?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Pharmacy technicians are exposed to volatile organic compounds, like the disinfectant isopropyl alcohol (IPA), during the process of aseptic compounding of parenteral cytotoxic drugs. The occupational exposure to nebulized IPA during aseptic compounding has not been investigated. The aim of this study was to investigate the exposure to IPA during aseptic compounding of parenteral cytotoxic drugs and to assess compliance with legal and regulatory limits. As a secondary endpoint, the difference between two disinfection methods was compared regarding the exposure to IPA. The exposure to IPA was measured during five working shifts of 8 hr and one shift of 4 hr. The concentration IPA was measured by using a six-gas monitor. Total daily exposure was calculated as 8-hr Time Weighted Average (TWA) air concentration in mg/m3 and compared with an Occupational Exposure Limit (OEL) value of 500 mg/m3 and incidental peak exposure of 5,000 mg/m3. To assess whether the 8-hr TWA air concentration meets the legal and regulatory limits the Similar Exposure Groups (SEG) compliance test was used. A paired sample t-test was conducted to assess difference in exposure between two disinfection methods. The average 8-hr TWA exposure to IPA during the six measurements varied from 2.6 mg/m3 to 43.9 mg/m3 and the highest momentary concentration measured was 860 mg/m3. The result of the SEG compliance test was 3.392 (Ur value) and was greater than the Ut value of 2.187 which means the exposure to IPA is in compliance with the OEL value. No significant difference in exposure was shown between two disinfection methods (p = 0.49). In conclusion, exposure to IPA during aseptic compounding of parenteral cytotoxic drugs showed compliance to the OEL values with no significant difference in exposure between two disinfection methods.
Introduction
Parenteral drugs, like cytotoxic drugs and antibiotics, are compounded daily under aseptic circumstances in cleanrooms of hospital pharmacies. Cytotoxic drugs are compounded in biohazard Laminar Air Flow (LAF) cabinets to protect the pharmacy technician against exposure to cytotoxic drugs and to protect the product against contamination with microorganisms (EudraLex Citation2015). Disinfection of products and materials before transferring them into the LAF cabinet will contribute to the protection form microbial contamination (Boom et al. Citation2021).
Alcohols like ethyl alcohol and isopropyl alcohol (IPA) are widely used in healthcare settings as a disinfectant because of their antimicrobial activity against a broad-spectrum of microorganisms (Yoo Citation2018). In most hospital pharmacies IPA is used in a concentration of 70% to disinfect drugs vials, materials, and surfaces of LAF cabinets (Salvage et al. Citation2014; World Health Organization Citation2010). The health and safety guideline of the Dutch federation of academic medical hospitals strongly recommends not to nebulize disinfectants (De Nederlandse Federatie van Universitair Medische Centra (NFU)), because exposure to nebulized IPA can result in irritating effects on the respiratory tract and eyes (The National Institute for Occupational Safety and Health (NIOSH)). IPA impregnated wipes can be used as first disinfection material to refrain from nebulizing disinfectants. On the other hand, avoiding IPA impregnated wipes can be necessary to prevent the carry-over or spreading of cytotoxic traces. Multiple studies showed that the outside of cytotoxic drugs vial contains traces of cytotoxic drugs (Connor et al. Citation2005; Favier et al. Citation2003; Fleury-Souverain et al. Citation2014; Gilbar Citation2005; Hedmer et al. Citation2005; Mason et al. Citation2003; Naito et al. Citation2012; Nygren et al. Citation2002; Schierl et al. Citation2010; Touzin et al. Citation2008). The use of wipes can result in occupational exposure as well as carry-over or spreading of the cytotoxic contamination to other surfaces, because the gloved fingers of the technician must touch the outside of these vials. Carry-over of cytotoxic drugs in the cleanroom is a known risk (Crul and Simons-Sanders Citation2018).
To prevent this risk of spreading cytotoxic drugs through carry-over, we developed the following method: cytotoxic drug vials are popped out from the secondary packaging into a plastic bag without touching it by the pharmacy technician. Inside the cleanroom the vial gets disinfected by spraying IPA into this plastic bag containing the drug vial(s). The disinfected vials are transferred to the LAF cabinet, again without touching them. The prevention of direct contact with potentially contaminated outside of vials, theoretically results in a lower risk of carry-over compared to using impregnated wipes. Carry-over could still be possible, because the compounding pharmacy technician in the LAF cabinet touches the cytotoxic vials as well as the compounded infusion bags. After compounding, the pharmacy technician in the cleanroom also touches the compounded infusion bags. However, Breukels et al. (Citation2018) showed that compounded infusion bags from a large panel of hospitals are not contaminated on the outside with cytotoxic traces, causing a low risk of carry-over (Breukels et al. Citation2018).
By applying our procedure, the use of nebulizing IPA in our cleanrooms significantly increased and the exposure to IPA during aseptic compounding could also increase. A previously published study with volunteers demonstrated that concentrations from 200–800 ppm result in acute symptoms, such as irritation of eyes and throat (National Research Council (US) Committee on Toxicology Citation1984). Serum concentrations IPA of 3–4 g/L can cause depression of the central nervous system and respiratory tract (Slaughter et al. Citation2014). It is not clear if IPA is carcinogenic (Eckardt Citation1974; Hueper Citation1996; Weil et al. Citation1952). However, from animal studies it is known that IPA could result in liver parenchymal cell dystrophy, hyperplastic ependymal cells, and degenerative changes in the cerebral motor cortex (Baikov et al. Citation1974).
Thus, the exposure of nebulized IPA is unknown during aseptic compounding of parenteral cytotoxic drugs. The aim of this study is to investigate the exposure to IPA of pharmacy technicians during aseptic compounding of cytotoxic drugs. Additionally, an assessment of compliance with legal and regulatory limits was conducted. In this respect, two disinfection methods were compared regarding the exposure to IPA. To our knowledge, this is the first study that investigates the exposure to IPA of pharmacy technicians during aseptic compounding of parenteral cytotoxic drugs in cleanrooms.
Methods
Occupational exposure limits
To assess compliance with regulatory limits the Derived No Effect Level (DNEL) of 500 mg/m3 for long term exposure was used as 8-hr time-weighted average (TWA) limit value, according to the REACH regulation of the European Chemicals Agency (ECHA) (European Chemicals Agency (ECHA) Citation2021). The DNEL was used as an Occupational Exposure Limit (OEL) because of the OEL value for IPA was not determined by regulatory authorities.
Instantaneous peak concentrations of ten times the 8-hr TWA should not result in narcotic or acute neurotoxic effects (Health Council of the Netherlands: Committee on Peak Exposures to Organic Solvents Citation1999). A tentative level of 5,000 mg/m3, logged as 1-min data, was used for incidental peak exposure according to the recommendations of the Health Council of the Netherlands.
Materials
In this study, Klercide 70/30 IPA (Ecolab, Saint Paul, MN) was used as a disinfectant. Klercide 70/30 IPA is a mixture of 70% IPA and 30% water for injection. The MX6 iBrid (Industrial Scientific Corporation, Pittsburgh, PA) was used to measure the exposure to nebulized IPA. The MX6 iBrid is a six-gas monitor with a Photo Ionization Detector (PID) meter. The six-gas monitor can detect and measure vaporized concentrations of volatile organic compounds (VOCs), like IPA. Aerosols with IPA cannot be detected by the six-gas monitor. The signal for measuring IPA was not amplified or dampened by other VOCs, because no materials containing VOCs, besides IPA, are present in the cleanroom.
The sensor of monitor was detached on the collar of pharmacy technician’s cleanroom coat, within the inhalation zone of 20 cm. All pharmacy technicians have read and signed an informed consent. The six-gas monitor has a measuring range of 0–2,000 ppm and a resolution of 0.1. The six-gas monitor logged every minute the concentration VOC as a TWA in ppm. The six-gas monitor was calibrated with isobutylene. By changing the response factor to 5.93 for IPA, the measured VOCs were converted to IPA. The measured concentrations were converted from in ppm to mg/m3, because of comparison with the OEL values according to the REACH regulation of the ECHA.
Disinfection methods
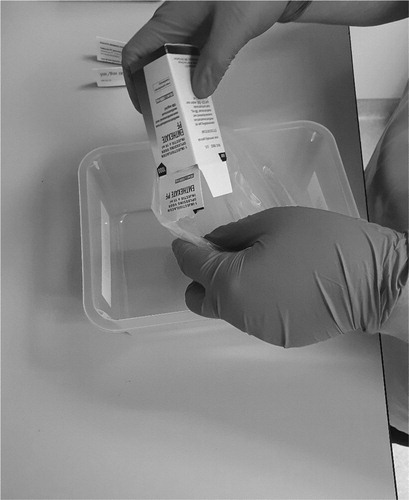
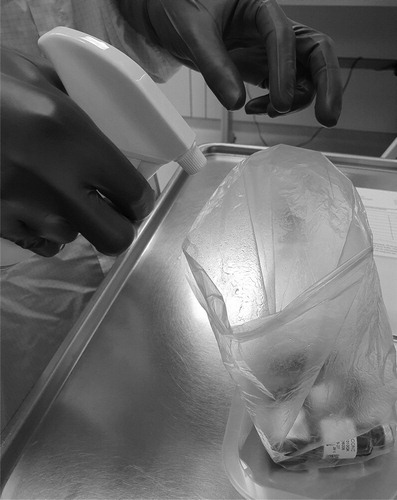
The vials of cytotoxic drugs were transferred from the original secondary packaging to a plastic bag without touching the primary packaging. In this plastic bag the cytotoxic drugs were transferred to the cleanroom (). The cytotoxic vials were disinfected by spraying IPA in the plastic bag containing the vials by the pharmacy technician in the cleanroom (). The number of sprays depended on the surface area of the vials. Complete wetting of the outside of the vials with the IPA was the standard. For small vials this means two to three sprays, but for multiple vials or large vials, this can be up to 7–8 sprays. However, different methods of spraying and the position of the pharmacy technician can affect the exposure to IPA. Two methods were studied.
The plastic bag with cytotoxic vial(s) and spray bottle with IPA were kept in front of the pharmacy technician at table height. The vials in the plastic were nebulized with IPA until they were fully humidified.
The plastic bag with cytotoxic vial(s) and spray bottle with IPA were kept as far as possible away from the face of the pharmacy technician by stretching both arms and turning away the head form the plastic bag and spray bottle. Also, the nozzle of the spray bottle was kept as close as possible to the bag. The vials in the plastic were nebulized with IPA until they were fully humidified.
The distance between the spray bottle and the monitor sampler differs between the two disinfection methods. The average distance difference is estimated at 30 cm, depending on the arm length of the pharmacy technician. After disinfection, the cytotoxic vials were transferred inside the LAF cabinet without touching them.
Measurements
In this study the exposure to IPA was measured during five working shifts of 8 hours and one working shift of four hours. Each day of the study the six-gas monitor was attached to a different pharmacy technician. The pharmacy technician did not perform other tasks than compounding cytotoxic drugs during the working shift. To investigate the difference in exposure between the two disinfection methods, each pharmacy technician was randomized to one disinfection method in the morning and the other in the afternoon. All measurements were performed in the cytotoxic compounding cleanroom at the Amsterdam UMC hospital, location VUmc. This cleanroom of 13.5 m2 is classified as grade D, has a negative pressure of −10 Pa, and has a ventilation rate of 29/hr.
Analysis
To assess compliance with the OEL value the Similar Exposure Groups (SEG) compliance test was used according to the NEN-EN 689 “Workplace exposure - Measurement of exposure by inhalation to chemical agents - Strategy for testing compliance with occupational exposure limit values” (Nederlands Normalisatie Instituut (NEN) Citation2018). The exposure to IPA was assessed with the SEG compliance test, regardless of the used method. Using a SEG compliance was suitable because tasks, the environment and circumstances were equal during both methods.
By using six measurements a reliable outcome of this statistical test was obtained. The test is based on the comparison of the 70% upper confidence limit (UCL) with the 95th percentile of the distribution of the results. If the UCL is below the OEL value, the exposure meets the conditions of compliance of IPA exposure. If the UCL is greater than the OEL value, the exposure does not meet these conditions. To demonstrate that the exposure measurements are log-normally distributed the Shapiro and Wilk test was performed. Compliance with the OEL was tested by using the following formulas:
(1)
(1)
(2)
(2)
(3)
(3)
By calculating the geometric mean (GM) and the geometric standard deviation (GSD) of the set of measurements the Ur can be calculated If Ur, real value of the test statistic, is greater or equal to Ut, critical value of the test statistic, then exposure complies with the OEL value. The Ut depends on the number of exposure measurements. In case of six measurements the Ut is 2.187 (Nederlands Normalisatie Instituut (NEN) Citation2018). To calculate difference between to disinfection methods the paired t-test was used, because the data were paired and were normally distributed.
Results
Exposure to IPA
No difference in the amount of prepared chemotherapy was observed during the study compared with the average prepared chemotherapy over the past years. The average 8-hr TWA exposure to IPA varied per 4 to 8-hr working shift from 2.6 mg/m3 to 43.9 mg/m3 (). The concentration-time plot in the Supplement (Supplementary Figure 1) shows the 1-min time-weighted mean concentrations of IPA of each measurement day. The highest concentration measured was 860 mg/m3, measured on Day Two ( and Supplementary Figure 1). On Day Six, the exposure was only measured in the afternoon instead of the whole day due to a staff shortage on that day.
Table 1. Average exposure to IPA and peak concentration IPA.
SEG compliance test
The p-value of the Shapiro-Wilk test is 0.42 which means the data of the exposure to IPA followed a lognormal distribution p-value. The normal distribution was also observed in a probability plot (Supplementary Figure 2). Because the data follows a log-normally distribution, the SEG compliance test was reliable to test compliance with OEL value. The geometric mean was 11.3 mg/m3 and the standard deviation was 3.1. The calculated Ur (3.392) was above the Ut value (2.187) resulting in an average exposure to IPA that did not significantly exceeds the OEL value. The average IPA complies the OEL value.
Two disinfection methods
The average 8-hr TWA exposure to IPA varied from 2.7 mg/m3 to 32.8 mg/m3 for Method One and from 1.2 to 37.5 mg/m3 for Method Two (). The p-value was 0.49 which means the exposure to IPA did not differ between the two methods.
Table 2. Average exposure to IPA of two disinfection methods.
Discussion
To our knowledge this is the first study which investigated the exposure to IPA in a pharmacy cleanroom during aseptic compounding of parenteral drugs and the compliance with the OEL. Pharmacy technicians in hospital pharmacies are a group of hospital employees that are frequently exposed to VOCs, including IPA, during their daily activities (LeBouf et al. Citation2014; Su et al. Citation2018). As stated before, it is unknown if exposure to IPA is carcinogenic, but occupational exposure could result in severe acute and long-term negative effects (National Research Council (US) Committee on Toxicology Citation1984; Slaughter et al. Citation2014). These potential hazards confirm the need to determine the exposure to IPA in health care settings.
The SEG compliance test demonstrated compliance with the OEL value, because the calculated Ur is greater than Ut. The probability that the OEL value will be exceeded is less than 5% with at least 70% confidence. It also confirms that the method in our hospital pharmacy for disinfecting potentially contaminated drug vials with cytotoxic drugs is safe in the context of VOC exposure. Protection from the cytotoxic drugs has been proven under the same working conditions in Dutch hospital pharmacies previously (Crul et al. Citation2020). By adopting this disinfection procedure, the staff is protected from both the cytotoxic drugs as well as the IPA vapor. In fact, the data is calculated by unverified conversion of VOC measurements by using a six-gas monitor to IPA as our specific compound of interest. This might even overestimate our measured concentrations.
Beside the average exposure to IPA, we measured peak concentration in relation to the tentative level of 5,000 mg/m3. The highest measured concentration of 860 mg/m3 is almost 6 times below the limit. Note that this study only compares the exposure with the legal and regulatory limits and does not monitor potential short- and long-term effects of the exposure to IPA. The most common acute effects of exposure to alcohols through inhalation are irritation of the eyes and the mucous membranes of the nose and throat (National Research Council (US) Committee on Toxicology Citation1984). Because of the incidental high exposure to IPA (0–860 mg/m3) and the potential exposure to cytotoxic drugs, pharmacy technicians can use protective eyewear and an FFP-3 mask when needed to prevent eye and airway irritation. These are used when the safety cabinet is opened after compounding for cleaning and disinfection.
The exposure to IPA did not significantly differ between the two disinfection methods. In other words, the position of the spray bottle and position of the head of the pharmacy technician, according to the two methods, did not significantly affect the exposure to IPA. Most likely the range of vaporized IPA by spraying one or multiple times has a much larger effect than the difference between the two methods.
Previous studies mainly investigated the exposure to IPA in healthcare settings by using alcohol-containing hand rubs. Based on these studies the exposure to IPA does not exceed legal and regulatory limits (Hautemanière, Ahmed-Lecheheb, et al. Citation2013; Hautemanière, Cunat, et al. Citation2013; Kramer et al. Citation2007). However, the exposure to IPA by using alcohol-containing hand rubs can be different from nebulized IPA. Literature data on exposure to alcohols by inhaling vaporized alcohols is limited and mostly concerns ethanol (Campbell and Wilson Citation1986; Dumas-Campagna et al. Citation2014; Nadeau et al. Citation2003) or methanol (Ernstgård et al. Citation2005). In one study, the toxicokinetics of inhaled IPA was studied in a controlled setting with a known exposure. This study showed that exposure to inhaled IPA differs between sexes (Ernstgård et al. Citation2003). However, none of these studies investigated the occupational exposure of inhaled IPA and other alcohols in healthcare settings nor did they compare the exposure with legal limits. This is the first study that investigated the occupational exposure to IPA by using nebulized IPA in a healthcare setting, in particular in a hospital pharmacy.
This study also has some limitations. First, at day six the exposure to IPA was only measured in the afternoon instead of the whole day due to a staff shortage on that day. As a result, the second disinfection method was not studied on the last measurement day. This has no consequences for the SEG compliance test because the exposure is divided by the measurement duration and a series of six exposure measurements remains.
Another variable factor on the exposure is the amount of planned compounding. The total number of compounded cytotoxic drugs can vary from day to day, which can result in more or less drug vials that need to be disinfected. This study was performed during the COVID-19 pandemic which caused a delay of chemotherapy treatments and thus less compounding than usual. However, on the six measurement days comparable amounts of chemotherapy were administered relative to the average of the daily administered parenteral chemotherapy over the past years.
Because exposure to IPA can vary from day to day and between employees it is possible that exposure to IPA also varies between different hospital pharmacies because of the amount of compounded parenteral cytotoxic drugs, the size of the cleanroom or ventilation differences. To confirm the data and safety of this method, it is recommended to perform the same study in other hospitals. In addition, future studies are needed to calculate the total exposure to alcohols for pharmacy technicians. A study of Maier et al. (Citation2015) demonstrated that the use of alcohol-containing hand rubs are safe because the exposure to alcohol by inhalation and dermal absorption are below toxic levels (Maier et al. Citation2015). However, the exposure to alcohols from nebulized alcohol and alcohol-containing hand rubs together is not known.
Conclusion
This study determined the exposure to IPA during aseptic compounding of parenteral cytotoxic drugs and confirms that exposure to IPA complies occupational exposure limits according to the applicable European guidelines. Nebulizing IPA into a bag with cytotoxic drug vials is a safe and proper method to disinfect potentially contaminated vials, which can be used in all hospital pharmacies.
Ethics approval
The pilot study was approved by the Medical Ethics Review Committee of VU University Medical Center (IRB00002991). The FWA number assigned to VU Medical Center is FWA00017598.
Supplemental Material
Download PDF (295.2 KB)Acknowledgments
The authors are grateful to the pharmacy technicians for their time and support of the study and F.F. van den Berg for his contribution to drafting the figures.
Funding
Regular institutional funding.
References
- Baikov BK, Gorlova OE, Gusev MI, IuV N, Iudina TV. 1974. Hygienic standardization of the average daily maximum permissible concentrations of propyl and isopropyl alcohols in the atmosphere. Gig Sanit. 1974(4):6–13.
- Boom FA, Le Brun PPH, Boehringer S, Kosterink JGW, Touw D. 2021. Improving the aseptic transfer procedures in hospital pharmacies part A: methods for the determination of the surface bioburden on ampoules and vials. Eur J Hosp Pharm. 28(1):35–38. doi:10.1136/ejhpharm-2018-001673
- Breukels O, van der Gronde T, Simons-Sanders K, Crul M. 2018. Antineoplastic drug contamination on the outside of prepared infusion bags. Int J Pharm Compd. 22(4):345–349.
- Campbell L, Wilson HK. 1986. Blood alcohol concentrations following the inhalation of ethanol vapour under controlled conditions. J Forensic Sci Soc. 26(2):129–135. doi:10.1016/S0015-7368(86)72458-4
- Connor TH, Sessink PJM, Harrison BR, Pretty JR, Peters BG, Alfaro RM, Bilos A, Beckmann G, Bing MR, Anderson LM, et al. 2005. Surface contamination of chemotherapy drug vials and evaluation of new vial-cleaning techniques: results of three studies. Am J Heal Pharm. 62(5):475–484. doi:10.1093/ajhp/62.5.475
- Crul M, Hilhorst S, Breukels O, Bouman-d'Onofrio JRC, Stubbs P, van Rooij JG. 2020. Occupational exposure of pharmacy technicians and cleaning staff to cytotoxic drugs in Dutch hospitals. J Occup Environ Hyg. 17(7-8):343–352. doi:10.1080/15459624.2020.1776299
- Crul M, Simons-Sanders K. 2018. Carry-over of antineoplastic drug contamination in Dutch hospital pharmacies. J Oncol Pharm Pract. 24(7):483–489. doi:10.1177/1078155217704990
- De Nederlandse Federatie van Universitair Medische Centra (NFU). Schoonmaak- en desinfectiemiddelen; [accessed 2021 Feb 14]. https://www.dokterhoe.nl/risicos/gevaarlijke-stoffen/de-richtlijn-gevaarlijke-stoffen-stoffen-met-extra-aandacht/schoonmaak-en-desinfectiemiddelen/.
- Dumas-Campagna J, Tardif R, Charest-Tardif G, Haddad S. 2014. Ethanol toxicokinetics resulting from inhalation exposure in human volunteers and toxicokinetic modeling. Inhal Toxicol. 26(2):59–69. doi:10.3109/08958378.2013.853714
- Eckardt RE. 1974. Annals of industry–noncasualties of the work place. J Occup Med. 16(7):472–477. 1974
- Ernstgård L, Shibata E, Johanson G. 2005. Uptake and disposition of inhaled methanol vapor in humans. Toxicol Sci. 88(1):30–38. doi:10.1093/toxsci/kfi281
- Ernstgård L, Sjögren B, Warholm M, Johanson G. 2003. Sex differences in the toxicokinetics of inhaled solvent vapors in humans 2. 2-propanol. Toxicol Appl Pharmacol. 193(2):158–167. doi:10.1016/j.taap.2003.08.005
- EudraLex. 2015. Good manufacturing practice (GMP). Chapter 5: production
- European Chemicals Agency (ECHA). 2021. Propan-2-ol; [accessed 2021 Feb 14]. https://echa.europa.eu/registration-dossier/-/registered-dossier/15339/7/1.
- Favier B, Gilles L, Ardiet C, Latour JF. 2003. External contamination of vials containing cytotoxic agents supplied by pharmaceutical manufacturers. J Oncol Pharm Pract. 9(1):15–20. doi:10.1191/1078155203jp102oa
- Fleury-Souverain S, Nussbaumer S, Mattiuzzo M, Bonnabry P. 2014. Determination of the external contamination and cross-contamination by cytotoxic drugs on the surfaces of vials available on the Swiss market. J Oncol Pharm Pract. 20(2):100–111. doi:10.1177/1078155213482683
- Gilbar PJ. 2005. External contamination of cytotoxic drug vials. J Pharm Pract Res. 35(4):264–265. doi:10.1002/j.2055-2335.2005.tb00359.x
- Hautemanière A, Ahmed-Lecheheb D, Cunat L, Hartemann P. 2013a. Assessment of transpulmonary absorption of ethanol from alcohol-based hand rub. Am J Infect Control. 41(3):e15–e19. doi:10.1016/j.ajic.2012.09.004
- Hautemanière A, Cunat L, Ahmed-Lecheheb D, Hajjard F, Gerardin F, Morele Y, Hartemann P. 2013b. Assessment of exposure to ethanol vapors released during use of alcohol-based hand rubs by healthcare workers. J Infect Public Health. 6(1):16–26. doi:10.1016/j.jiph.2012.09.015
- Health Council of the Netherlands: Committee on Peak Exposures to Organic Solvents. 1999. Peak exposures to organic solvents. The Hague: Health Council of the Netherlands.
- Hedmer M, Georgiadi A, Bremberg ER, Jönsson BA, Eksborg S. 2005. Surface contamination of cyclophosphamide packaging and surface contamination with antineoplastic drugs in a hospital pharmacy in Sweden. Ann Occup Hyg. 49(7):629–637. doi:10.1093/annhyg/mei042
- Hueper WC. 1996. Occupational and environmental cancers of the respiratory system. 1st ed. Berlin: Springer-Verlag; p. 105–107.
- Kramer A, Below H, Bieber N, Kampf G, Toma CD, Huebner N-O, Assadian O. 2007. Quantity of ethanol absorption after excessive hand disinfection using three commercially available hand rubs is minimal and below toxic levels for humans. BMC Infect Dis. 7:117. doi:10.1186/1471-2334-7-117
- LeBouf RF, Virji MA, Saito R, Henneberger PK, Simcox N, Stefaniak AB. 2014. Exposure to volatile organic compounds in healthcare settings. Occup Environ Med. 71(9):642–650. doi:10.1136/oemed-2014-102080
- Maier A, Ovesen JL, Allen CL, York RG, Gadagbui BK, Kirman CR, Poet T, Quiñones-Rivera A. 2015. Safety assessment for ethanol-based topical antiseptic use by health care workers: evaluation of developmental toxicity potential. Regul Toxicol Pharmacol. 73(1):248–264. doi:10.1016/j.yrtph.2015.07.015
- Mason HJ, Morton J, Garfitt SJ, Iqbal S, Jones K. 2003. Cytotoxic drug contamination on the outside of vials delivered to a hospital pharmacy. Ann Occup Hyg. 47(8):681–685. doi:10.1093/annhyg/meg078
- Nadeau V, Lamoureux D, Beuter A, Charbonneau M, Tardif R. 2003. Neuromotor effects of acute ethanol inhalation exposure in humans: a preliminary study. J Occup Health. 45(4):215–222. doi:10.1539/joh.45.215
- Naito T, Osawa T, Suzuki N, Goto T, Takada A, Nakamichi H, Onuki Y, Imai K, Nakanishi K, Kawakami J, et al. 2012. Comparison of contamination levels on the exterior surfaces of vials containing platinum anticancer drugs in Japan. Biol Pharm Bull. 35(11):2043–2049. doi:10.1248/bpb.b12-00628
- National Research Council (US) Committee on Toxicology. 1984. Emergency and continuous exposure limits for selected airborne contaminants: volume 2. Washington (DC): National Research Council (US) Committee on Toxicology.
- Nederlands Normalisatie Instituut (NEN). 2018. NEN-EN 689: 2018. Workplace exposure - measurement of exposure by inhalation to chemical agents - Strategy for testing compliance with occupational exposure limit values. Delft (The Netherlands).
- Nygren O, Gustavsson B, Ström L, Friberg A. 2002. Cisplatin contamination observed on the outside of drug vials. Ann Occup Hyg. 46(6):555–557. doi:10.1093/annhyg/mef074
- Salvage R, Hull CM, Kelly DE, Kelly SL. 2014. Use of 70% alcohol for the routine removal of microbial hard surface bioburden in life science cleanrooms. Future Microbiol. 9(10):1123–1130. doi:10.2217/fmb.14.73
- Schierl R, Herwig A, Pfaller A, Groebmair S, Fischer E. 2010. Surface contamination of antineoplastic drug vials: comparison of unprotected and protected vials. Am J Health Syst Pharm. 67(6):428–429. doi:10.2146/ajhp080621
- Slaughter RJ, Mason RW, Beasley DMG, Vale JA, Schep LJ. 2014. Isopropanol poisoning. Clin Toxicol (Phila). 52(5):470–478. doi:10.3109/15563650.2014.914527
- Su F-C, Friesen MC, Stefaniak AB, Henneberger PK, LeBouf RF, Stanton ML, Liang X, Humann M, Virji MA. 2018. Exposures to volatile organic compounds among healthcare workers: modeling the effects of cleaning tasks and product use. Ann Work Expo Health. 62(7):852–870. doi:10.1093/annweh/wxy055
- The National Institute for Occupational Safety and Health (NIOSH). Isopropyl alcohol; [accessed 2021 Feb 14]. https://www.cdc.gov/niosh/npg/npgd0359.html.
- Touzin K, Bussières J-F, Langlois E, Lefebvre M, Gallant C. 2008. Cyclophosphamide contamination observed on the external surfaces of drug vials and the efficacy of cleaning on vial contamination. Ann Occup Hyg. 52(8):765–771. doi:10.1093/annhyg/men050
- Weil CS, Smyth HFJ, Nale TW. 1952. Quest for a suspected industrial carcinogen. AMA Arch Ind Hyg Occup Med. 5(6):535–547.
- World Health Organization. 2010. WHO best practices for injections and related procedures toolkit. Geneva (Switzerland). Report No: WHO/EHT/10.02.
- Yoo JH. 2018. Review of disinfection and sterilization - back to the basics. Infect Chemother. 50(2):101–109. doi:10.3947/ic.2018.50.2.101