) Studied organizations varied significantly in the assignment of SNs; these variances occurred even though the SN assignment was in essence a process of hazard identification, and required a lesser amount of quantitative decision making compared to the traditional OEL derivation process. These analyses indicate that OELs and related notations can vary significantly among occupational health organizations. Thus, informed use of OELs requires an understanding of the basis for the underlying differences in the approaches used by OEL-deriving organizations.
Table 2 Variability in Exposure Limits Derived for n-hexane
The Basis for Differences in OELs
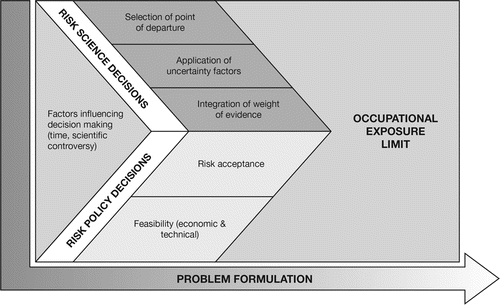
The variability in traditional OELs can present a confusing landscape within which the occupational hygienist must navigate. To properly assess the appropriateness of an OEL for a specific occupational exposure scenario, an understanding of the different decisions that can be made in the risk assessment process is helpful. Because OELs are derived from a series of complex decisions, many of which are based on limited data and require scientific assumptions, they are inherently imprecise.(Citation1) Although the OEL-setting process rests on a scientific foundation, many of the decisions can be influenced by an organization's science judgment practices. Although different decisions might be made among organizations, this does not invalidate the results when considered in the context of the risk assessment and risk management policies and practices of individual organizations. The sources of variation in OELs derived among organizations, as identified in , can help an OEL user understand the differences among values and the implications for their own occupational exposure and risk assessment scenarios. According to this scheme, contributions to the differences in OELs can be divided into two broad categories—risk science and risk policy. The assumptions that are made and decisions that are taken when confronted with each of these sources of uncertainty vary among organizations, leading to differences in derived OELs.(Citation43)
Problem formulation
The problem formulation stage of risk assessment can influence differences in OELs among organizations. The goal of problem formulation is to design risk assessments to be able to answer specific risk management questions,(Citation20) which might be different for each type of OEL. It follows that even though values can differ, they can be equally appropriate—fitting the purpose of the organization that developed them. Although traditional OELs are generally derived based on continuous inhalation exposure for 8 hr per day, 5 days per week, over a working lifetime, slight aspects of the exposure scenarios can differ, including the definition of the duration of a working life. The breadth of the population considered in the values can also vary among organizations. Whereas the U.S. Occupational Safety and Health Act of 1970 prescribes that “ … medical criteria will assure insofar as practicable that no employee will suffer diminished health, functional capacity, or life expectancy as a result of his work experience,”(Citation44) the ACGIH TLVs “represent conditions under which it is believed that nearly all workers may be repeatedly exposed, day after day, over a working lifetime, without adverse health effects,”(Citation45) [emphasis in the original] and will not necessarily prevent discomfort or injury for a small percentage of workers that are especially sensitive to an agent.
Risk Science Decisions
Different decisions can be made in the area of risk science, which can create variability in OELs derived by different organizations for the same chemical. Diversity in decision making can occur for many reasons, including differences in problem formulation among organizations (resulting from differing goals and needs), the evolution of risk science over time, and the capabilities of different organizations. In selecting an OEL, it should be borne in mind that one decision (or resulting OEL) is not necessarily “better” than another; the decisions could be equally defensible, but one might be more appropriate than another for a specific occupational exposure scenario, linking back to the problem formulation stage. Several key risk science decisions are often at the root of the differences in OELs, including selection of the point of departure, application of uncertainty factors, and integration of weight of evidence.
Selection of the Point of Departure
A point of departure (POD) is the no-observed-adverse-effect level (NOAEL), lowest-observed-adverse-effect level (LOAEL), benchmark dose (BMD), or some other similar value derived from critical health effects in key studies, which is used as the basis of an OEL calculation. Both the selection of the critical study and of the POD on which the OEL is based can vary among organizations. If organizations develop OELs at different times, the critical study for a newer value might not have been available at the time that older OELs were derived. Practices in some organizations might limit the selection of the critical studies to those that are available in the open literature, whereas others might allow for the use of data sourced outside the international public domain (e.g., industrial research, internal reports), which can stimulate controversy due to limited transparency and selectivity being suspected or inferred.(Citation17,Citation46) Moreover, some organizations might use the highest quality studies available, resulting in a large percentage of OELs based on animal studies (e.g., in 2009, approximately 50% of the ACGIH TLVs were based on animal data),(Citation3) whereas others might favor key study selection based on human data. Once a critical toxicity endpoint (e.g., the most sensitive effect) has been selected, organizational practices can also affect the selection of the POD, a specific exposure level that is derived from the critical studies and upon which the OEL is grounded. For example, organizations could identify adversity at different points along the continuum of severity (i.e., no effect < no observable effect < compensatory effects that are not adverse < borderline effects with an unknown significance to health < early adverse effect < overt disabling effect < death),(Citation47) leading to the selection of different PODs for deriving an OEL. Other factors that could impact the selection of the POD include the use of a threshold approach vs. linear extrapolation, basing the POD on exposure levels used in the study (i.e., selecting a LOAEL or NOAEL) vs. performing dose–response modeling (e.g., BMD modeling), and various quantitative choices, such as the use of a specific response level when performing dose–response modeling.
It should also be noted that the types of health effects upon which OELs are based sometimes do not include the full range of health effects that are possible. This issue has been addressed in part by the development and implementation of the United Nations Globally Harmonized System of Classification and Labeling of Chemicals (GHS). The GHS includes criteria internationally negotiated and agreed upon for identifying the hazards of a broad range of health effects that may be encountered in the workplace, which is most useful for data-poor compounds. These criteria also address the degree or severity of the hazard in the classification scheme. Thus the GHS can now be employed as a tool for countries when considering the development of an OEL. Prior to addressing risk assessment issues, the GHS classification criteria can be used to fully characterize the health, physical, and environmental hazards of a chemical. This complete hazard assessment can facilitate the process of further considering exposure and risk when deriving an OEL from available data, as well as ensure that all health effects, and relevant physical and environmental hazards, are addressed when establishing risk management.(Citation48) The GHS also provides an approach to classify mixtures of chemicals. Mixed exposures are prevalent in workplaces, and proper protection includes consideration of how to deal with combined exposures.
Application of Uncertainty Factors
Many OELs account for variability, uncertainty or weakness in a substance-specific literature database using a combination of uncertainty and adjustment factors that are typically selected from a standard set of values.(3,11,49-54) Data-derived adjustment factors can also sometimes be used instead of default uncertainty factors.(Citation55,Citation56) Although most organizations start with a standard group of values, the methods of selecting and applying the uncertainty factors are not fully harmonized.(Citation7) First, most OEL-setting organizations provide little quantitative guidance on uncertainty factors. Second, if advice is given (as is the case for REACH guidance on DNELs),(Citation57) this advice is limited to default conditions and provides little quantitative guidance on when and how to depart from the defaults. The lack of quantitative guidance might result in arbitrary choices in the range of applicable uncertainty factors, leading to inconsistent OELs.(Citation58)
Integration of Weight of Evidence
Even if an OEL is derived mainly from a single study, the entire body of available scientific literature is usually considered during the hazard assessment process. To integrate the totality of evidence in OEL development, some organizations might use a formal hierarchical approach, whereas others might be less regimented. Frameworks for systematically evaluating weight of evidence exist and are receiving emphasis in risk assessment; as noted previously, the GHS provides criteria for hazard assessment, which includes consideration of weight of evidence. Some approaches focus on overall holistic methods for integrating complex data. Data fusion is a formal method using specialized techniques to gather and integrate data from a variety of sources to decrease uncertainty in the risk assessment process.(Citation59,Citation60) In addition, there has been an increased emphasis on providing decision tools or frameworks that assist development of risk decisions in a systematic way. For example, the International Programme on Chemical Safety (IPCS) mode of action framework can be used to systematically evaluate the degree of human relevance of adverse effects that are observed in animal studies.(Citation61) The IPCS methods incorporate principles of the Bradford Hill criteria to assess the body of literature (e.g., strength of association, consistency, specificity, temporality, presence of dose–response relationship, and plausibility).(Citation62) A hypothesis-based weight of evidence process has also been proposed as a way of assessing and communicating a body of data, and the uncertainties therein, for the evaluation of chemical toxicity.(Citation63) Further areas where organizations might vary in their weight of evidence analyses include in their evaluations of quality, reliability, and relevance of each study—tools to harmonize such systems used in the context of chemical registration include the Klimisch et al.(Citation64) criteria for toxicology studies and the Money et al.(Citation65) criteria for epidemiology studies. Severity scoring and categorical regression affords an objective means of integrating data from diverse toxicity endpoints into a single analysis, as has been previously performed.(Citation66,Citation67) Expert elicitation may also be used in integrating scientific evidence that is subject to uncertainty.(Citation68) The organizations might also have different ways of dealing with conflicting data, or in using supporting studies to help resolve uncertainty. The degree to which documented and systemic processes for decision-making use formal decision tools is not consistent; at present, most organizations develop OELs using peer input and review methods, rather than formal decision tools.
Risk Policy Decisions
The essential elements of risk policy decisions are also an important factor in generating a landscape of varying OELs. Risk policy decisions differ from risk science decisions in that they are largely extra-scientific and hence more value-laden. As with the risk science decisions, one risk policy decision is not inherently better than another. Two important types of risk policy decisions that affect OEL values are risk acceptance and feasibility.
Risk Acceptance
Various organizations and jurisdictions tolerate different levels of risk, which contributes to inter-organization variability in OELs. Risk acceptance is inherently a trans-scientific issue,(Citation69) with differences dependent on subjective responses to adverse effects.(Citation17) To set a numerical value by using uncertainty factors or performing dose–response modeling implies an agreement upon what frequency of injury, disease, or discomfort is deemed acceptable. Past regulatory decisions indicate that risk levels in the range of 1/1,000 to 1/10,000 (10−3 to 10−4) are considered acceptable for occupational risk scenarios.(Citation70) For example, the 1980 Benzene decision by the Supreme Court noted that risks of 1 in 1,000 (10−3) and 1 in 1,000,000,000 (10−9) might be considered by a reasonable person to be significant and insignificant, respectively; based on this decision, a lifetime mortality risk of 1 in 1,000 is considered by OSHA to present a clearly significant risk to workers.(Citation71) However, organizations with different views on acceptable levels of risk might derive different OELs, even if using the same data sources. Risk acceptance considerations are typically considered in the context of non-threshold compounds (e.g., genotoxic carcinogens), but might also influence decisions made in the evaluation of threshold effects (e.g., in the application of uncertainty factors).(Citation1,Citation11)
Feasibility
One major factor contributing to differences between OELs is the consideration of feasibility. A distinction can be made between health-based and regulatory-adjusted OELs, with the former being generally more precautionary than the latter because they are based on health considerations only. For the “regulatory adjusted” OELs, health-based OEL values might be modified to include non-health based considerations. Because non-health considerations—primarily economics and technical feasibility, including engineering controls and analytical measurement capability—might vary by geographic region, a regulatory adjusted OEL developed in one country is not necessarily universally applicable. These factors lead to differences not only between health based and regulatory adjusted OELs, but also between jurisdictions with different socioeconomic contexts and technological capabilities.(Citation8)
Other Sources of Differences in Decisions
Other differences between OELs might not be easily explained by scientific or policy differences between organizations. In the comparative study of OELs by Schenk and coworkers,(Citation36) the authors found no evidence of variability of OELs among organizations that could be associated with risk assessment or management principles, health vs. feasibility approaches, level of health protection, or whether a value was legally mandated. The authors also postulated that “ … there might be scientific controversy regarding some substances that lead to different conclusions being drawn from the risk assessments.”(Citation36) This implies a need to examine processes used when deriving the OELs available for each chemical before selecting it for application to a risk assessment to ensure the validity of the risk assessment methods used, and to identify whether the OEL is truly health based or modified by other technical, social and economic considerations.
The time at which the assessment was performed can also drive differences in OELs. Although the age of an assessment does not directly fit into the categories of risk science or risk policy decisions, it can influence both. Dose–response assessment approaches evolve over time, and the introduction of new epidemiology and toxicology studies broadens the database available for the derivation of OELs. Moreover, as a society's willingness to accept risk can change, risk acceptance might also vary correspondingly. Finally, for OELs that account for economic and technological feasibility, economic growth and technological advancements can decrease the burden of lower guideline values. In general, the progression of time has resulted in lower OELs. As demonstrated by Hansson,(Citation18) in the years since the original publication of the ACGIH TLVs, the levels gradually decreased over time; by 1996, the geometric mean of the ratios of the most recent TLVs to those on the 1946 list was only 0.23. OEL-setting organizations also differ in the degree to which they have ongoing work programs to maintain the values current based on availability of new health studies.
INTERNATIONAL HARMONIZATION OF OELS
Selecting an OEL for occupational hygiene applications presents a challenge when the processes used by OEL-setting organizations differ significantly around the world. Not only do the risk science and risk policy decision-making processes differ, but the ways of presenting and communicating these decisions can also vary between organizations, adding another barrier for occupational hygienists who are charged with gathering, interpreting and applying such information. Harmonization of the OEL derivation processes applied around the world has been suggested as a means of minimizing variability in approaches. Harmonization, as defined in the IPCS Harmonization Project Strategic Plan, is the establishment of “common principles, understanding and approaches and enhanced transparency in risk assessment, facilitating use for regulatory purposes.”(Citation72) A goal of international harmonization of OELs is to have compatible—and not necessarily exact or standardized—values in different countries as a result of the application of convergent methods and practices by different organizations. Thus, the application of harmonization principles to the OEL development processes from organizations around the world could help in making the selection of appropriate exposure guideline values less complicated for occupational hygienists. Both risk policy and risk science drivers for varying OELs could be the subject of harmonization efforts. Although there are examples of existing harmonization initiatives to build upon, the advantages and challenges of harmonization merit a more detailed discussion.
Harmonization of Decision-Making Processes
Various elements of the OEL derivation process can be harmonized so that similar approaches are applied by different organizations. As previously framed by the International Council on Mining & Metals (ICCM),(Citation73,Citation74) aspects for which standardized criteria can be provided include:
review and evaluation of relevant scientific literature;
selection of critical health endpoint(s);
determination of whether critical effects are threshold or non-threshold;
selection of key studies and PODs for dose–response assessments;
selection of uncertainty factors that most appropriately represent the uncertainty and variability associated with a literature database; and
calculation of the OEL.
Standardized criteria for the consideration of policy decisions in the OEL-derivation process, including risk acceptance and technological and economic feasibility, could also be developed.
Harmonization of OEL Derivation Documentation
ICCM also recommended standardized criteria for the documentation and publication of all key steps in the derivation process.(Citation73,Citation74) Common templates could also be developed for the documentation of the processes involved in the derivation of an OEL (e.g., criteria documents), improving consistency in the documentation of the OEL derivation processes among different organizations.(Citation2,Citation69) An ideal format for a standardized scientific supporting document might easily be agreed upon, because there are often only minor differences between existing scientific documentation for OELs. Proposed characteristics of an ideal “standardized” supporting document that might increase the likelihood of acceptance as the scientific basis for an OEL by organizations around the world are presented in . Commonly accepted definitions for the terms used in the OEL documentation could also help lead to the harmonization of scientific supporting documents.(Citation75)
Table 3 Ideal Characteristics of Standardized Scientific Supporting Documents for Occupational Exposure Limits (OELs)
Existing Harmonization Initiatives
There has been a long history of attempts to harmonize the OEL derivation process among countries around the world. A successful international harmonization initiative was a 1989 workshop held in The Hague, Netherlands, organized by the Directorate General of Labour in the Netherlands and the Commission of the European Communities. The workshop had the objective of initiating the examination of harmonization and cooperation in the preparation of scientific supporting documents for OELs, both within the Europe and elsewhere.(Citation2) This international discussion ultimately paved the way for establishment of the Concise International Chemical Assessment Documents (CICADs), which were first published in 1998.(Citation76) CICADs are technical documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals on human health and the environment, including the characterization of hazard and dose-response from exposure to a chemical, which countries can then use to develop an OEL. The documents are based on selected national or regional evaluation documents or on existing Environmental Health Criteria assessments published by WHO. Similarly, at the level of the European region, the European Commission created the Scientific Experts Group (now the SCOEL) in 1990. This committee has been proposing Indicative Limit Values (ILVs; now IOELVs) and Binding Limit Values (BLVs; now BOELVs) for adoption by EC Member States since 1991.(Citation7) Other agreements have been developed between a few organizations or countries; one example of this is the Nordic Expert Group (NEG)—a collaboration between Sweden, Norway, Denmark, Finland and Iceland that develops criteria documents for the establishment of OELs.(Citation6) Activities of the NEG include describing the scientific database for a chemical; using these data, the Scandinavian countries derive their own OEL values. The NEG has also furthered its collaborations in the establishment of agreements with NIOSH and the Dutch Expert Committee on Occupational Safety.(Citation6,Citation69)
Steps toward harmonization of OELs have also been taken by many organizations to promote mutual awareness of other organizations’ activities, priorities, and thought processes, as well as the exchanging of information. To date, the extent of harmonization efforts regarding OELs has been based largely on information sharing. The ILO is a key organization encouraging international collaboration, as it promotes information and data sharing among countries. Perhaps as a result of data sharing, many of the OELs adopted around the world are based on those from other organizations, such as ACGIH, NIOSH, OSHA, and the EU.(Citation77) Cross-membership among OEL groups, for example with SCOEL members acting as representatives for other OEL-deriving organizations, also provides a significant opportunity for shared information. In addition, significant efforts have been initiated to improve the transportability of toxicity and health effects data that serve as the input to the OEL derivation process. For example, the concept of a toxicity data portal with exposure response arrays has been described.(Citation78) To date, no single effort has seen global acceptance, but the trend is to increase data sharing and transparency.
Benefits and Drawbacks of Harmonization Initiatives
Harmonization of OELs can have many advantages. The process of developing OELs is complex, lengthy, and resource intensive.(Citation69) The time-consuming process of OEL development can restrict the number of values that are derived, with few updates to existing OELs for many organizations,(Citation36) leading to aging of OELs. Strong international collaboration efforts could reduce the need for multiple OEL-setting entities,(Citation12) or could encourage work sharing between organizations, thereby preventing duplication of personnel and financial resources that results when multiple organizations derive OELs for the same chemicals.(Citation2) Harmonization principles can also reduce confusion and economic inefficiencies that can occur, which can particularly affect multi-national companies that are required to comply with many different mandatory OELs.(Citation12) Inconsistent OEL derivation practices can also result in discrepancies in worker protection amongst countries.(Citation12) Harmonization could be particularly beneficial to workers in smaller countries. If performed properly, harmonization can also lead to greater transparency and use of best practices.
A unified scientific approach to the setting of OELs is one part of the path toward increased harmonization, but many impediments can stall progress. Many differences among organizations and countries can hinder the development of consistent OELs, such as legal, regulatory, economic, political, and cultural distinctions.(Citation8) Even if harmonized guidance on deriving OELs were available, inconsistencies between organizations might still occur—because of the nature of the data used in their derivation, exposure limits would be difficult to derive using one standardized approach.(Citation3) Caution must also be taken to ensure that harmonization does not magnify existing problems with the OEL development process. Centralization of the decision process, if done improperly, could lead to decreased transparency and increased distance between regulators and the public, including business owners and workers.(Citation36) Consistency in OEL development could also be a concern if less desirable approaches are promoted, or if it leads to a lower margin of safety.(Citation79) An important value of harmonization is the sharing of information on methods, while recognizing the value of flexibility available through the application of alternative approaches.
FRAMEWORK FOR THE SELECTION OF APPROPRIATE OELs
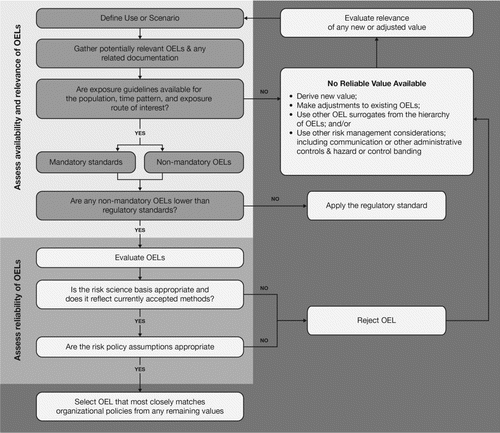
As previously noted, greater harmonization in the development of OELs has many advantages, including increased congruity in the approaches used by different organizations to derive the values. However, even with harmonization, some of the policy differences outlined in the section entitled “The Patchwork Landscape of OELs” will likely always exist among the OEL-setting organizations, resulting in a number of defensible OELs that can be used. This can lead to complexities when occupational hygienists need to select the most appropriate value from several potentially relevant OELs. Because little guidance exists in such cases, a framework is proposed to aid in the systematic selection of the most appropriate OEL for a particular situation. A schematic representation of this process is presented in , which is elaborated upon in the remainder of this section. The framework was designed to provide occupational hygienists with a guide of the decision logic process of assessing reliability and relevance of existing OELs.
Although much of this document has focused on traditional OELs, additional exposure limits might be useful for consideration in the framework. As discussed in the section entitled “Hierarchy of OELs,” tools such as IDLHs, AEGLs, and environmental health exposure guidelines can have data requirements and scientific validity that are similar to traditional OELs, but are derived for different exposure scenarios. Moreover, in data-poor situations, occupational exposure benchmarks that are lower on the OEL hierarchy—such as company- or vendor-derived values for internal use, or DNELs calculated under REACH legislation—might also be useful for consideration in the framework. However, as these values are not traditional OELs, occupational hygienists should apply the entire framework to carefully assess whether the benchmarks are appropriate for the relevant exposure scenario.
Although detailed knowledge of the science behind OEL derivation is not necessary for the application of the framework, familiarity with the processes might be helpful. Other articles from this journal issue will be helpful to occupational hygienists,(Citation10,Citation11) and comprehensive analyses of the processes for setting OELs have been developed elsewhere.(3,17,23,51,52,73,81,83) As the derivation of environmental exposure guidelines parallels that for OELs, occupational hygienists might also find guidelines for the derivation of these values to be useful.(Citation80,Citation82)
In many situations, the framework does not need to be followed in its entirety. After a cursory review of each of the eligible OELs, the occupational hygienist might immediately identify that most do not address the predefined scenario of use. Moreover, rather than evaluating each of the risk science and risk policy decisions, the occupational hygienist might decide to select the regulatory OEL, the most conservative guideline, or the newest value (if the assumption is that the most recent assessment will contain the most current risk assessment approaches and key studies). If the occupational hygienist has adequate time and sufficiently understands the processes behind the derivation of OELs, the entire framework should be followed, wherever possible. However, the most important aspects of the decision-making process, independent of how the framework is applied, are consistency and proper documentation of the approach.
For occupational hygienists who are able to apply the framework in its entirety, a series of key elements should be considered throughout the decision-making process. As described below, the steps begin with defining the scenario and gathering relevant OELs, and culminate in evaluation of the risk science and risk policy bases of the selected OELs.
Define Use or Scenario
Prior to identifying relevant OELs for a compound, the nature of exposure should be defined. This involves identifying how the exposure primarily occurs and who is exposed. Although traditional OELs are generally derived based on continuous inhalation exposure of healthy adult workers for 8 hr per day, 5 days per week, over a working lifetime, occupational risk assessments increasingly require variable exposure scenarios to be addressed. For example, occupational hygienists might have to consider exposures of an intermittent or infrequent nature, or exposures via dermal absorption. Moreover, in addition to a healthy workforce, occupational hygienists might also need to consider more susceptible populations in the workforce (e.g., workers who are potentially pregnant) or in the general population (in cases of community stewardship, or assessments of para-occupational or “take-home” exposures). Consideration of co-exposures to other agents might also be important. The goal is to identify traditional OELs or other exposure benchmarks that match the usage patterns and target population for the scenario being evaluated, because the type of exposure for which the benchmark is designed can influence the key studies that are used or the scientific assumptions and adjustments that are made.
Gather Potentially Relevant OELs and Related Exposure Benchmarks
Attempts should be made to identify as many potentially relevant OELs for the compound(s) of interest as possible. This process would include gathering applicable mandatory standards from state/provincial, national, or regional levels, as well as non-mandatory recommended OELs from organizations, such as the ACGIH TLVs, NIOSH RELs, and AIHA or OARS WEELs. OELs that are used in other jurisdictions could also be obtained. More extensive lists can be found in the online GESTIS database(Citation84)—a collection of occupational OELs gathered from various EU member states, Canada (Québec, Ontario), Japan, Switzerland, the United States, and other countries—and in books with collections of OELs.(Citation35,Citation45) Internet links to many countries’ OEL programs can also be found on the ILO (http://www.ilo.org/safework/info/publications/WCMS_151534(Citation85) and TERA OARS (http://www.tera.org/OARS) websites. Labels and safety data sheets provided by suppliers in accordance with the GHS will also provide the occupational hygienist with information identifying some existing OELs for a workplace. Depending on the nature of exposure, AEGLs and IDLH values might also be useful for inclusion. For compounds with a paucity of OELs, obtaining documentation for non-traditional benchmarks lower in the hierarchy of OELs might provide useful information for the exercise. In these cases, working provisional OELs can be sought from product manufacturers or relevant trade associations, and DNELs can be obtained from the ECHA database;(Citation86) however, these benchmarks should be considered with caution, and should undergo a thorough review (following the steps in the framework) prior to their application, because peer review or public consultation might not have been involved in their establishment. The occupational hygienist might also consider obtaining environmental health exposure guidelines from organizations such as the Environmental Protection Agency and Agency for Toxic Substances and Disease Registry in the United States, Health Canada, and IPCS. In addition to simply gathering the OELs and related exposure benchmarks, any documentation behind the derivation or establishment of these values should be obtained, when available.
For simplification of the presentation of the framework, the collective title of the gathered exposure benchmarks—including traditional OELs, acute values, environmental health exposure guidelines, and tools further down the hierarchy of OELs—will be referred to hereafter as OELs.
Assess the Relevance of OELs
As previously discussed in this article, the problem formulation stage of risk assessment—which ensures that risk assessments answer specific risk management questions—can influence the decisions made in the OEL derivation process. The exposure scenario defined in the first stage should be compared to the existing OELs to ensure that the assumptions used to derive the value align with the target population and exposure pattern assumed in the scenario of interest. If the problem formulation for certain OELs is sufficiently different from that of the defined scenario, the OEL might not be relevant. Key considerations in determining relevancy include:
target populations—potentially sensitive worker subpopulations (such as women who are pregnant or of a childbearing age, or workers with pre-existing conditions that might increase their susceptibility to a chemical) vs. healthy workers;
route of exposure—inhalation vs. dermal vs. oral routes; and
use patterns—intermittent vs. continuous use, and short-term vs. chronic exposures.
Even if all of these elements do not align, it might still be possible to adapt the OEL for the particular circumstances of interest. For example, if the target population of the OEL is broader—and encompassing more sensitive subpopulations—than those that will actually be exposed, the occupational hygienist could review the details behind the derivation of the value to identify if the uncertainty factor for human variability in susceptibility had been applied and could be modified for the current application of the OEL. If the key study used as the basis for the OEL has a different exposure pattern to the defined scenario, further adjustments for exposure duration could be made. A variety of techniques for the adjustment of OELs for differing exposure durations and temporal patterns are available.(Citation3) Moreover, an overly conservative approach could be applied, if feasible; for example, if the scenario of interest is limited to acute or short-term exposures, but maintaining exposures below a chronic OEL is achievable, this lower value could be retained in the assessment process. Any OELs that are not applicable to the defined scenario, or that cannot be adjusted to better match the defined scenario, should be eliminated from further consideration. If no values remain, and no other OELs can be found from a more extensive search of the literature, it might be necessary to derive a new value or adopt a value modified from an alternative scenario (including those derived for environmental exposures for the general population).
Compare Mandatory Standards to Non-mandatory OELs
For legal reasons, it is important to ensure exposure is maintained below all mandatory OELs as a minimum practice. However, further consideration of non-mandatory OELs is highly recommended if they are lower than mandatory values. Phase 2 of the assessment should be completed with these non-mandatory OELs to determine if they represent more appropriate guides for worker health protection.
Assess the Reliability of OELs
Scenario-relevant OELs must then be evaluated with respect to their scientific bases and the science policy assumptions applied in their derivation. The reliability assessment steps are to be completed for each OEL that has not been excluded based on misalignment with the problem formulation statement. To increase validity and transparency in the selection process, a list of acceptable and unacceptable scientific approaches and science policy decisions should be developed prior to performing the assessment, wherever possible. Detailed information on the bases of each of the OELs is needed for this assessment; consequently, it might be necessary to gather various technical documents that provide this information, or to contact the OEL-setting organization for more information if these details are not published. Gathering the documentation for each of the OELs or obtaining as many details as possible about the derivation is critical to identifying whether the appropriate risk science and policy methods and approaches were applied.
Table 4 Overview of Steps Involved in the Derivation of an Occupational Exposure Limit
Evaluate the Risk Science Basis for the OEL
Prior to evaluating the validity of the scientific approaches applied by each of the OEL-developing organizations, an important step is to obtain an update on the body of literature that exists for the chemical. Many easily accessible databases are within reach of the practicing occupational hygienists for this purpose (e.g., U.S. National Library of Medicine Toxnet.)(Citation87) In particular, knowledge of the toxicological and epidemiological publications that could be used as key studies for the assessment—as well as the strengths and limitations of each study—is important, since these resources will inform the judgment as to whether currently listed OELs are adequately up to date. For users with more experience with OELs, such information can also be used to verify that the most relevant adverse endpoint(s) for the OEL and the key studies that form acceptable bases for the OEL are documented. Such data serve a number of purposes to: (1) ensure the key effects based on newer data are addressed; (2) verify that the effects the OEL is based on are relevant to the scenario of interest; (3) guide decisions on the margin between exposure and OEL that might be suitable for initiating risk management (i.e., for severe toxicity a bigger margin between exposure and the OEL is typically desired); and (4) inform additional preventive risk management strategies such as medical surveillance needs. OELs that are developed based on adverse effects that are not currently the most relevant (e.g., if more conservative or acceptable endpoints have been identified, or if an endpoint in animals is no longer considered relevant to humans) or inappropriate studies (e.g., studies that are no longer based on current scientific principles, or that are no longer the most conservative or relevant in the body of literature for the compound) can be eliminated from further review. The occupational hygienist should then review the remaining OELs to ensure that the choice of the point of departure (NOAEL, LOAEL, BMD, or cancer slope factor) and any applied uncertainty factors are acceptable.
As part of the risk science evaluation of the OEL, the occupational hygienist should also identify whether the OEL was peer reviewed. Peer review is important when developing an OEL, as it increases the validity and reliability of the guideline value. When experts not directly involved in the OEL derivation process review the value, they can help to identify any missing considerations and potential biases that could negatively impact its calculation. Public consultation or other similar external review processes might also be considered to be a sufficient peer review activity. The occupational hygienist should identify whether each remaining OEL has undergone peer review, and, wherever possible, obtain comments from the peer review process that could help to identify limitations with the value. If an OEL under consideration has not been peer reviewed, the occupational hygienist could consider obtaining such a review to ensure its validity.
Evaluate the Risk Policy Assumptions for the OEL
The occupational hygienist will evaluate all OELs that were deemed to have an adequate scientific basis to ensure the policy assumptions for the OEL are relevant. If the OEL is based on direct estimates of risk (including, but not limited to, linear extrapolations of cancer risk), ensure that the assumed acceptable risk level (e.g., 1 in 1000) is in line with organizational policies. Moreover, feasibility of the OEL should also be considered. If the OELs under review have been evaluated for economic, engineering, and analytical feasibility, ensure that the assumptions made in these assessments are consistent with the target organization's capabilities.
Select the Appropriate OEL
If more than one OEL was retained from the assessment, the most appropriate OEL should be selected. The occupational hygienist should rank the OELs to identify which value was derived using assumptions that are most similar to those used within the target organization. Using pre-identified criteria, the OELs based on risk science and risk policy decisions that are most closely aligned with the occupational hygienist's organizational policies should be selected. If some risk science or risk policy decisions are of particular importance for the organization, the criteria can be arranged so that greater weight is placed on these higher priority options. The occupational hygienist must ensure that the selected value is equivalent to or more conservative than any legally mandated standards. Although many organizations select the lowest among the pool of “relevant and reliable” OELs, others actually review the literature and points of departure of the existing OELs and select the most robust OELs for their purposes. Such strategies are appropriate—the primary recommendation is that the process is documented to ensure consistency.
If no OELs are deemed appropriate during the assessment, the occupational hygienist can consider deriving a working provisional OEL for the organization. Using existing OELs, a new value could be derived by selecting a combination of appropriate risk science and risk policy assumptions from the various OELs. Another potential approach would involve selecting a single OEL or other exposure benchmark as a starting point and changing the risk science and risk policy decisions to ones that are aligned with the organization's practices. Typical adjustments might address assumptions related to the duration and route of exposure and areas of uncertainty included in current exposure guidelines. An overview of the steps involved in deriving an OEL can be found in and more detailed guidance has been provided elsewhere in the literature.(3,10,11,17,23,51,52,73,81,83) Any provisional OELs should be peer reviewed to strengthen the confidence in the value.
If no OELs can be found for a particular chemical, or if it is not possible to derive a new value based on existing OELs, the OEL hierarchy concept can be applied. In the absence of quantitative limits, risk management strategies can be applied that address prior handling experiences with similar compounds using techniques such as control banding. Control banding strategies have a proven utility when there is uncertainty, whether with a lack of firm toxicological and exposure information or in the absence of OELs.(Citation88) Both the pharmaceutical and nanotechnology industries highlight control banding's utility under these circumstances and there is a strong research basis internationally for its role in implementing controls commensurate to risk in the absence of OELs for nanomaterials.(89-91) As more materials become available in bulk and nanosized dimensions, multiple OELs might need to be developed for the same material, as NIOSH has done for titanium dioxide.(Citation92) The hazard classifications provided under the GHS can be used to accomplish this approach. The vast number of potential nanomaterials will likely necessitate the development of categorical approaches based on mode of action or physical and chemical characteristics.(Citation93,Citation94)
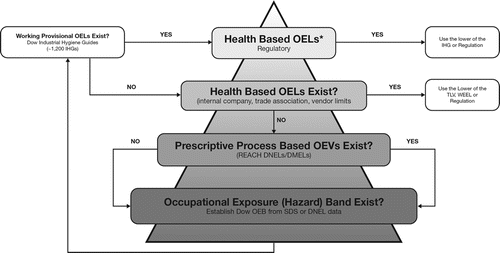
Although a decision framework for selecting an appropriate (relevant and reliable) OEL is proposed in this article, the concepts presented in the framework are not novel. Many organizations already have similar decision processes in place; one example of this is the process used by The Dow Chemical Company, displayed in .
The selection process should be guided by a knowledgeable occupational hygienist; however, participatory occupational hygiene approaches can be used to involve workers in the selection of the most appropriate OEL. Participatory approaches have previously been recommended for application in other areas of occupational hygiene, including the use of control banding in a Risk Level-Based Management System,(Citation95) and could also be extended for the use of more traditional OELs. Workers can help in the gathering and presentation of information for each of the OELs. Decision-making activities can provide an opportunity to empower workers to participate in the process of preventing diseases that might result from exposures to chemical substances.(Citation96)
CONCLUSION
OELs are developed by many organizations around the world and provide an essential tool for occupational health risk assessment. Because of the uncertain nature of risk assessment and differing levels of data availability, a patchwork of OELs has evolved over time. OELs have been developed for only a small fraction of the universe of chemicals; where OELs have been derived, multiple values exist for most substances. With many OEL-setting organizations currently in existence around the world, each with their own approaches, these values can differ appreciably. The need to identify the most appropriate OEL for a compound when a range of values exists presents a challenge for occupational hygienists; to address this challenge, a systematic framework to guide the process of evaluating and selecting the most appropriate OEL for use in specific circumstances has been proposed. To further simplify this process, it is recommended that international harmonization activities be expanded to promote commonalities and transparency in the approaches used by organizations around the world in their development of OELs.
ACKNOWLEDGMENTS
The authors would like to thank the peer reviewers (including H. Griem, C. Laszcz-Davis, T.J. Lentz, K. MacMahon, and J. Perkins) for their valuable expertise and many helpful comments that served to improve the initial draft of this manuscript. The work of staff at the National Institute for Occupational Safety and Health (NIOSH) who assisted with technical aspects of this paper, including graphic design (G. Fazio) and reference database maintenance (D. Baker), is also gratefully acknowledged.
This work, in part, was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344, LLNL-JRNL-651212. D. Krewski is the Natural Sciences and Engineering Research Chair in Risk Science at the University of Ottawa. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
REFERENCES
- Waters, M., L. McKernan, A. Maier, M. Jayjock, V. Schaeffer, and L. Brosseau: Exposure estimation and interpretation of occupational risk: Enhanced information for the occupational risk manager. J. Occup. Environ. Hyg. 12(0):S99–S111 (2015).
- Zielhuis, R.L., P.C. Noordam, C.L. Maas, J.J. Kolk, and H.P. Illing: Harmonization of criteria documents for standard setting in occupational health: a report of a workshop. Regul. Toxicol. Pharmacol. 13(3):241–262 (1991).
- Paustenbach, D.J., D.M. Cowan, and J. Sahmel: The history and biological basis of occupational exposure limits for chemical agents. In Patty's Industrial Hygiene, R. V and B. Cohrssen (eds.). Hoboken, NJ: John Wiley & Sons Inc, 2011.
- “Derivation of MAK values.” Available at: http://www.dfg.de/en/dfg_profile/statutory_bodies/senate/health_hazards/structure/working_groups/derivation_mak/index.html2013).
- Ripple, S.: History of occupational exposure limits. Exposure Setting Processes—An International Challenge, Workshop at the International Occupational Hygiene Assocation (IOHA) Conference, Rome, Italy2010).
- “The Nordic Expert Group.” Available at: http://www.av.se/arkiv/neg/the_neg/2013).
- European Commission: Methodology for the Derivation of Occupational Exposure Limits: Key Documentation (Version 7). (2013).
- Adkins, C., L. Booher, D. Culver, et al.: “Occupational exposure limits: do they have a future?” Available at http://www.ioha.net/assets/files/MASTER%20OEL%20Green_Paper%2009%2018%2009(1).pdf.
- Paustenbach, D.J.: Occupational Exposure Limits. In ILO Encyclopaedia of Occupational Health and Safety, pp. 30.27–30.34. Geneva: International Labour Organisation, 1997.
- Wheeler, M.W., R. Park, A.J. Bailer, and C. Whittaker: Historical context and recent advances in exposure-response estimation for deriving occupational exposure limits. J. Occup. Environ. Hyg. 12(0):S7–S17 (2015).
- Dankovic, D.A., B.D. Naumann, A. Maier, M.L. Dourson, and L. Levy: The scientific basis of uncertainty factors used in setting occupational exposure limits. J. Occup. Environ. Hyg. 12(0):S55–S68 (2015).
- Howard, J.: Setting occupational exposure limits: are we living in a post-OEL world? U. Pa. J. Lab. Empl. L. 7(3):513–528 (2005).
- United States Department of Labor: Occupational Health and Safety Standards 1910.1000 Z Toxic and Hazardous Substances, Washington DC.
- European Commission: “Council Directive 98/24/EC of 7 April 1998 on the protection of the health and safety of workers from the risks related to chemical agents at work” Available at https://osha.europa.eu/en/legislation/directives/75.
- Salter, L., and E. Levy: In the Eye of the Storm: Case Study One: The American Conference of Governmental Industrial Hygienists. In Mandated Science: Science and Scientists in the Making of Standards, L. Salter (ed.), pp. 36–66. Dordrecht, Netherlands: Kluwer Academic Publishers, 1988.
- Piney, M.: Exposure limits, practicability and health risks: Arguments for a paradigm shift. In The Politics of Chemical Risk: Scenarios for a Regulatory Future, R. Bal and W. Halffman (eds.), pp. 27–73. Dordrecht, The Netherlands: Kluwer Academic Publishers, 1998.
- Vincent, J.H.: International occupational exposure standards: a review and commentary. Am. Ind. Hyg. Assoc. J. 59:729–742 (1998).
- Hansson, S.O.: Setting the Limit: Occupational Health Standards and the Limits of Science. New York: Oxford University Press, 1998.
- American Conference of Governmental Industrial Hygienists (ACGIH): Sources, Abbreviations, Definitions and Terms Used Within the Database and Its Reports. In Documentation of the TLVs and BEIs with Other Worldwide Occupational Exposure Values: 2006 CD-ROM, ACGIH (ed.), pp. 1–15. Cincinnati, OH: ACGIH, 2006.
- National Academy of Sciences (NAS): Science and Decisions: Advancing Risk Assessment. Washington, D.C.: National Academy Press, 2009.
- Krewski, D., M. Westphal, M.E. Andersen, et al.: A framework for the next generation of risk science. Environ. Health Perspect. 122(8):796–805 (2014).
- Laszcz-Davis, C., A. Maier, and J. Perkins: The hierarchy of OELs: A new organizing principle for occupational risk assessment. Synergist: 26–30 (March 2014).
- Nielsen, G.D., and S. Ovrebo: Background, approaches and recent trends for setting health-based occupational exposure limits: a minireview. Regul. Toxicol. Pharmacol. 51(3):253–269 (2008).
- Kreider, M.L., and E.S. Williams: Interpreting REACH guidance in the determination of the derived no effect level (DNEL). Regul. Toxicol. Pharmacol. 58(2):323–329 (2010).
- Ader, A.W., J.P. Farris, and R.H. Ku: Occupational health categorization and compound handling practice systems: roots, application and future. Chem. Health Saf. 12(4):20–26 (2005).
- American Industrial Hygiene Association (AIHA) Control Banding Working Group: Guidance for Conducting Control Banding Analyses. Fairfax, VA: AIHA, 2007.
- Naumann, B.D.: “Control banding in the pharmaceutical industry.” Occupational Hygienists Conference Proceedings 2005. Available at: http://www.aioh.org.au/downloads/documents/ControlBandingBNaumann.pdf (accessed November 15, 2014).
- National Institute for Occupational Safety and Health (NIOSH): Qualitative Risk Characterization and Management of Occupational Hazards: Control Banding (CB). DHHS (NIOSH) Publication No. 2009-152 (2009).
- Ripple, S.: Hyped about hazard banding. Synergist: 43–45:60 (2009).
- Zalk, D.M., and D.I. Nelson: History and evolution of control banding: a review. J. Occup. Environ. Hyg. 5(5):330–346 (2008).
- Barlow, S.: Threshold of Toxicological Concern (TTC): A Tool for Assessing Substances of Unknown Toxicity Present at Low Levels in the Diet. Brussels, Belgium, ILSI Press, 2005.
- Kroes, R., J. Kleiner, and A. Renwick: The threshold of toxicological concern concept in risk assessment. Toxicol. Sci. 86(2):226–230 (2005).
- Dolan, D.G., B.D. Naumann, E.V. Sargent, A. Maier, and M. Dourson: Application of the threshold of toxicological concern concept to pharmaceutical manufacturing operations. Regul. Toxicol. Pharmacol. 43(1):1–9 (2005).
- National Institute for Occupational Safety and Health (NIOSH): Current intelligence bulletin 66: derivation of immediately dangerous to life or health (IDLH) values. DHHS (NIOSH) Publication No. 2014–100 (2013).
- Brandys, R.C., and G.M. Brandys: Global Occupational Exposure Limits for Over 6,000 Specific Chemicals. 2nd Edition. Hingdale, IL: Occupational and Environmental Health Consulting Services Inc., 2008.
- Schenk, L., S.O. Hansson, C. Rudén, and M. Gilek: Occupational exposure limits: a comparative study. Regul. Toxicol. Pharmacol. 50(2):261–270 (2008).
- Chemical Abstracts Service: “CAS REGISTRY(SM) surpasses 75 million small molecules2013).
- “TSCA Chemical Substance Inventory: Basic Information.” Available at: http://www.epa.gov/oppt/existingchemicals/pubs/tscainventory/basic.html2013).
- Health Canada: Proposal for Priority Setting for Existing Substances on the Domestic Substances List Under the Canadian Environmental Protection Act, 1999: Greatest Potential for Human Exposure. Ottawa, ON, Canada: Health Canada, Existing Substances Division, (2003).
- European Chemicals Agency (ECHA): “Classification and Labelling Notification Report on 4 January.” Available at http://echa.europa.eu/documents/10162/13585/clp_final_report_20110104_en.pdf.
- Schenk, L.: Comparison of data used for setting occupational exposure limits. Int. J. Occup. Environ. Health 16(3):249–262 (2010).
- Chen, C.P., H.W. Ahlers, G.S. Dotson, et al.: Efficacy of predictive modeling as a scientific criterion in dermal hazard identification for assignment of skin notations. Regul. Toxicol. Pharmacol. 61(1):63–72 (2011).
- Haber, L.T. and A. Maier: Scientific criteria used for the development of occupational exposure limits for metals and other mining-related chemicals. Regul. Toxicol. Pharmacol. 36(3):262–279 (2002).
- United States Department of Labor: “OSH Act of 1970.” Available at https://www.osha.gov/pls/oshaweb/owasrch.search_form%3Fp_doc_type=OSHACT.
- American Conference of Governmental Industrial Hygienists (ACGIH): 2014 TLVs and BEIs. Cincinnati, OH: ACGIH, 2014.
- Castleman, B.I., and G.E. Ziem: Corporate influence on threshold limit values. Am. J. Ind. Med. 13(5):531–559 (1988).
- Henschler, D.: Evaluation of adverse effects in the standard-setting process. Toxicol. Lett. 64–65:53–57 (1992).
- United Nations Economic Commission for Europe: Globally Harmonized System of Classification and Labelling of Chemicals. Fifth revised edition. Geneva: United Nations Economic Commission for Europe, 2013.
- ACUTEX: Methodology to develop AETLs (2006).
- European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC): Guidance on Assessment Factors to Derive a DNEL. Technical Report No. 110. Brussels: European Centre for Ecotoxicology and Toxicology of Chemicals, 2010.
- Illing, H.P.: Extrapolating from toxicity data to occupational exposure limits: some considerations. Ann. Occup. Hyg. 35(6):569–580 (1991).
- Ku, R.H.: An overview of setting occupational exposure limits (OELs) for pharmaceuticals. Chem. Health Saf. 7(1):34–37 (2000).
- Ritter, L., C. Totman, K. Krishnan, R. Carrier, A. Vezina, and V. Morisset: Deriving uncertainty factors for threshold chemical contaminants in drinking water. J. Toxicol. Environ. Health B Crit. Rev. 10(7):527–557 (2007).
- Schenk, L., and G. Johanson: Use of uncertainty factors by the SCOEL in their derivation of health-based occupational exposure limits. Crit. Rev. Toxicol. 40(9):791–798 (2010).
- Renwick, A.G., J.C.M. Dorne, and K. Walton: Pathway-related factors: The potential for human data to improve the scientific basis of risk assessment. Hum. Ecol. Risk Assess. 7(1):165–180 (2001).
- International Programme on Chemical Safety (IPCS): Chemical-Specific Adjustment Factors for Interspecies Differences and Human Variability: Guidance Document for Use of Data in Dose/Concentration–Reponse Assessment. Harmonization Project Document No. 2. Bussels: IPCS, 2005.
- European Chemicals Agency: Guidance on information requirements and chemical safety assessment Chapter R.8: Characterisation of dose [concentration]-response for human health. ECHA-2010-G-19-EN Helsinki, Finland: European Chemicals Agency, 2012.
- Schenk, L., and G. Johanson: A quantitative comparison of the safety margins in the european indicative occupational exposure limits and the derived no-effect levels for workers under REACH. Toxicol. Sci. 121(2):408–416 (2011).
- Dyck, R., M.S. Islam, A. Zargar, A. Mohapatra, and R. Sadiq: Application of data fusion in human health risk assessment for hydrocarbon mixtures on contaminated sites. Toxicology 313(2–3):160–173 (2013).
- Islam, M.S., A. Zargar, R. Dyck, A. Mohapatra, and R. Sadiq: Data fusion-based risk assessment framework: An example of benzene. Int. J. Syst. Assur. Eng. Manage. 3(4):267–283 (2012).
- International Programme on Chemical Safety (IPCS): Part 1: IPCS Framework for Analysing the Relevance of a Cancer Mode of Action for Humans and Case-Studies; Part 2: IPCS Framework for Analysing the Relevance of a Non-Cancer Mode of Action for Humans. Harmonization Project Document No. 4. Geneva, Switzerland: IPCS, 2007.
- Tao, T., Y. Bhuller, Y. Bonvalot, et al.: Weight of Evidence: General Principles and Current Applications at Health Canada (2011).
- Rhomberg, L.R., L.A. Bailey, and J.E. Goodman: Hypothesis-based weight of evidence: a tool for evaluating and communicating uncertainties and inconsistencies in the large body of evidence in proposing a carcinogenic mode of action–naphthalene as an example. Crit. Rev. Toxicol. 40(8):671–696 (2010).
- Klimisch, H.J., M. Andreae, and U. Tillmann: A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul. Toxicol. Pharmacol. 25(1):1–5 (1997).
- Money, C.D., J.A. Tomenson, M.G. Penman, P.J. Boogaard, and R.J. Lewis: A systematic approach for evaluating and scoring human data. Regul. Toxicol. Pharmacol. 66(2):241–247 (2013).
- Chambers, A., D. Krewski, N. Birkett, et al.: An exposure-response curve for copper excess and deficiency. J. Toxicol. Environ. Health B Crit. Rev. 13(7–8):546–578 (2010).
- Dourson, M.L., L.K. Teuschler, P.R. Durkin, and W.M. Stiteler: Categorical regression of toxicity data: a case study using aldicarb. Regul. Toxicol. Pharmacol. 25(2):121–129 (1997).
- Tyshenko, M.G., S. El Saadany, T. Oraby, et al.: Expert elicitation for the judgment of prion disease risk uncertainties. J. Toxicol. Environ. Health A 74(2–4):261–285 (2011).
- Lundberg, P.: National and international approaches to occupational standard setting within Europe. Appl. Occup. Environ. Hyg. 9(1):25–27 (1994).
- Travis, C.C., and H.A. Hattemer-Frey: Determining an acceptable level of risk. Environ. Sci. Technol. 22(8):873–876 (1988).
- United States Government Accountability Office (USGAO): Chemical risk assessment: Selected federal agencies’ procedures, assumptions, and policies. No. GAO-01-810 (2001).
- International Programme on Chemical Safety (IPCS): “International Programme on Chemical Safety Harmonization Project Strategic Plan: Harmonization of Approaches to the Assessment of Risk from Exposure to Chemicals.” Available at: http://www.who.int/entity/ipcs/methods/harmonization/strategic_plan.pdf2013).
- International Council on Mining and Metals: “The Setting and Use of Occupational Exposure Limits: Current Practice.” Available at https://www.icmm.com/document/17.
- International Council on Mining and Metals: Towards a Harmonized Approach to Setting Occupational Exposure Limits: Report of an ICMM-Sponsored Workshop, London, November 9–11, 2005 (2006).
- Mikheev, M.I.: Toward WHO-recommended occupational exposure limits. Toxicol. Lett. 77(1–3):183–187 (1995).
- International Programme on Chemical Safety (IPCS): 1,2-Dichloroethane. Concise International Chemical Assessment Document No. 1 (1998). . Geneva: IPCS.
- Ding, Q., L. Schenk, K. Malkiewicz, and S.O. Hansson: Occupational exposure limits in Europe and Asia–continued divergence or global harmonization? Regul. Toxicol. Pharmacol. 61(3):296–309 (2011).
- Woodall, G.M. and R.B. Goldberg: Summary of the workshop on the power of aggregated toxicity data. Toxicol. Appl. Pharmacol. 233(1):71–75 (2008).
- Schenk, L., S.O. Hansson, C. Rudén, and M. Gilek: Are occupational exposure limits becoming more alike within the European Union? J. Appl. Toxicol. 28(7):858–866 (2008).
- Dourson, M.L., and J.F. Stara: Regulatory history and experimental support of uncertainty (safety) factors. Regul. Toxicol. Pharmacol. 3(3):224–238 (1983).
- Seeley, M.R., L.E. Tonner-Navarro, B.D. Beck, et al.: Procedures for health risk assessment in Europe. Regul. Toxicol. Pharmacol. 34(2):153–169 (2001).
- WHO: Assessing Human Health Risks of Chemicals: Derivation of Guidance Values for Health-Based Exposure Limits. Environ. Health Criteria 170 (1994).
- Haber, L.T., J.E. Strawson, A. Maier, I.M. Baskerville-Abraham, A. Parker, and M.L. Dourson: Noncancer Risk Assessment: Principles and Practice in Environmental and Occupational Settings. In Patty's Toxicology, E. Bingham and B. Cohrssen (eds.), Hoboken, NJ: John Wiley & Sons, 2012. pp. 89–132.
- “GESTIS International Limit Values.” Available at: http://limitvalue.ifa.dguv.de/Webform_gw.aspx2013).
- “Chemical exposure limits.” Available at: http://www.ilo.org/safework/info/publications/WCMS_1515342013).
- “Information on Chemicals.” Available at: http://echa.europa.eu/information-on-chemicals2013).
- “TOXNET: Toxicology Data Network.” Available at: http://toxnet.nlm.nih.gov2013).
- Zalk, D.M.: Control Banding: A Simplified, Qualitative Strategy for the Assessment of Risks and Selection of Solutions. Delft, Netherlands: TU Delft, 2010.
- Brouwer, D.H.: Control banding approaches for nanomaterials. Ann. Occup. Hyg. 56(5):506–514 (2012).
- Van Duuren-Stuurman, B., S.R. Vink, K.J. Verbist, et al.: Stoffenmanager Nano Version 1.0: a web-based tool for risk prioritization of airborne manufactured nano objects. Ann. Occup. Hyg. 56(5):525–541 (2012).
- Zalk, D.M., S.Y. Paik, and P. Swuste: Evaluating the Control Banding Nanotool: a qualitative risk assessment method for controlling nanoparticle exposures. J. Nanopart. Res. 11(7):1685–1704 (2009).
- National Institute for Occupational Safety and Health (NIOSH): Current Intelligence Bulletin 63: Occupational Exposure to Titanium Dioxide. DHHS (NIOSH) Publication No. 2011-160 (2011).
- Kuempel, E., V. Castranova, C. Geraci, and P. Schulte: Development of risk-based nanomaterial groups for occupational exposure control. J. Nanopart. Res. 14(9):1–15 (2012).
- Schulte, P., V. Murashov, R. Zumwalde, E. Kuempel, and C. Geraci: Occupational exposure limits for nanomaterials: state of the art. J. Nanopart. Res. 12(6):1971–1987 (2010).
- Zalk, D.M., R. Kamerzell, S. Paik, J. Kapp, D. Harrington, and P. Swuste: Risk level based management system: a control banding model for occupational health and safety risk management in a highly regulated environment. Ind. Health 48(1):18–28 (2010).
- Zalk D.M.: Participatory occupational hygiene: a path to practical solutions. Asia. Pac. Newsl. Occup. Health Saf. 9(3):51 (2002).