Abstract
Cement workers are exposed to various kinds of occupational hazards, dust being the most hazardous. Despite certain exposure limits on the emission of air pollutants in place, several people die each year due to complications from respiratory disease. This study aimed to assess the prevalence of chronic respiratory symptoms among workers exposed to cement dust. A quantitative, descriptive cross-sectional design was employed among 81 workers from two cement production companies in Gauteng, South Africa in 2018. A self-administered questionnaire, anthropometric measurements, and a spirometry test were used as data collection tools. Data were analyzed using Wilcoxon rank sum, binary logistic regression, Pearson’s chi-squared, and Fischer’s exact tests. Respiratory symptoms such as wheezing, recurring blocked nose, sneezing/stuffy nose, fatigue/tiredness, rapid breathing, soreness/watery eyes, and breathlessness were significantly prevalent among participants from both facilities. Engineering and housekeeping control measures such as the use of High-Efficiency Particulate Air (HEPA) vacuums to clean up dust and proper use of Personal Protective Equipment (PPE) where workers are exposed to dust particles should be implemented.
Introduction
In developing countries, millions of people work in dusty environments daily, and this includes cement industry workers (Ballal et al. Citation2004). One of the major illnesses in the cement industry is occupational lung disease, which is usually exacerbated by long-term exposure to toxic and irritating substances and has a long latency period (Akanbi et al. Citation2014). Despite the passage of environmental emission regulations for hazardous air pollutants, the number of people who die each year due to complications from respiratory disease has continued to rise (Lage et al. Citation2015).
Workplace exposure to cement dust contribute substantially to the burden of multiple chronic respiratory diseases, including asthma (population attributable fraction (PAF), 16%); chronic obstructive pulmonary disease (COPD, PAF, 14%); chronic bronchitis (PAF, 13%); idiopathic pulmonary fibrosis (PAF, 26%); hypersensitivity pneumonitis (occupational burden, 19%); other granulomatous diseases, including sarcoidosis (occupational burden, 30%); pulmonary alveolar proteinosis (occupational burden, 29%); tuberculosis (occupational burden, 2.3% in silica-exposed workers and 1% in healthcare workers); and community-acquired pneumonia in working-age adults (PAF, 10%) (Blanc et al. Citation2009). It is estimated that about 4% of the entire gross national product of all countries globally is spent on annual losses stemming from work-related diseases and accidents (Akanbi et al. Citation2014).
Cement is one of the world’s most essential building materials, and exposure to cement dust has been shown to negatively affect human health (Zeleke et al. Citation2011; Rathebe Citation2023). Cement mill workers are exposed to dust during various manufacturing and production processes, such as quarrying, handling of raw materials, grinding, blending, packing, and shipping the finished products (Rafeemanesh et al. Citation2015), and the highest concentrations of airborne particulates are typically in the crane, packing, and crusher departments of cement factories (Aminian et al. Citation2014).
Workers in cement factories are exposed to health hazards from exposure to calcium oxide, aluminum trioxide, silica, silicon dioxide, ferric oxide, dicalcium silicate, magnesium oxide, selenium, thallium, hexavalent chromium, high temperatures, and noise (Manjula et al. Citation2013; Aminian et al. Citation2014; Adeyanju and Okeke Citation2019). These hazards occur at various stages of the production process, with the highest concentrations of workplace exposures occurring in the crane, packing, and crusher areas of the production process and are associated with diseases such as silicosis, preterm delivery, psychasthenia, COPD, and infertility (Manjula et al. Citation2013; Aminian et al. Citation2014; Adeyanju and Okeke Citation2019).
Clinical and epidemiological studies have shown an increased incidence of respiratory impairment and potential adverse effects on the respiratory system among cement production workers (Nordby et al. Citation2011; Rahmani et al. Citation2018) with inhalation and/or ingestion as the main routes for worker exposure. Cement dust particles created by crushing, grinding, and pulverizing, are corrosive and in the respirable (4 um or less) dust fraction, which creates an exposure hazard for the eyes, mucous membranes, and respiratory tract. Exposures may be reduced using personal protective equipment (PPE) thereby minimizing the likelihood of exposure. PPE includes, but is not limited to, laboratory coats, gowns, full-body suits, gloves, protective footwear, safety glasses, safety goggles, masks, and respirators (World Health Organization (WHO) Citation2020). Long-term exposure can lead to occupational lung disease (Sana et al. Citation2013), and diseases associated with inhalation of airborne dust are the most prominent group of occupational respiratory diseases (Blanc et al. Citation2009; Rahmani et al. Citation2018).
Exposure to respirable crystalline silica (RCS) dust is a common occupational hazard in many industrial settings across the world, and RCS is present in cement dust (Hnizdo and Vallyathan Citation2003). The morbidity and mortality from silicosis and silica dust-associated tuberculosis (TB) have significantly decreased because of the reduction of RCS exposure levels in most developed countries during the past century. Despite this, workers exposed to RCS continue to be at risk for developing COPD, silicosis, and other lung disorders (Hnizdo and Vallyathan Citation2003). RCS is a major component of concrete, therefore during construction work airborne respirable quartz may be produced which may put workers at risk for developing silicosis, a permanent lung disease that can be fatal (Linch Citation2002). A study conducted in Malaysia found that exposure to breathable cement dust significantly increases the likelihood of developing respiratory health symptoms as well as reducing lung function levels and resulting in elevated levels of fractional exhaled nitric oxide among highly exposed workers (Jalaludin Citation2018). Chronic exposure to cement dust has contributed to a greater prevalence of chronic respiratory symptoms and a reduction of ventilatory capacity (Al-Neaimi et al. Citation2001).
Previous studies have shown that chronic respiratory diseases, including COPD, account for most of the public health challenges in both developing and industrialized countries due to associated negative health and economic impacts (Aït-Khaled et al. Citation2001; Rahmani et al. Citation2018). Chronic exposure to cement dust can ultimately cause chronic bronchitis, asthma, lung cancer, pneumonia, and tuberculosis, and an increased prevalence of respiratory symptoms and reduced lung function indices were observed amongst workers following a single work shift (Aminian et al. Citation2014). Cement dust is considered to have the potential to induce malignant-related respiratory illnesses such as laryngeal and respiratory cancer as well as silicosis (Wilken et al. Citation2011). Several studies have reported associations between cement dust exposure, chronic respiratory symptoms, silicosis, and COPD (Gizaw et al. Citation2016; Fell et al. Citation2017). A study among Norwegian cement factory workers revealed airway inflammation and an increase in the percentage of neutrophils in induced sputum after dust exposure (Tungu et al. Citation2013).
There is limited literature on the prevalence of respiratory symptoms in workers exposed to cement dust in South Africa. Previous studies conducted on cement workers in Gauteng focused on the prevalence of chronic bronchitis amongst nonsmokers (Acutt Citation2004), thus necessitating the need to include smokers and nonsmokers to see if smoking was associated with any clinic respiratory symptoms as well as the prevalence of other chronic respiratory diseases in cement workers in this study. The purpose of this study was (1) to identify the prevalence of respiratory symptoms among cement workers, (2) to evaluate worker practices regarding the use of respiratory protection gear, and (3) to identify whether demographics and other factors were associated with respiratory symptoms.
Methods
Study design
A cross-sectional, quantitative study designed to examine the relationship between chronic respiratory illnesses and lung functioning among study participants was conducted. The study was conducted between 14 August 2018 and 31 October 2018. Ethics approval was obtained from the University of Johannesburg, Faculty of Health Sciences Research Ethics Committee (REC no. 01-21-2018) and Higher Degrees Committee (HDC no. 01-41-2018).
Study area
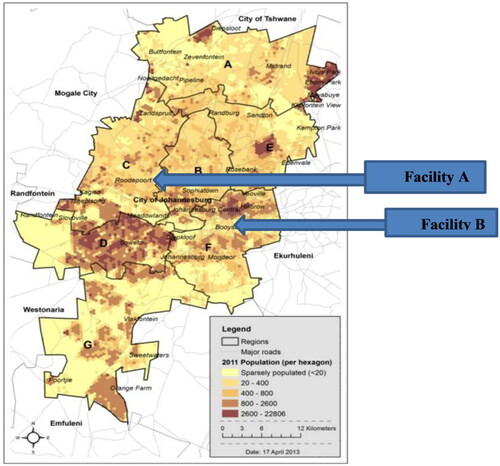
This study took place in Johannesburg where Facility A, a cement factory based in Roodepoort, and Facility B, a ready-mix concrete manufacturing facility based in Booysens in the Gauteng Province, South Africa (). These regions are home to various mines and mine dumps, and the sampled facilities are substantive generators of dust due to typical cement processes that are known to present exposure hazards to the respiratory system. Data collection took place at the participant’s workplace in the presence of only the participant and researcher.
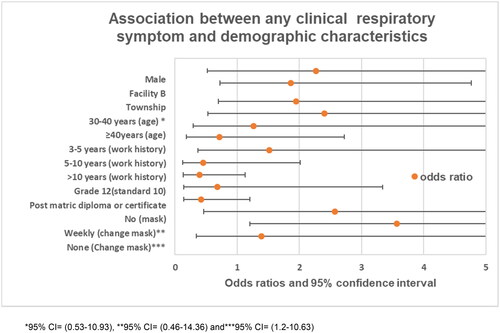
Figure 1. Location of the companies that participated in this study (map obtained from www.joburg.org) *alphabets represent regions. *95% CI= (0.53–10.93), **95% CI= (0.46–14.36), and ***95% CI= (1.2–10.63).
Sampling
A systematic sampling technique was used. A list of all employees working in both facilities was requested from the Human Resources Department and every fifth employee on the list was selected to participate by starting with a randomly chosen participant. All participants were approached face to face and none of the workers refused to participate in the study. Workers with confirmed respiratory illness and on treatment before study commencement and those below the age of 18 or above the age of 60 years at the time of the study were excluded from participating to ensure the reliability of results.
Data collection
Questionnaire
Data were collected utilizing a self-administered questionnaire to assess the subjective respiratory symptoms of exposure to cement dust. The questionnaire was developed based on other validated tools such as the British Medical Research Council Questionnaire on Respiratory Symptoms (BMRC), a questionnaire for employees exposed to respiratory sensitizers as well as an occupational safety and health respiratory questionnaire for occupational medicine and this was in English. This study was piloted on 10% of the sample size in a different facility in Johannesburg. Daily experiences of respiratory symptom complaints were used to obtain information on occupational history, demographics, smoking history, and any other acute or chronic respiratory symptoms. Based on the literature, the following factors that may influence the effects of exposure were considered in the questionnaire: demographic characteristics (age, sex, ethnicity, education, occupation, and years of experience) and contextual factors (occupational setting and area of residence). Workers were to complete the questionnaires in their place of employment with the assistance of trained field research assistants who were conversant in different languages spoken in South Africa.
Anthropometric and spirometry measurements
The body weight, height, and blood pressure were measured before lung function test. Then pulmonary functioning parameters were measured using an electronic DMS brand spirometer with the help of a qualified occupational health nurse. Accentuation was put on forced expiratory volume (FEV) and forced vital capacity (FVC). The lung function tests were conducted after the instruments were successfully calibrated daily and when the temperature changed according to the manufacturer’s specification and checked for leaks, daily cleaning, and regular maintenance. The equipment was compliant with the criteria set in the South African National Standard –SANS 451. FEV was measured considering FEV1/FVC ratios.
The spirometry test procedure was explained and demonstrated to the participants before performing the test. Participants were asked to follow procedure instructions from the American Thoracic Society (ATS) guidelines (1994). A minimum of three acceptable maneuvers were performed taking into consideration acceptability and repeatability criteria. Participants were only allowed to have up to eight attempts per session if their maneuvers were not acceptable, repeatable, and usable and were allowed to come for reassessment at a later date if they coughed during the maneuver. All participants with dentures were asked to remove the dentures before the test and those participants wearing tight or restrictive clothes were asked to loosen the clothing.
The following contra-indications for performing the spirometry test were taken into consideration which included recent myocardial infarction, unstable angina, known aneurism, uncontrolled hypertension, blood pressure above 180/110 mmHg, and a pulse above 110 beats per minute, recent cerebrovascular accident (stroke), hemoptysis, recent surgery of the eye, abdomen or chest, pneumothorax, pulmonary embolism, as well as pregnancy complications such as placenta previa, and glaucoma. None of the participants had any contra-indications.
The spirometry tests were conducted in a well-ventilated, temperature-controlled room. Work surfaces and equipment were cleaned and disinfected daily. A new disposable mouthpiece was used for every subject tested, after the test participants were asked to remove the mouthpiece and dispose of it in a medical waste bag.
Review criteria for the review of spirometry results were adapted from the South African Thoracic Society Standards of Spirometry (Van Schalkwyk et al. Citation2004). The normal measurement ranges for lung function via spirometry are as follows: FVC % predicted 80% and above, FEV1% predicted 80% and above, and FEV1/FVC ratio 75% and above. Thus, mild obstructive lung function was characterized by values of FEV1% predicted 60–79%, FEV1/FVC ratio 75% and above; moderate obstructive lung function FEV1% predicted 41–59%, FEV1/FVC ratio 41–59% and above; severe obstructive lung function FEV1% predicted <40% and FEV1/FVC ratio <40% while mild restrictive lung function is characterized by values of FVC predicted 61–79%; moderate restrictive lung function FVC % predicted 40–60% and severe restrictive lung function characterized by values of FVC% predicted <50%.
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS) software version 26. The baseline characteristics for each group were summarized using median and interquartile ranges for all continuous data. Simple proportions with confidence intervals were determined using exact methods for categorical variables. Continuous variables were compared using the Wilcoxon rank sum test between the two groups. Two-way comparisons of frequencies of categorical data were done using Pearson’s chi-squared tests, where the expected value was greater than or equal to five. Fischer’s exact test was used where the expected cell value was less than five. Logistic regression was used to assess the association between any clinical respiratory symptoms (e.g., sneezing, coughing, wheezing, etc.) and demographic variables, and a p-value of <0.1 was considered to be significant in all analyses.
Pearson and Spearman correlation coefficients were used to assess the relationship between lung function tests, age, and work history, number of cigarettes smoked per day, and body mass index (BMI). Data from open-ended questions were tabulated and categorized and a text analyzer was used to group the same answers.
Results
Study population characteristics and clinical results
presents the demographic and clinical characteristics of participants. A total of 81 workers from two cement facilities were interviewed, 31 from facility A and 50 from facility B were physically examined and underwent a pulmonary function test. In both facilities, the majority of the workers were males, 87% and 91% in facility A and facility B respectively. The median age was 39 years (IQR 31-49) in Facility A and 37 years (IQR 33-47) in Facility B. In Facility A, 41% of workers had a normal body index (BMI), 19% were overweight, and 39% were obese. In Facility A, only 6% have consulted a medical practitioner after referral regarding respiratory symptoms and 77% of workers confirmed having done screening tests regarding respiratory symptoms.
Table 1. Clinical and demographic characteristics of the study population.
Association between job titles and respiratory symptoms
presents a comparison of job titles at high risk for respiratory systems (operators) and job titles at low risk for respiratory symptoms (administration, clerk, driver, maintenance, etc.). Bout of coughing, chest pain, blocked nose, chest tightness, wheezing, phlegm, sneezing, fatigue, soreness or watery eyes and breathlessness were reported in 20%, 20%, 22%,14%, 9%, 10%, 33%, 35%, 9%, and 9% of all workers across both facilities respectively. Rapid breathing, which is also known as hyperventilation or more than 20 breaths per minute was reported in 6 workers (7%). There was a significant association between job titles and blocked nose, wheezing, phlegm, and breathlessness, with p-value(s) < 0.05 ().
Table 2. Association between job title and respiratory symptoms amongst workers from both facilities.
A chi-squared test was performed to assess the association between the facility and respiratory symptoms. Twenty-two percent of workers from Facility A reported having bouts of coughing and 18% from Facility B, while a blocked nose was reported in 9.7% of workers in Facility A and 30% of workers in Facility B, with a p-value of 0.032. Wheezing, sneezing or stuffy nose, fatigue, rapid breathing, soreness or watery eyes, and breathlessness were significantly associated with the facility ().
Table 3. Reported symptoms of the respiratory system.
presents the association between the facilities and respiratory symptoms. Binary logistic regression was used to assess the association between experiencing any clinical respiratory symptoms and risk factors.
Mean FEV1 and FVC were significantly different between both facilities. Facility A had a high mean FEV1 (101.07, SD: 16.41) compared to Facility B (87.94, SD: 14.50) (p-value < 0.001). Mean FVC was significantly different between the facilities. Facility A had a mean FVC of 103.5 (SD: 15.39) compared to 87.32 (SD: 12.26) in Facility B (p-value< 0.001). However, there was no difference in mean FEV1/FVC between facilities. The correlation between pulmonary function indices and demographic characteristics is outlined in ().
Table 4. Correlation between pulmonary function indices and demographic characteristics.
FEV1/FVC had a reverse relationship with smoking (p-value < 0.007). In addition, FVC and FEV had a similar relationship with the number of cigarettes smoked per day (p-value = 0.004 and p-value = 0.004 respectively). Medical recommendations and referrals were made amongst all workers who presented with abnormal lung function tests.
In Facility A, four workers revealed abnormal results, two workers presented with mild restriction, and two workers presented with mild to moderate obstruction. Most of the workers in Facility A who showed abnormal results were from the packaging department and have been with the company for over 5 years. In Facility B, 12 workers showed abnormal lung function results. Most of those workers presented with mild to moderate restriction in their lung function test results and their work history ranged from 3 months to 11 years. Emphysema, asthma, pneumonia, bronchitis, and sinusitis were more common among Facility B workers and these were confirmed by tests from the doctors after they were referred for further tests such as chest x-rays and other tests. Lung disease (Tuberculosis) was detected in one participant from Facility B. One worker was diagnosed with Emphysema in Facility B, this was diagnosed by different doctors after the workers were referred for further management.
Evaluation of workers’ practices regarding the use of respiratory protection
This objective was achieved by using a questionnaire, workers answered questions regarding respiratory protection, and a walk-through was conducted at the facilities to observe worker practices concerning the use of respiratory protection. Most workers in Facility A used dust masks (filtering facepieces (FFP2)) provided by the employer as a respiratory protective measure compared to Facility B (96.8% and 46.0%, respectively). Of those individuals that used FFP2s, 93.55% in Facility A reported changing their mask daily while 21% reported changing their FFP2 daily in Facility B. During the walk-through survey, most workers in Facility A were wearing an FFP2 during working hours and understood the health benefits of PPE. The company’s policy on PPE was visibly displayed and discussed during toolbox talks, and the importance of using PPE was emphasized. In Facility B, the majority of workers were not wearing respiratory protection and an increased amount of dust was the only factor that prompted the use of the FFP2.
Discussion
The prevalence and severity of silicosis depend on the duration and magnitude of exposure to free-silica powders as well as individual susceptibility (Méndez-Vargas et al. Citation2013) Exposure to dust is unavoidable in cement factories; however, exposure can be reduced through effective engineering control measures and the proper use of appropriate respiratory protection equipment (Ahmed and Abdullah Citation2012). Additionally, the regular use of appropriate PPE has been shown to protect cement workers from adverse respiratory health effects (Al-Neaimi et al. Citation2001). However, for various reasons, industrial workers in rapidly developing countries do not always use or have access to the appropriate PPE. A study conducted in a cement factory in the United Arab Emirates (Ahmed and Abdullah Citation2012) reported that 19.5% of workers who used N95 particulate respirators consistently had a lower prevalence rate of respiratory symptoms than those who did not use respirators.
The results of this study highlighted important values and predictions. In this study, participants reported the prevalence of emphysema, bronchitis, and tuberculosis. Results of this study showed a) similarities to the findings of a study conducted in Tanzania (Mwaiselage et al. Citation2006), in that acute respiratory symptoms, are associated with exposure to cement dust and b) that the prevalence of respiratory symptoms was high among workers who were exposed to cement dust, with similar findings to a study conducted in Greece (Rachiotis et al. Citation2018).
Another study reported that dysplasia, squamous metaplasia, and acute inflammatory infiltrated cells were detected (Hommi et al. Citation2014). The results of this study indicate an association between smoking habits and respiratory symptoms, as well as an association between cement dust exposure and the prevalence of respiratory symptoms. Symptoms such as wheezing, sneezing/stuffy nose, fatigue, rapid breathing, recurring blocked nose, soreness/watery eyes, and breathlessness were prevalent among participants from both facilities.
The difference in the mean values of FVC declined with a subsequent increase in the number of cigarettes smoked per day and the number of years that an individual had been smoking. Furthermore, FEV1/FVC% results suggested a reversed relationship with smoking cigarettes. However, gender, residential area where participants lived, age, and level of education were not significantly associated with having any of the respiratory clinical symptoms reported in this study. One study conducted in the UK suggested that smoking may lead to clinically recognized COPD in 15–20% of people who smoke (Willemse et al. Citation2004) Smoking is the most important risk factor for developing COPD, and smoking cessation is the only effective treatment for slowing down the associated accelerated decline in FEV1.
The strength of this study includes the availability of spirometry measurements conducted to confirm respiratory symptoms and lung function indices among the participants. However, the study was only able to investigate relative differences in symptom prevalence between the exposed groups since a non-exposed group was not included.
Limitations
Industrial hygiene exposure assessments were not conducted and the sample size was significantly smaller due to the limited study budget. In addition, the area air quality monitoring data could not be obtained since the proximate monitoring station has been dysfunctional for the past five years. No data about environmental, secondary, involuntary, and passive smoke exposure. The cross-sectional nature of this study affected its ability to establish the temporality of the symptoms and exposure to cement dust. The healthy worker effect might have affected the study results since only current workers in the cement industry were studied. Workers who had developed respiratory symptoms might have left their jobs or changed industries, thus underestimating the effect that cement dust exposure might have on individuals.
Conclusions and recommendations
This study revealed that there is a higher prevalence of chronic respiratory symptoms among smokers. It is therefore recommended that smoking cessation be encouraged for all workers. Smoking cessation will improve respiratory symptoms and prevent an accelerated decline in lung function indices. Wheezing, sneezing, fatigue, rapid breathing, recurring blocked nose, soreness or watery eyes, and breathlessness were significantly prevalent in both facilities. FVC, FEV1, and FEV1/FVC were higher in nonsmokers in each age group. BMI, age, level of education, area of residence, and gender were not significantly associated with the spirometric values. The cross-sectional nature and small sample size of the current study affect its generalizability.
The results of the study indicate poor health and safety measures among workers, it was observed that participants in Facility B did not use respiratory protection as recommended by company policy.
Engineering, PPE, and administrative control measures to prevent exposure to cement dust should be used. The provision of and training on the proper use of PPE respiratory protection where workers are exposed to cement dust particles should be carried out by employers. These interventions will help improve the work environment, protect worker health, and promote sustainable practices within the industry.
Ethical approval
Ethical clearance was sought and received from the University of Johannesburg, Faculty of Health Sciences Research Ethics Committee (REC-01-21-218), and permission was obtained from two cement facilities before this study was conducted. All the information retrieved from this study was kept confidential and anonymous by allocating random numbers to selected participants to replace personal identifiers. Informed consent was obtained from study participants before the commencement of this study and all fundamentals were discussed through an information letter.
Consent to participate
Informed consent was obtained from study participants before commencement of this study and all fundamentals were discussed through an information letter. The authors are also thankful to both cement companies for providing access to conduct this study.
Consent for publication
Consent has been obtained from both companies to publish the data.
Acknowledgments
The authors would like to acknowledge the University of Johannesburg and the National Institute for Communicable Disease for their support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Funding
The study received no external funding.
Data availability statement
None.
References
- Acutt J. 2004. Airflow limitations and the prevalence of chronic bronchitis amongst cement workers in South Africa Doctoral thesis, University of the Witwatersrand.
- Adeyanju E, Okeke CA. 2019. Exposure effect to cement dust pollution: a mini review. SN Appl Sci. 1(12):1572. doi: 10.1007/s42452-019-1583-0.
- Ahmed HO, Abdullah AA. 2012. Dust exposure and respiratory symptoms among cement factory workers in the United Arab Emirates. Ind Health. 50(3):214–222. doi: 10.2486/indhealth.ms1320.
- Aït-Khaled N, Enarson D, Bousquet J. 2001. Chronic respiratory diseases in developing countries: the burden and strategies for prevention and management. Bull World Health Organ. 79(10):971–979.
- Akanbi O, Olasunkanmi I, Oriolowo K, Odusote A, Olusegun A, Olaoniye W, Eng M. 2014. Assessment of post-work peak expiratory flow rate of workers in cement company. Sigurnost. 56:315–322.
- Al-Neaimi YI, Gomes J, Lloyd OL. 2001. Respiratory illnesses and ventilatory function among workers at a cement factory in a rapidly developing country. Occup Med (Lond). 51(6):367–373. doi: 10.1093/occmed/51.6.367.
- Aminian O, Aslani M, Sadeghniiat Haghighi K. 2014. Cross-shift study of acute respiratory effects in cement production workers. Acta Med Iran. 52(2):146–152.
- Ballal SG, Ahmed HO, Ali BA, Albar AA, Alhasan AY. 2004. Pulmonary effects of occupational exposure to Portland cement: a study from eastern Saudi arabia. Int J Occup Environ Health. 10(3):272–277. doi: 10.1179/oeh.2004.10.3.272.
- Blanc PD, Iribarren C, Trupin L, Earnest G, Katz PP, Balmes J, Sidney S, Eisner MD. 2009. Occupational exposures and the risk of COPD: dusty trades revisited. Thorax. 64(1):6–12. doi: 10.1136/thx.2008.099390.
- Fell AKM, Fell AKM, Nordby KC. 2017. Association between exposure in the cement production industry and non-malignant respiratory effects: a systematic review. BMJ Open. 7(4):e012381. doi: 10.1136/bmjopen-2016-012381.
- Gizaw Z, Yifred B, Tadesse T. 2016. Chronic respiratory symptoms and associated factors among cement factory workers in Dejen town, Amhara regional state, Ethiopia, 2015. Multidiscip Respir Med. 11(1):13. doi: 10.1186/s40248-016-0043-6.
- Hnizdo E, Vallyathan V. 2003. Chronic obstructive pulmonary disease due to occupational exposure to silica dust: a review of epidemiological and pathological evidence. Occup Environ Med. 60(4):237–243. doi: 10.1136/oem.60.4.237.
- Hommi B, Abdelaziz MS, Supervisor HG-EA. 2014. Effect of occupational cement dust pollution on the respiratory epithelium in Amran cement factory - Yemen. J Sci Technol. 15:25–32.
- Jalaludin J. 2018. Association between respirable dust exposure and respiratory health among cement workers. Malays J Med Health Sci. 14:78–86.
- Lage ML, Melanes Ramos L, Oliveira T, Da C, Rocha V. 2015. The atmospheric pollution and repercussions on human health: a brief review of toxicological environmental effects on respiratory system. Brazil J Surg Clin Res. 13(2):29–34.
- Linch KD. 2002. Respirable concrete dust–silicosis hazard in the construction industry. Appl Occup Environ Hyg. 17(3):209–221. doi: 10.1080/104732202753438298.
- Manjula R, Praveena RV, Clevin RR, Ghattargi CH, Dorle AS, Lalitha DH. 2013. Effects of occupational dust exposure on the health status of portland cement factory workers. Int J Med Public Health. 3(3):192–196. doi: 10.4103/2230-8598.118963.
- Méndez-Vargas MM, Báez-Revueltas FB, López-Rojas P, Tovalín-Ahumada JH, Zamudio-Lara JO, Marín-Cotoñieto IA, Villeda F. 2013. Silicosis and industrial bronchitis by exposure to silica powders and cement. Rev Med Inst Mex Seguro Soc. 51(4):384–389.
- Mwaiselage J, Moen BE, Bråtveit M. 2006. Acute respiratory health effects among cement factory workers in Tanzania: an evaluation of a simple health surveillance tool. Int Arch Occup Environ Health. 79(1):49–56. doi: 10.1007/s00420-005-0019-x.
- Nordby K-C, Fell A, Notø H, Eduard W, Skogstad M, Thomassen Y, Bergamaschi A, Kongerud J, Kjuus H. 2011. Exposure to thoracic dust, airway symptoms and lung function in cement production workers. Eur Respir J. 38(6):1278–1286. doi: 10.1183/09031936.00007711.
- Rachiotis G, Kostikas K, Pinotsi D, Hadjichristodoulou C, Drivas S. 2018. Prevalence of lung function impairment among Greek cement production workers: a cross-sectional study. Ind Health. 56(1):49–52. doi: 10.2486/indhealth.2017-0005.
- Rafeemanesh E, Alizadeh A, Afshari Saleh L, Zakeri H. 2015. A study on respiratory problems and pulmonary function indexes among cement industry workers in Mashhad, Iran. Med Pr. 66(4):471–477. doi: 10.13075/mp.5893.00115.
- Rahmani AH, Almatroudi A, Babiker AY, Khan AA, Alsahly MA. 2018. Effect of exposure to cement dust among the workers: an evaluation of health related complications. Open Access Maced J Med Sci. 6(6):1159–1162. doi: 10.3889/oamjms.2018.233.
- Rathebe PC. 2023. Occupational exposure to silicon dioxide and prevalence of chronic respiratory symptoms in the cement manufacturing industries: A review. J Public Health Res. 12(4). doi: 10.1177/22799036231204316.
- Sana S, Bhat GA, Balkhi HM. 2013. Health risks associated with workers in cement factories. Inter J Scient Res Public. 3(5):1–5.
- Tungu AM, Bråtveit M, Mamuya SD, Moen BE. 2013. Fractional exhaled nitric oxide among cement factory workers: a cross sectional study. Occup Environ Med. 70(5):289–295. doi: 10.1136/oemed-2012-100879.
- Van Schalkwyk EM, Schultz C, Joubert JR, White NW; South African Thoracic Society Standards of Spirometry Committee. 2004. Guideline for office spirometry in adults, 2004. S Afr Med J. 94(7 Pt 2):576–587.
- Wilken D, Velasco Garrido M, Manuwald U, Baur X. 2011. Lung function in asbestos-exposed workers, a systematic review and meta-analysis. J Occup Med Toxicol. 6(1). doi: 10.1186/1745-6673-6-21.
- Willemse BW, Postma DS, Timens W, ten Hacken NH. 2004. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J. 23(3):464–476. doi: 10.1183/09031936.04.00012704.
- World Health Organization (WHO). 2020. Laboratory biosafety manual. https://healthyms.com/msdhsite/index.cfm/14,4538,188,pdf/WHO_Lab_Biosafety_Manual.
- Zeleke ZK, Moen BE, Bråtveit M. 2011. Lung function reduction and chronic respiratory symptoms among workers in the cement industry: a follow up study. BMC Pulm Med. 11(1). doi: 10.1186/1471-2466-11-50.