Abstract
McIntyre Powder (MP) is a finely ground aluminum powder that was used between 1943 and 1979 as a prophylaxis for silicosis. Silicosis is a chronic lung disease caused by the inhalation of crystalline silica dust and was prevalent in the Canadian mining industry during this time period. The McIntyre Research Foundation developed, patented, and produced the MP and distributed it to licensees in Canada, the United States, Mexico, Chile, Belgian Congo, and Western Australia. In the province of Ontario, Canada it is estimated that at least 27,500 miners between 1943 and 1979 were exposed to MP. The present study was undertaken to examine the chemical and physical characteristics of two variations of MP (light grey and black). Chemical analyses (using X-ray Fluorescence and Inductively Coupled Plasma approaches) indicate that the black MP contains significantly higher concentrations of aluminum and metal impurities than the light grey MP (p < 0.001). X-ray diffractometry shows that while aluminum hydroxide dominates the aluminum speciation in both variations, the higher total aluminum content in the black MP is attributable to a greater proportion of elemental aluminum. Physical characterization (using electron microscopy, light microscopy, and dynamic light scattering) indicates that the light grey MP consists of particles ranging from 5 nm to 5 µm in diameter. Atomic Force Microscopy shows that the light grey MP particles in the nanoparticle range (<100 nm) have a mode between 5 and 10 nm. Consequently, it is possible that inhaled smaller MP nanoparticles may be transported via blood and lymph fluid circulation to many different organs including the brain. It is also possible for inhaled larger MP particles to deposit onto lung tissue and for potential health effects to arise from inflammatory responses through immune activation. This MP characterization will provide crucial data to help inform future toxicological, epidemiological, and biological studies of any long-term effects related to the inhalation of aluminum dust and nanomaterials.
Introduction
In the 1930s, the Canadian mining industry experienced a growing number of chronic lung diseases being reported in uranium and gold mine workers. The most prevalent lung disease was silicosis, a chronic fibrosis of the lung caused by the inhalation of crystalline silica dust.[Citation1,Citation2] The notion of using a fine aluminum powder for the treatment of silicosis was first published in 1937 as a preliminary report which was followed by a full report in 1939.[Citation3,Citation4] In the reports by Denny et al.,Citation4 the authors proposed that aluminum reduced the solubility of crystalline silica in the lung and formed a coating around silica particles to aid their clearance from the lung. Early in the 1940s, the McIntyre Research Foundation was formed to investigate possible preventative treatments for lung disease such as silicosis, which prompted the establishment of the Porcupine Silicosis Clinic in Timmins, Ontario, Canada, which conducted the first human trials using aluminum dust to treat silicosis. The fine aluminum powder now called “McIntyre Powder” officially came into use in December 1943 at the McIntyre Porcupine mine in Timmins, Ontario. The McIntyre Research Foundation developed, patented and produced the McIntyre Powder (MP) and distributed it to licensees in Canada, the United States, Mexico, Chile, Belgian Congo, and Western Australia.
In the province of Ontario, Canada, it is documented in the Mining Master Record that at least 27,500 miners were exposed to MP between 1943 and 1979. MP was administered before the start of every underground work shift. Miners would be isolated in a custom-designed airtight locker room (the mine dry) into which the airborne MP was dispersed for the miners to deliberately inhale. The MP in-air concentration recommended by the McIntyre Research Foundation in 1944 to prevent silicosis was one gram of MP per 1,000 ft3 (35.6 mg/m3) of locker room air volume for an exposure time of 10 min.[Citation5] The MP prophylaxis program was discontinued in 1979 on the recommendation of a task force commissioned by the Ontario government.[Citation6] Although evidence of either positive or negative health effects associated with inhaling MP was considered inconclusive, the task force report did recommend that mortality and morbidity studies be carried out to investigate possible long-term effects.[Citation6] At the time, two government reports of studies on Ontario miners from 1955–1977 found increased mortality rates amongst gold and uranium miners which were attributed to respiratory disorders, including silicosis, but found no evidence of altered mortality rates in miners exposed to MP compared to non-exposed miners.[Citation1,Citation2]
Subsequently, a 1990 study by Rifat et al.[Citation7] investigated potential effects of MP inhalation on cognitive function. The authors concluded that miners exposed to MP (n = 261) had significantly increased cognitive impairment that was correlated with their duration of exposure when compared to unexposed miners (n = 346). Peters et al.[Citation8] also described long-term cardiovascular and neurological effects related to MP inhalation. The study found no significant difference in mortality in the MP exposed miner group (n = 647) attributable to Alzheimer’s and cardiovascular diseases when compared to the unexposed miners (n = 1247). Peters et al.[Citation8] concluded that there was no significant difference in mortality from silicosis between the aluminum exposed group and unexposed group, suggesting that the MP had no protective effects against the inhalation of crystalline silica dust or fibrosis of the lung.
It is well established that both particle size and chemical composition of particles greatly affect the biological response to foreign inhaled particles in the body.[Citation9,Citation10] Animal models have shown that nanoparticles elicit inflammatory responses when inhaled or injected and that chronic exposures can cause chronic inflammatory diseases such as fibrosis and cancer.[Citation11–15] Occupational exposures to aluminum have been linked to a variety of neurologic, pulmonary, and cardiovascular effects and aluminum concentrations in brain tissues have been shown to accumulate with age and exposure level.[Citation10,Citation16,Citation17]
Although early studies indicated that a substantial proportion of the particles were nanoscale (≤100 nm),[Citation5,Citation18,Citation19] a key aspect of MP that has not been examined to date is its particle size distribution. Consequently, the chemical and physical characteristics of the MP needed to be evaluated. Recent acquisition of a collection of MP samples provided a unique opportunity to conduct a thorough chemical and physical characterization of various forms of the powder that were produced from the 1940s to the 1970s. The characterization work will help elucidate possible mechanisms underlying possible long-term health effects related to chronic or acute inhalation of aluminum dust (especially in the nanoscale level). The objective of this study is to characterize MP powders to provide crucial information that will support future toxicological, epidemiological, and biological studies on the possible long-term health effects related to the inhalation of MP.
Materials and methods
Source of McIntyre Powder samples
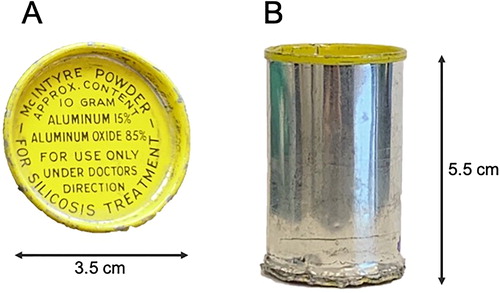
Samples of black McIntyre Powder (MP) used in this study were obtained from the Timmins Museum National Exhibition Centre, Timmins, Ontario, Canada. The black powder was subsampled from a previously opened MP canister which resembled the canister in Citation1. Four canisters of light grey MP (which also resemble the canister in ) were donated by United Steel Workers International (USW). All the canisters donated for this study were reported to be recovered from various mine sites in Ontario, Canada where MP had been used. The MP composition was labeled on the original canister as 15% aluminum and 85% aluminum oxide (). The first description of the commercial manufacturing of MP was published by A.W. Jacob in 1944.[Citation5] Solid aluminum pellets were ground in a ball-mill one meter in diameter and two meters in length.[Citation5] During grinding the powder was transported to a hopper by air currents produced by a fan.[Citation5] The powder was then cloth filtered to ensure only the smallest particles were collected for packaging in canisters.[Citation5] The protocol for manufacturing MP was modified in 1956 and later patented in 1958.[Citation18,Citation19] As presented at a conference held by the McIntyre Research Foundation in Toronto, Ontario, the key modifications were (a) manipulating air humidity during grinding to reduce the particle size, and (b) controlling the metallic aluminum content. The new (modified) MP was described as consisting of 13% metallic aluminum, with particle size less than 1.2 µm and 60% of the particles below 400 nm in diameter.[Citation18,Citation19]
Figure 1. Example of a McIntyre Powder aluminum canister. (A) Top view. (B) Side view. All McIntyre Powder canisters obtained for this study did not display a date of manufacturing, serial number, or lot number information.
Although all the McIntyre Powder Research Foundation canisters subsampled for the present study were labelled without dates as shown in , it is assumed that these materials post-date the 1958 patent. Atomic Force Microscopy (AFM), Dynamic Light Scattering (DLS), and Transmission Electron Microscopy (TEM) analyses were performed on the <200 nm size range of the light grey powder only to characterize particle size distribution and particle morphology. The black powder variant was excluded from these analyses due to the lack of available sample. Both the light grey and black powders were analysed using X-ray diffraction (XRD), X-ray fluorescence (XRF), and inductively coupled plasma mass spectrometry/optical emission spectroscopy (ICP-MS/OES) to determine the chemical and mineralogical composition of the samples.
Atomic force microscopy (AFM)
AFM analysis was conducted to measure the smallest size fraction present in the light grey MP. A suspension of light grey MP was prepared in Milli-Q filtered water, followed by sonication and centrifugation at 400 g for 10 min to separate larger particles (>200 nm) from smaller particles (<200 nm). Only the supernatant was sampled for AFM analysis as larger particles (≥200 nm) will interfere with the AFM probe. The MP suspension supernatant was deposited on mica. After particle deposition, non-adsorbed particles were removed by rinsing with water and the mica was dried with a filter paper. After the drying step, imaging was performed in Tapping Mode with a Multimode AFM (Veeco, Santa Barbara, CA, USA) operating with a Nanoscope IIIa controller. Silicon cantilevers AC160TS (Olympus, Hambourg, Germany) were used with resonance frequencies of approximately 300 kHz. All images were collected at a scan frequency of 1.5 Hz and a resolution of 512x512 pixels. A first- or second-order polynomial function was used to remove the background slope.
Dynamic light scattering (DLS)
DLS analysis was conducted to measure the average particle size of the three different size fractions present in the light grey MP as well as the surface charge of the corresponding fractions. A suspension of light grey MP in Milli-Q filtered water was prepared by sonication followed by centrifugation at 400 g for 10 min. One mL of the supernatant was removed for analysis. Then a small pipette was used to sample the top and bottom of the pelleted material which had collected at the bottom of the vial. These more concentrated subsamples were diluted by a factor of 10 into Milli-Q filtered water to yield an appropriate particle concentration for DLS analysis. Particle size distribution and zeta potential of the light grey MP particles were measured using DLS (Delsa Nano C Particle Size apparatus; Beckman Coulter, Villepinte, France) equipped with two 658 nm laser diode sources (power 30 mW) and a temperature controller (from 15–90 °C). The particle size was measured in triplicate at a constant scattering angle (165°) and a temperature of 25 °C and averaged for each subsample. The zeta potential was determined by measuring the electrophoretic movement of the charged particles under an applied electric field, with scattered light detected at a 15° angle.
Transmission electron microscopy (TEM)
TEM analysis was conducted to confirm the chemical composition of particles present in the smallest size fraction of the MP as well as to gather information about the morphology of the particles. A light grey MP suspension as previously described was deposited on a copper grid (3.05 mm, 250 Mesh, Agar Scientific Ltd., Stansted, Essex, UK), covered with carbon for imaging. Electron microscopy observations were made using a high-resolution transmission electron microscope (HR-TEM-STEM, Thermo Scientific Tecnai F20ST) (ThermoFisher Scientific, Materials and Structural Analysis Division, Eindhoven, Netherlands), operating at 200 keV with a field emission gun). Energy-dispersive X-ray (EDX) analysis coupled with STEM was used to identify the elemental composition of the MP particles. High resolution images were recorded at Scherzer defocus on a CCD multiscan camera (Gatan Quantum System, Gatan France, Evry, France), after astigmatism corrections, and eventually filtered via the Digital Micrograph software (GMS-3, Gatan GmbH, München, Germany).
X-ray diffraction (XRD)
XRD identifies phases within materials based on crystallographic lattice spacing and was chosen to identify the dominate minerals present in the two variants of the MP. Two samples of MP, one light grey and one black, were analysed by powder X-ray Diffraction (XRD) at the XPS Glencore Laboratory (Sudbury, Ontario, Canada). The instrument used to analyse the MPs was a Bruker D8 Advance, equipped with a cobalt X-ray tube (Bruker, Madison, WI, USA). The powders were mounted with grains in random orientations to ensure that all crystallographic directions are sampled by the beam. The resulting diffraction pattern is plotted as 2θ (peak spacing) versus intensity (X-ray counts). Identification of mineral phases is determined using EVA software (Bruker); the software compares the XRD spectra of the unknown sample to a published database containing over 200,000 powder diffraction files (International Centre for Diffraction Data). Note, due to low sample mass, the powders were mounted on a “zero-background” holder secured with petroleum jelly. In some cases, the jelly produced an amorphous signal (referred to as an artifact in ), however it was found that the amorphous signal was easily distinguishable from the sample signal and was removed from the dataset.
X-ray fluorescence (XRF) and loss-on-ignition (LOI)
XRF solid sample measurements were conducted to obtain a quantitative “total Al” concentration (which is difficult to achieve with wet digestion methods). Four samples of MP (three light grey and one black) were analyzed by X-ray Fluorescence (Panalytical Axios advanced wavelength dispersive XRF, Royston, UK) and loss-on-ignition by Activation Laboratories (Ancaster, Ontario, Canada). For XRF analysis, the sample is prepared into a pressed pellet and scanned with a primary X-ray source. Resulting secondary X-rays (fluorescence) are emitted upon excitation and are characteristic of the sample chemistry. Detection limits of XRF are in the order of 0.001–0.01 wt%. Al recoveries using XRF averaged 103% (± 1.5%) for five certified reference materials and ranged from 99–103%. Loss on ignition (LOI), which includes H2O+, CO2, S and other volatiles, was determined from the weight loss after roasting the sample at 1000 °C for 2 hr.
Inductively coupled plasma-mass spectrometry (ICP-MS) and inductively coupled plasma-optical emission spectroscopy (ICP-OES)
ICP-MS and ICP-OES analyses were performed to compare the trace elements present in the light grey and black MP and also to gather information about the variability of the trace elements amongst different canisters of light grey MP. Five canisters of MP (four light grey and one black) were subsampled in triplicate (approx. 50 mg per subsample) to obtain a measure of heterogeneity for each canister and shipped for elemental analysis to Health Canada (Tunney’s Pasture, Ottawa, Ontario, Canada). The MP subsamples were extracted using microwave digestion (Ethos Touch Control Advanced Microwave Lab station), using 50 mg samples in a mixture of 1.5 mL of 16 N HNO3 and 0.2 mL of 12 N HCl, based on the digestion method of Beltran-Huarac et al.[Citation20] Following microwave-assisted acid digestion, elemental concentrations were determined in the diluted extracts (1:10) using ICP-MS (Perkin Elmer NexION300s; Waltham, MA, USA) for trace elements (Zn, Ga, V, Cu, Ti, Pb, Mn, Sn, Zr, Ni, Mo, Cr, B, Br, Ce, Ba, Hf, Sr, and U) and ICP-OES (Agilent 5100 SVDV; Santa Clara, CA, USA) for elements occurring at higher concentrations (Al, Fe, Si, Mg, Na, and Ca). Difficulties were encountered obtaining quantitative recoveries for Al using certified reference materials as reported previously when HF is not used in the digestion.[Citation21,Citation22] Al concentrations determined using microwave digestion/ICP-OES were about 7% lower than results obtained using XRF (92.8% of XRF results for the black powder and 93.3% of XRF results for the light grey powder).
Results
Particle size distribution
This section describes particle size analysis of the nanoscale fraction of the light grey MP sample using AFM, DLS, and TEM. The International Organization for Standardization (ISO) defines a nanoparticle as an object having a diameter of less than 100 nm in at least one of three dimensions (ISO/TS 80004‐2:2015). It should be noted that the nanoscale MP materials characterized in the present study are considered incidental nanoparticles, as they were not deliberately manufactured (engineered) nanomaterials. To put the characterization of the nanoscale MP particles into perspective, and Citation1 provide the particle size characterization of aerosolized black MP samples by the McIntyre Research Foundation in the 1950s using electron microscopy () and thermal precipitator (). These analyses demonstrate the effectiveness of the 1956 modifications to the manufacturing process, in achieving a smaller particle size distribution. Results indicated that the complete particle size range of the prototype MP was from 5 µm to ≤ 200 nm (). The aerosolization tests showed that 60 min after dispersal of the modified MP, 23.36% of the collected particles smaller than 200 nm remained suspended in air (). A 1956 report from the McIntyre Research Foundation noted that the smallest particles that remained airborne were visible but too small to be counted using a thermal precipitator at 1,500x magnification.[Citation18] After the powder manufacturing protocol changed in 1956, it was reported that the modified MP contained no detectible particles larger than 2 µm.[Citation18]
Figure 2. A 1944 electron micrograph of McIntyre Powder particles (10,000X magnification). This image was taken directly from the paper entitled “The engineering aspects of aluminium prophylaxis” by A.W. Jacob published in Transactions, XLVII:185–202 (1944). Reprinted with permission of the Canadian Institute of Mining, Metallurgy and Petroleum.
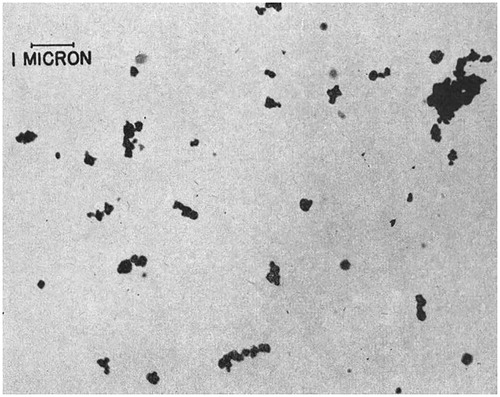
Table 1. Characterization of prototype and modified McIntyre Powder samples by the McIntyre Research Foundation in 1950s.[Citation18,Citation19] Particle size distribution after dispersal in air, using light microscopy (1500X magnification). Both powders analyzed were reported to be black in color. Modified technique received patent in 1958.
Atomic force microscopy (AFM)
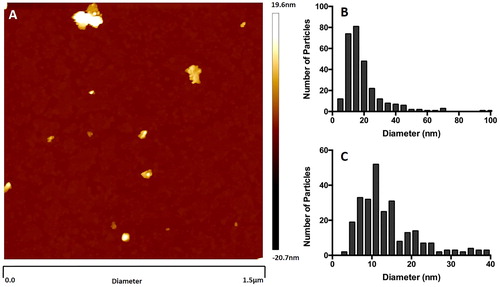
Citation3 shows the particle size distribution of the MP fraction smaller than 100 nm, obtained using AFM analysis of the suspension obtained after centrifugation. Although not representative of the entire MP sample, provides detailed characterization of the nanoscale size range, whereas previously the smallest MP particles to be characterized were classified only as ≤200 nm.[Citation18] Image analysis of the MP suspension shows the smallest particles being approximately 5 nm in diameter (. The particle size distribution generated from the analysis of the supernatant show particle sizes ranging from 100 nm to <10 nm (. The particle size distribution was skewed towards the smaller end of the scale with the mode occurring between 10 and 15 nm (.
Figure 3. Atomic Force Microscopy (AFM) particle size analysis of light grey McIntyre Powder: 1 mg/mL suspension of McIntyre Powder in Milli-Q filtered water. (A) Example of an image generated during the AFM analysis; (B) particle size distribution within supernatant; and (C) particle size distribution of particles smaller than 40 nm in diameter.
Dynamic light scattering (DLS)
Subsamples of the MP supernatant were analysed after centrifugation to prevent larger particles from interfering with the DLS measurements. The average particle size (hydrodynamic diameter) within the suspension was 132 ± 37 nm (mean ± standard deviation). Particle size averaged 327 ± 90 nm within the top subsample of the pelleted MP material and 638 ± 142 nm within the bottom subsample (). Agglomeration of MP particles that was observed with light microscopy and TEM ( Citation4) are likely to occur in liquid suspension, which could affect the effective particle size when analyzed by the DLS technique. The zeta potential of the supernatant, top pellet subsample, and bottom pellet subsample were +49 ± 6 mV, +37 ± 7 mV, and +14 ± 3 mV, respectively (). This suggests that the different size fractions may consist of particles with variable surface characteristics.
Table 2. Dynamic Light Scattering (DLS) particle size analysis of light grey McIntyre Powder: Zeta potential and size (hydrodynamic diameter) of particles in supernatant, and in top and bottom subsamples of the centrifugate. All values are mean ± SD; n = 3.
Transmission electron microscopy (TEM)
TEM images of MP particle morphologies () displayed agglomerates of rod and lamella shaped particles and varying degrees of porosity. The TEM analysis confirmed the composition of the nanoparticles to be aluminum (. Consistent with the DLS and AFM findings, the TEM analysis revealed numerous particles ≤50 nm in diameter ().
Figure 4. Transmission Electron Microscopy (TEM) image of light grey McIntyre Powder on a carbon grid: TEM images show the presence of agglomerates that include lamellae and rod-like structures, and that the powder is characterized by variable porosity.
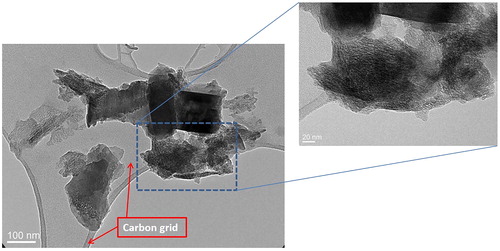
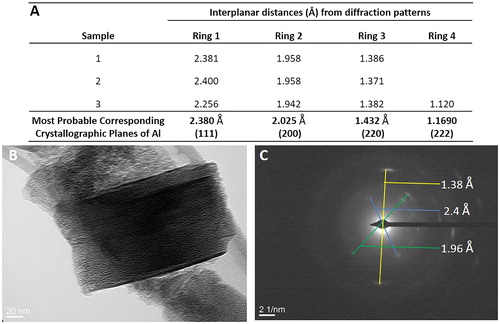
Figure 5. Transmission Electron Microscopy (TEM) analysis of light grey McIntyre Powder particles: (A) table of crystallographic data confirming the particle in image B is primarily composed of aluminum; (B) TEM image of McIntyre Powder particle; and (C) example of interplanar distances and diffraction pattern image.
Mineralogy
X-ray diffraction (XRD)
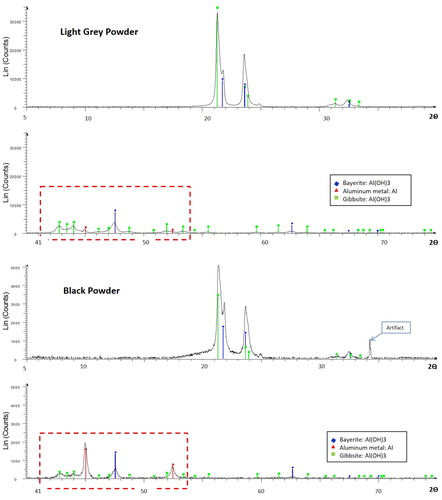
XRD spectra comparing the mineralogical composition of light grey and black MP are shown in Citation6. Similar peaks for gibbsite and bayerite, which are polymorphs of aluminum hydroxide (Al(OH)3), occur between 200 and 250 2θ in both the light grey MP () and the black MP (. These peaks indicate that aluminum hydroxide dominates the mineralogy of both black and light grey MP. The key difference between the mineralogy of black and light grey MP lies in the range between 410 and 540 2θ (red rectangles in ), in which there are significant peaks for elemental aluminum in the black MP only (consistent with the manufacturer’s label of 15% aluminum; ). In contrast, the elemental aluminum peaks in the light grey MP are very small (close to background). These results are similar to previous XRD analyses of a different set of black and grey MP samples presented in the Appendices of the 1990 Rifat thesis. In the previous study, the black MP contained a mixture of pure aluminum and bayerite, whereas the grey powder contained different polymorphs of aluminum hydroxide (gibbsite, bayerite and possibly nordstrandite) but no elemental aluminium.[Citation23]
Elemental composition
X-ray fluorescence and loss-on-ignition
The XRF analyses () indicated that aluminum content was higher in the black powder (44.3%) compared to the light grey powder (34.7%), which is attributable to the higher proportion of elemental aluminum in black MP, as revealed by the above XRD analyses. The amount of sample mass lost on ignition (LOI) was significantly higher in the light grey powder (∼32–34%) than in the black powder (15.8%; ). The higher LOI values, and lower total Al values, for the light grey MP compared to the black MP are consistent with the differences in Al speciation identified by XRD, specifically (a) the lower elemental aluminum abundance and (b) the higher proportion of hydroxides in the light grey MP. Most other elements were below XRF limits of detection (LOD) with the exception of Si, Fe, Ca, Ti, P, Cr, and V which were above or close to LOD (not presented in ).
Table 3. Chemical Composition of light grey and black McIntyre Powders.
ICP-MS and ICP-OES
Concentrations of metal impurities determined by ICP-MS and ICP-OES are averaged for the four light grey samples in , and individual results are presented in supplementary Tables A and B. The between-sample variability of metal impurities in the light grey MP, represented by relative standard deviation (%RSD = standard deviation divided by the mean, expressed as percent) is very high for some metals (e.g., RSD= 72% for Pb, 38% for Cr, and 50% for Ni) and very low for others (e.g., RSD ≤ 5% for Fe, Si, Na, Ga, V, Mn, Zr, Mo, Hf, and U). With respect to the black MP sample, within-sample variability is notably higher for Pb (RSD = 103%) and Cr (RSD = 59%) compared to other metal impurities ().
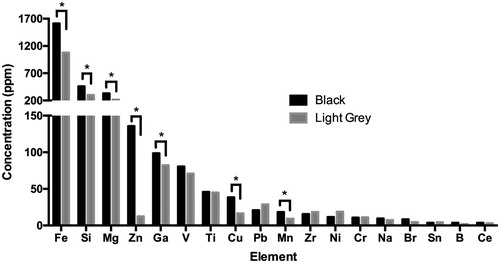
Citation7 compares concentrations of impurities in black vs. light grey MP, for elements present at concentrations exceeding 1 ppm. Elements displaying significantly higher concentrations in the black MP compared to the light grey MP (p < 0.001) include Fe (1,612 vs. 1,078 ppm), Si (459 vs. 300 ppm), Zn (136 vs. 12.4 ppm), Ga (98.5 vs. 82.3 ppm), Cu (38.4 vs. 16.5 ppm), and Mn (18.2 vs. 9.2 ppm). Other elements displaying significantly higher concentrations in the black MP include Ba, Ca, Mo, and U at p < 0.001; and Hf and Mg at p < 0.05 ( Citation4). Although not included in the bar graph, the aluminum content of the black MP sample determined by ICP-OES was 41.1% ± 0.7%, which was slightly lower than the XRF value (by 7%) caused by the difficulty of achieving quantitative recovery using acid digestion.[Citation21,Citation22] The difference in Al content between black MP and light grey MP observed using XRF also was confirmed using ICP-OES: Al content was significantly lower (p < 0.001) in the light grey samples (32.4% ± 0.6%; caption). These results confirm the XRF comparison of aluminum concentrations in black vs. light grey MP (shown in ). The significantly higher Al value for the black MP is caused by the difference in aluminum speciation (i.e., greater proportion of elemental aluminum in black MP compared to light grey MP) indicated by XRD analysis ().
Figure 7. Comparison of elemental contaminant concentrations in black vs. light grey McIntyre Powder (MP) determined by ICP-MS and ICP-OES. Note that primary y-axis refers to Fe, Si and Mg concentration, and secondary y-axis refers to concentration of remaining elements from Zn to Ce. *Asterisk indicates elements displaying statistically significant difference in concentration between light grey MP and black MP. Although not included in the bar graph, the Al content of the black MP sample determined by ICP-OES (41.1% ± 0.7%) was also significantly higher (p < 0.001) than the average Al of the four light grey samples (32.4% ± 0.6%; expressed as ppm in Supplementary Table A).
Discussion
From 1943–1979, tens of thousands of mine workers in Canada and worldwide were exposed to MP. The results of the present study confirm that the composition of the black MP is distinct from that of the light grey MP, in terms of both aluminum speciation and the concentrations of elemental impurities. It is known that miners were exposed to the black form of MP from 1943, as described in the earliest published characterizations of MP.[Citation24] It is not apparent from the literature why the light grey variation of the MP exists, or the extent of usage of the light grey powder.
With respect to chemical composition, the difference in chemistry between the black and light grey MP is likely related to differences in original source material and/or processing, manufacturing, and packaging. Given the significantly higher iron and manganese content of the black MP, it is possible that the colour difference may be related to the type of iron oxide and/or manganese oxide present (both Fe-II oxide and Mn-IV oxide are black in powder form). The grinding process itself could have introduced elemental contamination to the MP, and the type of contamination would have been governed by the composition of the balls and of the mill walls, both of which could have contributed metals. The fact that certain elements (for example Cr and Pb) display higher within-sample variability (indicated by high %RSD) could be explained by the presence of contaminant metal particles. Such heterogeneity could reflect contamination of the MP introduced during processing (such as paint chips and rust particles from equipment) or from the canister itself (e.g., Pb solder). Previous mineralogical studies, presented as Appendices in the 1990 Rifat thesis, identified particles of iron oxide/hydroxide and lead chromate (possibly paint) in MP samples using scanning electron microscopy.[Citation23] Further analyses using synchrotron X-ray approaches would be useful to better understand the speciation of the metal impurities in MP. It is also important to note that the black powder contains more Al per volume than the light grey powder, due to the greater proportion of elemental Al in the black powder.
The recommended in-air concentration of MP set by the McIntyre Research Foundation was 35.6 mg/m3 for 10 min. The American Conference of Government Industrial Hygienists (ACGIH®) and the Ontario Ministry of Labour (MOL) recommend a threshold limit value (TLV®) for Al metal and insoluble compounds of 1.0 mg/m3 time weighted average (TWA) for the respirable fraction.[Citation25,Citation26] The Ontario Occupational Health and Safety Act (OHSA) Regulation 833/90 includes excursion criteria for substances that do not have a short term (15 min) exposure limit (STEL). The excursion criteria state that if a substance does not have a STEL, the exposure shall not exceed the following excursion limits: three times the TWA TLV for any 30-min period or 5 times the TWA TLV at any time (O. Reg. 833/90). Therefore, hypothetically both the light grey and black MP exposures would exceed the current excursion limits for aluminum and insoluble aluminum compounds based on the recommended dose.
With respect to particle size distribution of MP, it was the stated goal of the McIntyre Research Foundation[Citation5,Citation18,Citation19,Citation24] to generate the smallest possible particle size (<2 µm) in order to maximize penetration of the MP into the lungs. As early imaging technologies did not permit characterization of particles <200 nm diameter, the present study addressed this data gap by characterizing the nanoscale fraction of MP which is capable of entering the alveolar region of the lung. Inhalation and deposition of particles in the human respiratory tract is a size-dependent cascade in which the size distribution of particles plays a major role in determining airborne particle behavior. Larger particles often are deposited in the nasopharyngeal region (5–30 µm), and smaller particles (1–5 µm) are deposited in the tracheobronchial region.[Citation27] However, very small particles including submicron particles (<1 µm) and nanoparticles (<100 nm) are able to penetrate deeply into the alveolar region, where the particle clearance mechanisms are not adequate.[Citation27] Particles below 50 nm can interact directly with the lung epithelium and have been shown to enter cells and cell organelles as well as cross the blood brain barrier.[Citation27]
Conclusion
Our physical-chemical characterization of MP has revealed the presence of Al nanoparticles (<100 nm), which is the size range considered to be potentially more biologically reactive. The preliminary evidence collected to date supports the notion that further research is required to help determine the effects of MP inhalation. This characterization of MP provides important information to support future toxicological, epidemiological, and biological studies of the long-term effects related to the inhalation of aluminum dust and nanomaterials.
Supplemental Material
Download PDF (89.6 KB)Acknowledgments
We are grateful to Janice Martell, founder of the McIntyre Powder Project, for her unwavering support and wealth of knowledge and for supplying samples. We would also like to thank Dave Wilken and Kimberly O’Connell from OHCOW for their support and for supplying samples. We would also like to thank Michelle Kelvin from XPS Glencore, Canada for conducting the XRD analysis. We are grateful to Christine Levesque and Marc Chénier of Health Canada for conducting the ICP analyses, and we thank Kristin Macey, Larbi El Bilali, and Daryl Smith of Health Canada for valuable review comments on an earlier version of the manuscript. We are also grateful to Loïc Hamon of SABNP laboratory at Évry University, Paris, France for conducting the AFM analysis. We thank Mohamed Sennour from Centre des Matériaux of MINES Paris Tech, Évry, France for the TEM analysis. Mitacs, NSERC, and Bruce Power had no role in the preparation of this article.
Additional information
Funding
References
- Muller, J., W.C. Wheeler, J.F. Gentleman, G. Suranyi, and R.A. Kusiak: Study of mortality of Ontario miners 1955–1977 Part I. Toronto: Ontario Ministry of Labour Special Studies Branch (1983).
- Muller, J., R.A. Kusiak, G. Suranyi, and A.C. Ritchie: Study of mortality of Ontario gold miners 1955–1977 Part II. Toronto: Ontario Ministry of Labour Specisal Studies Branch (1986).
- Denny, J.J., W.D. Robson, and D.A. Irwin: The prevention of silicosis by metallic aluminum: I. A preliminary report. Can. Med. Assoc. J. 37(1):1–11 (1937).
- Denny, J.J., W.D. Robson, and D.A. Irwin: The prevention of silicosis by metallic aluminum Ii. Can. Med. Assoc. J. 40(3):213–228 (1939).
- Jacob, A.W.: The engineering aspects of aluminum prophylaxis. Can. Instit. Min. Metal. XLVII:185–202 (1944).
- Gent, M., C.C. Grey, D. Hewitt: Report of the scientific task force on aluminum inhalation therapy to the Ontario Ministry of Labour. Toronto: Ministry of Labour (1980).
- Rifat, S.L., M.R. Eastwood, D.R. McLachlan, and P.N. Corey: Effect of exposure of miners to aluminium powder. Lancet 336(8724):1162–1165 (1990).
- Peters, S., A. Reid, L. Fritschi, N. de Klerk, and A.W. Musk: Long-term effects of aluminium dust inhalation. Occup. Environ. Med. 70(12):864–868 (2013).
- Bakand, S., and A. Hayes: Toxicological considerations, toxicity assessment, and risk management of inhaled nanoparticles. Int. J. Mol. Sci. 17(6): (2016).
- Krewski, D., R.A. Yokel, E. Nieboer, et al.: Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J. Toxicol. Environ. Health B Crit. Rev. 10(Suppl 1):1–269 (2007).
- Crepeaux, G., H. Eidi, M.O. David, et al.: Highly delayed systemic translocation of aluminum-based adjuvant in CD1 mice following intramuscular injections. J. Inorg. Biochem. 152:199–205 (2015).
- Crepeaux, G., H. Eidi, M.O. David, et al.: Non-linear dose-response of aluminium hydroxide adjuvant particles: Selective low dose neurotoxicity. Toxicology. 375:48–57 (2017).
- Eidi, H., M.O. David, G. Crepeaux, et al.: Fluorescent nanodiamonds as a relevant tag for the assessment of alum adjuvant particle biodisposition. BMC Med. 13:144 (2015).
- Gherardi, R.K., H. Eidi, G. Crepeaux, F.J. Authier, and J. Cadusseau: Biopersistence and brain translocation of aluminum adjuvants of vaccines. Front. Neurol. 6:4 (2015).
- Exley, C.: The pro-oxidant activity of aluminum. Free Radic. Biol. Med. 36(3):380–387 (2004).
- Campbell, A., D. Hamai, and S.C. Bondy: Differential toxicity of aluminum salts in human cell lines of neural origin: implications for neurodegeneration. Neurotoxicology 22(1):63–71 (2001).
- Lukiw, W.J., M.E. Percy, and T.P. Kruck: Nanomolar aluminum induces pro-inflammatory and pro-apoptotic gene expression in human brain cells in primary culture. J. Inorg. Biochem. 99(9):1895–1898 (2005).
- Newkirk, T.E., J.W.G. Hannon, and A.D. Campbell: The physical and chemical characteristics and the commercial manufacture of a new McIntyre Aluminium Powder. Toronto:McIntyre Research Foundation (1956).
- Hannon, J.W.G.: Method of Producing an Atmosphere Protective Against Silicosis, U.S.P. Office (ed.): McIntyre Research Foundation, 1958.
- Beltran-Huarac, J., Z. Zhang, G. Pyrgiotakis, et al.: Development of reference metal and metal oxide engineered nanomaterials for nanotoxicology research using high throughput and precision flame spray synthesis approaches. NanoImpact 10:26–37 (2018).
- Hassan, N.M., P.E. Rasmussen, E. Dabek-Zlotorzynska, V. Celo, and H. Chen: Analysis of environmental samples using microwave-assisted acid digestion and inductively coupled plasma mass spectrometry: Maximizing total elemental recoveries. Water, Air Soil Pollut. 178:323–334 (2007).
- Rasmussen, P.E., C. Levesque, M. Chénier, and H.D. Gardner: Contribution of metals in resuspended dust to indoor and personal inhalation exposures: Relationships between PM10 and settled dust. S1-2 Analytical Notes. Build. Environ. 143:513–522 (2018).
- Rifat, S.: Evidence regarding effects of exposure to McIntyre Powder. Appendices consisting of McIntyre Powder analytical reports by Dr. Mitchell, Department of Geology, Lakehead University (dated 1988 and 1990). In Preventative Medicine and Biostatistics, p. 301. Toronto, Ontario, Canada: University of Toronto, 1990.
- Crombie, D.W., J.L. Blaisdell, and G. Macpherson: The treatment of silicosis by aluminum powder. Can. Med. Assoc. J. 50(4):318–328 (1944).
- ACGIH: “Aluminum metal and insoluble compounds.” 2008.
- “Current Occupational Exposure Limits for Ontario Workplaces Required under Regulation 833.” Available at https://www.labour.gov.on.ca/english/hs/pubs/oel_table.php#a (accessed January 2, 2018).
- Maher, B.A., I.A. Ahmed, V. Karloukovski, et al.: Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA 113(39):10797–10801 (2016).