abstract
Declining function of the immune system, termed “immunosenescence,” leads to a higher incidence of infection, cancer, and autoimmune disease related mortalities in the elderly population.Citation1 Increasing interest in the field of immunosenescence is well-timed, as 20% of the United States population is expected to surpass the age of 65 by the year 2030.Citation2 Our current understanding of immunosenescence involves a shift in function of both adaptive and innate immune cells, leading to a reduced capacity to recognize new antigens and widespread chronic inflammation. The present review focuses on changes that occur in haematopoietic stem cells, macrophages, and T-cells using knowledge gained from both rodent and human studies. The review will discuss emerging strategies to combat immunosenescence, focusing on cellular and genetic therapies, including bone marrow transplantation and genetic reprogramming. A better understanding of the mechanisms and implications of immunosenescence will be necessary to combat age-related mortalities in the future.
INTRODUCTION TO IMMUNOSENESCENCE
Human life expectancy has increased from 40 to 80 years of age just over the past 2 centuries largely due to medical advances.Citation3 However, it is likely that the human immune system did not evolve to protect the host over such an extended lifespan. Immunosenescence is a term that describes the changes in the immune system that are seen in the aging population. The hallmarks of immunosenescence include a reduced capability to respond to new antigens, increased memory responses, and a lingering level of low-grade inflammation that has been termed “inflamm-aging.”Citation4 Decline of the immune system is associated with increased incidence of infection, immune disease, and cancer in the elderly.Citation5 While immunosenescence is often described as a decline in the number and function of immune cells, myeloid cells have been shown to increase in the aged population and some secreted peptides are also expressed in greater amounts. Therefore, it is important to keep in mind that immunosenescence is more appropriately conceptualized as a change in the actions of the immune system, rather than an overall decline of all functions and constituents.
The term “immunosenescence” does not accurately describe the cellular mechanisms responsible for the age-related changes of the immune system. Immunosenescence can only be explained in part by cellular senescence, and may also be influenced by cumulative history of antigen exposure, environmental stressors, and dealings with acute or latent pathogens. Mechanisms of cellular senescence have been rigorously described in some cell types, namely fibroblasts, but there are likely differences in the characteristics of cellular senescence observed between organisms, tissues, and even cell microenvironments. In general, senescence occurs when a cell permanently or irreversibly exits the cell cycle.Citation6 Hayflick and colleagues first observed that human fibroblasts have a limited capacity to proliferate in culture, a principle known as the Hayflick limit. After several population doublings, the cultured fibroblasts lost the ability to divide, although sufficient space and nutrients were provided. Interestingly, the cells remained viable in the non-dividing state for many weeks and were thus termed senescent. These findings suggested that senescent cells might represent a physiological response to prevent cellular immortality and growth, as is common in cancer, and could help to explain the decline of tissue regeneration and repair that is observed with aging. More recent findings have shown cellular senescence can be triggered by many stimuli, including telomere shortening (also known as replicative senescence), DNA damage, chromatin perturbation, oncogene expression, and stress.
While senescent cells are not actively growing, they are distinct from quiescent or dormant cells. Senescent cells undergo extreme changes in gene expression and become resistant to apoptosis. Cell cycle inhibitors including cyclin-dependent kinase inhibitors (CDKIs), such as p21 and p16, are often expressed by senescent cells. Senescent cells secrete many factors, including extracellular matrix remodeling proteins and inflammatory cytokines, which lead to changes in the microenvironment. These inflammatory cytokines may serve to recruit cells from the immune system that will subsequently remove the senescent cells from the surrounding tissue.Citation7 However in the aging population, it has been suggested that the declining immune system cannot keep up with the need to eliminate senescent cells.Citation8 The increase in senescent cells then secrete more inflammatory cytokines and reactive oxygen species, which may drive neighboring cells into senescence and contribute to the cycle of inflamm-aging.Citation9 Over time, the accumulation of non-functional senescent cells may lead to organ failure and death of the host.Citation10 Further studies are needed to better understand cellular senescence and how it relates to the functional decline of the immune system and the chronic, low-grade inflammation that is linked to many diseases in the elderly population.
When considering the mechanisms of immunosenescence, it is important to remember that changes in the function of immune cells can be cell-intrinsic or cell-extrinsic. Cell-intrinsic changes in the genome might arise as a result of epigenetics or DNA damage in the form of oxidative damage, telomere shortening, or impairment of DNA repair responses.Citation11 Cell-extrinsic changes, such as the increased inflammatory milieu, may also play a role in immunosenescence.Citation12 It is likely that both cell intrinsic and extrinsic changes are involved in immunosenescence, but an understanding of the exact mechanisms affecting certain cell types will allow scientists to appropriately design targeted therapies that can counteract immunosenescence.
Mouse models have predominantly been used in the study of immunosenescence because of their close evolutionary relationships to humans and the availability of several immunologically relevant genetic and infectious disease models. In addition to using naturally aged models to study immunosenescence, immune diseases and infections such as rheumatoid arthritis and HIV have also shed light into mechanisms of immunosenescence.Citation13,14 There are many important differences in aging between humans and mice that must be addressed. First, 24 month-old mice may not be physiologically equivalent to elderly 80-year old human beings, which is particularly relevant as cellular replication is a important mechanism of aging and senescence.Citation4 In contrast to most human cells, murine cells contain telomeres that are 10 times longer.Citation15 Despite this, murine cells senesce after only a few doublings in culture due to supraphysiological oxygen concentrations, while human cells are not as sensitive. Finally, immunological aging is shaped by infection and antigenic load, and it is important to keep in mind the difference in pathological history, especially viral infection, of mice and men.
HSCS AND AGING
The immune system is generated and maintained by asymmetric division of multipotent haematopoietic stem cells (HSCs) in the bone marrow.Citation16 The immune system has 2 arms, the innate and the adaptive systems, which work together to eliminate pathogens and neoplastic cells, respond to vaccination, and regulate processes such as tissue turn over and wound healing.Citation16 The cells of the innate immune system include monocytes/macrophages, dendritic cells, natural killer cells, and polymorphonuclear leukocytes. The cells of the adaptive immune system include antigen-specific lymphocytes, T-cells and B-cells.Citation16 This review will focus on changes in HSCs, macrophages, and T-cell lineages, as these have been the most widely studied.
Increasing evidence shows that HSCs themselves undergo age-related changes and have a limited replicative lifespan. HSC aging was demonstrated by serial transplantation of whole bone marrow, which only supported 4–6 rounds of transplantation, suggesting the possibility of stem cell exhaustion or replicative senescence.Citation17 Interestingly, HSCs from long-lived C57BL/6 mice have relatively slow replication, whereas short-lived DBA/2 mice exhibit faster replication, associating lifespan with replicative senescence of HSCs.Citation18 In addition, accumulation of DNA damage has a profound impact on HSCs, leading to loss of proliferation, diminished self-renewal, increased apoptosis, and subsequent exhaustion.Citation19 Differentiation of the HSCs is also affected by aging, where HSCs committed to the myeloid lineage outnumber lymphoid cells in both mice and men.Citation20
MACROPHAGES AND AGING
The increased incidence of infections in the elderly suggests defects in the ability of innate immunity, particularly macrophages, to function as effective barrier cells. Classically activated macrophages recognize pathogens, perform phagocytosis, and produce cytotoxic bursts of nitric oxide and superoxide. These classically activated macrophages have been termed “M1,” for the production of Th1 cytokines. Alternatively, macrophages can express Th2 cytokines, and are termed “M2,” and can also play roles in tissue surveillance, wound healing, and tissue regeneration.Citation21 While macrophages are commonly classified as being in an “M1 or M2 activation state,” in vivo data suggests that macrophages are heterogeneous and may have characteristics of both M1 and M2 activation states at any given time. Therefore macrophage activation may exist along a spectrum rather than an “all-or-nothing” response, largely controlled by the micro-environment.Citation22
Inflammatory macrophages are derived from circulating blood monocytes which enter the tissue due to chemoattractant signals during injury or infection.Citation16 Monocytes themselves are formed from HSCs in the bone marrow along the common myeloid progenitor cell pathway.Citation16 While monocyte-derived macrophages are short-lived and are not believed to proliferate in sites of infection, tissue resident macrophages, which develop from the embryonic yolk sac or fetal liver, can survive for at least 6 weeks and maintain a presence in the tissue through homeostatic proliferation.Citation23 Tissue-resident macrophages are present in many tissues with slightly different phenotypes including liver (Kupffer cells), brain (microglia), lungs (alveolar macrophages), bone (osteoclasts), skin (Langerhans's cells), Peyer's patches in the gut, red and white pulp in the spleen, the peritoneal cavity, and the interstitium of organs such as kidney, heart, and pancreas.Citation23 Because of their widespread presence throughout the body and their heterogeneous functions, it is not surprising that macrophages are a key component of the innate immune system. The increase in infection in the aging population may be a result of decreased macrophage number and function.Citation23
Despite the increased output of myeloid progenitor cells in the elderly, the number of macrophage precursors in the blood is reduced compared to the younger population.Citation24 In addition, the number and function of Langerhans's cells in the epidermal tissue, has been reported to decline with age.Citation25 Alternatively activated M2 macrophages have been reported to increase in aged skeletal muscle and are correlated with the detrimental replacement of functional muscle with fibrotic tissue.Citation26 Bone marrow transplantation of old mice with donor cells from young mice reduced the number of M2 cells in skeletal muscle and subsequently reduced fibrosis, suggesting a cell-intrinsic mechanism of the aged bone marrow or M2 macrophages.Citation26 However, these mice were not tracked over the long term, so reemergence of fibrosis from cell-extrinsic factors cannot be ruled out. From these studies, it is likely that the number and function of macrophages in an aged organism will differ based on the tissue and activation state of interest, further complicating our understanding of immunosenescence.
Classically activated macrophages are also affected in aging. Like dendritic cells, macrophages are professional antigen-presenting cells (APCs) that can stimulate T-cell activation.Citation16 Aged macrophages have reduced MHC class II molecules, reported in both humans and mice, likely contributing to the known decline in T-cell response in the elderly.Citation27,28 Some of the most classic features of the macrophage are phagocytosis and respiratory burst. Phagocytosis, the process of engulfing a pathogen or cellular debris for subsequent destruction in the phagosome, was found to be decreased in aged murine peritoneal macrophages.Citation29 Receptors that mediate phagocytosis may have changes in expression or function with aging, however this has not yet been examined. The decline of phagocytic ability has also been correlated to a decline in macrophage-derived chemokines such as macrophage inflammatory protein (MIP) and eotaxin.Citation30 In addition, decreases in nitrous oxide and superoxide have been reported in aged rats.Citation31 Decreases in adherence, opsonization, and tumor cell killing by aged macrophages in aged mice were also observed.Citation32-34
As for additional cytokines and chemokines secreted by macrophages during aging, the literature has shown mixed results. Some studies find that aged macrophages secrete more pro-inflammatory cytokines such as IL-1 and IL-6,Citation35,36 other studies determine aged macrophages secrete less,Citation37,38 while others find no change.Citation39 These discrepancies are likely the result of different tissue sources and activation states of the macrophages tested, as well as differences in the health of the test population. It is, however, accepted that aged macrophages secrete more prostaglandin E2 than younger counterparts.Citation40 Prostaglandin E2 may be responsible for the decrease in MHC class II molecules on macrophages, as well as increased production of IL-10 which suppresses T-cell activation, thereby weakening the response to vaccination and the elimination of pathogens.Citation41
Toll-like receptors (TLRs), which play an important role in pathogen detection, have decreased surface expression on peritoneal and splenic macrophages in aging mice.Citation42 This decrease in TLR expression may be responsible, in part, for the susceptibility of the elderly to bacterial, mycotic, and viral infections. Loss of the TLR signaling pathway may also reduce pro-inflammatory cytokine secretion that is crucial for mounting an attack against a pathogen by recruiting other cellular and humoral components of the immune system.
As alluded to above, macrophages play important roles in wound healing in both the M1 and M2 activation states. Studies in humans and rodents have revealed poor cutaneous wound healing, characterized by enhanced platelet aggregation, delayed re-epithelialization, delayed angiogenesis, delayed collagen deposition, and decreased wound strength. The decline in wound healing is positive correlated with a delayed infiltration of macrophages.Citation43 Ashcroft et al. collected punch biopsies of the wounds of healthy human subjects, aged 19-96, at various time points up to 3-months post-wounding.Citation44 The authors found that macrophage and lymphocyte infiltration was delayed in the older populations, with cell numbers peaking at Day 84 as opposed to Day 7 in young patients for macrophage/monocytes.Citation44 Another study showed that the rate of wound repair in aged mice could be partially restored by transplanting peritoneal macrophages from young mice, demonstrating the importance of macrophages in the wound healing process that becomes unsynchronized in aging.Citation45 Decreases in angiogenesis of the wounds may be a result of decreased TLR expression on macrophages, which are also involved in VEGF signaling.Citation46 In addition, a decrease in adhesion molecules VLA-4 on monocytes or the receptor VCAM-1 on endothelial cells may explain the delayed rate of macrophage infiltration to the wound.Citation44 In the future, it will be important to unite the field of immunosenescence by characterizing differences in aged macrophages from different tissue sources using the same functional assays and conditions to reduce some of the contradictories currently present in the literature. It is clear, however, that delayed infiltration of macrophages and decreased functions such as phagocytosis, respiratory burst, and antigen-presentation are likely responsible, at least in part, for the increase in infection-related deaths in the elderly population.
T-CELLS AND AGING
Lymphoid progenitor cells that will differentiate into T-cells are formed in the bone marrow from HSCs then migrate to the thymus for maturation.Citation16 The developing T-cells in the thymus, known as thymocytes, undergo a series of processes to become fully mature, antigen-recognizing cells. Developing thymocytes, which lack both CD4 and CD8, are known as double negative cells. Double negative cells that lack expression of CD44, but do express CD25, undergo β-selection, which is the formation of antigen-specific T-cell receptors (TCRs). Gene rearrangement leads to the production of a repertoire of over 108 TCRs, which is sufficient to protect against the range of pathogens likely encountered throughout life.Citation16 Formation of TCR with CD3 molecules leads to survival, proliferation, and differentiation of thymocytes into double positive cells that express both CD4 and CD8. Cells that do no undergo β-selection are eliminated by apoptosis. Double positive cells undergo positive selection for their antigen by interacting with cortical epithelial cells. Thymocytes that engage in antigen/MHC with appropriate affinity survive, whereas others that bind too weakly are eliminated. Then thymocytes migrate into the medulla of the thymus where they are presented self-antigens by APCs, including macrophages and dendritic cells. Cells that interact too strongly with APCs undergo apoptosis, the process of negative selection, to prevent potential autoimmune reactions. Following these maturation steps, mature T-cells become singly positive for either CD4 or CD8, and exit the thymus for circulation in the blood stream. CD8 cells are known as effector T-cells, which are cytotoxic killers, and recognize antigens by MHC Class I restricted molecules. CD4 cells are known as helper cells, which are professional cytokine factories that can enhance or subdue other functions of the immune system, and recognize antigens by MHC Class II restricted molecules.Citation16 T-cells are major contributors to immunosenescence related complications, especially infection and cancer. Aging results in low numbers of CD8 naïve cells, persistent memory cells, and reduced diversity of the TCR repertoire.Citation1 Several studies have reported that naive T-cells are increasingly dysfunctional with aging, whereas the functions of memory cell populations are preserved.Citation47
The genesis of naïve T-cells in adults is entirely dependent on ongoing but vastly diminished thymic function.Citation48 In C57/BL6 mice, the thymus begins to involute at puberty, where thymic cellularity decreases drastically from 1–3 months of age followed by less extreme involution from 3–7 months of age.Citation49 In humans, function of the thymus decreases beginning as early as the first year of life at a rate of 3% per year, then slows to a rate of 1% per year around middle age until death.Citation50 Involution of the thymus is likely responsible for loss of naïve T-cells and reduced T-cell receptor diversity observed with aging.Citation51 Defects in T-cell synapses with antigen-presenting cells are also reported. The defects are likely a result of glycosylation of cell-surface molecules such as CD28 or changes in cell membrane lipid properties.Citation52,53 Reduced production of IL-2 after T-cell activation may be the single most important consequence of the synapse defect in mice, since addition of exogenous IL-2 can rescue many of the age-related deficits of the T-cell activation.Citation54 In humans, diminished signaling through Erk as a result of the phosphatase DUSP6 has been identified as a mechanistic explanation to decline in T-cell activation.Citation55
Recently some studies have challenged the importance of thymic involution to immunosenescence. An in silico study found that even complete loss of thymic function at the age of 20 may not influence TCR repertoire diversity.Citation56 According to the model, multiple changes in growth behavior are required for TCR contraction. Restoration of thymic output cannot prevent or rescues shrinkage of the TCR repertoire. While this may be the case, a large proportion of the elderly have undergone depletion of peripheral T-cells due to chemotherapy or antiretroviral therapy, and therefore reintroduction of a thymus would still prove useful to combat age related mortalities.Citation4
Lower abundance of TCR diversity, and diminished ability to clonally expand and produce cytotoxic mediators in CD8 T-cells may be responsible for increased mortality from bacterial and viral infections in the elderly. Old mice succumb more readily to infection with West Nile virus or Listeria than younger mice.Citation57 The occurrence of intrinsic defects in naïve cells, versus memory cells, is counter-intuitive since naïve T-cells have lower turnover and are therefore less likely to develop replicative senescence. However, studies in mice have reported increased longevity of naïve CD4 T-cells, correlating with a decrease in proapoptotic Bim expression, and suggests that this longer cell lifespan may lead to accumulation of defects.Citation58
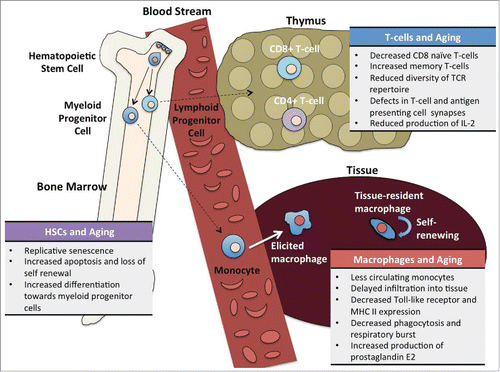
In Sweden, 2 longitudinal studies have been carried out to study the very elderly (>85 year old) and develop an immune risk profile (IRP) that can predict mortality.Citation59,60 The first study, known as OCTO, followed people in very good health over the age of 85. The OCTO study discovered an immune risk profile predictive of mortality that included high levels of CD8 T-cells, low levels of CD4 T-cells, and poor proliferative response to concanavalin A. The second study, termed NONA, selected a more representative population, with only 10% in of enrolled participants in perfect health. Interestingly a similar IRP was discovered. The NONA study found that increased amounts of memory and effector CD8-positive, CD28-negative T-cells and depleted quantities of naïve T-cells able to recognize new antigens are associated with mortality. The small number of subjects who reached the ages of 90 or even 100 years old never entered the IRP group during the 2-6 years of follow ups. One unexpected result was correlation of the IRP with persistent cytomegalovirus infection (CMV). CMV is generally considered a harmless infection, although 50-80% of adults over the age of 40 are likely to have CMV infection in the United States.Citation61 The accumulation of CMV specific CD8 T-cells and large clonal expansions associated with CMV antigens provide additional support for the hypothesis that CMV contributes markedly to immune dysfunction with aging. It is currently unclear if the findings of the OCTO and NONA study will apply universally to younger populations or elderly populations outside of Sweden. Changes in HSCs, macrophages, and T-cells are summarized in .
FIGURE 1. Summary of Age-Related Changes in the Immune System. Steady-state turnover of the immune system begins in the bone marrow with haematopoietic stem cells (HSCs), which give rise to both myeloid and lymphoid lineages. Along the lymphoid lineage, T-cells mature in the thymus and become positive for CD4 or CD8. Monocytes, derived from myeloid progenitor cells, travel through the blood steam to sites of recruitment and enter tissues as elicited macrophages. HSCs, T-cells, and macrophages all experience cellular changes with aging.
TARGETED THERAPIES FOR AGED IMMUNE CELLS
Currently, there are no clinically used therapies aimed at rejuvenating aged HSCs, macrophages, or T-cells to prevent or treat age-related disease. Emerging cellular and genetic therapies that could be applied to immunosenescence are being explored using animal models with some success.
Rejuvenating Aged Immune Cells
Many age-associated defects have been observed in HSCs, which give rise to all downstream effectors cells of the immune system, including macrophages and T-cells. Therefore, rejuvenating the HSCs might improve some of the dysfunction of both macrophages and T-cells, as well as many other cell types, observed in aging. Bone marrow transplantation from a young donor to an elderly patient could be used to rejuvenate the exhausted, aged progenitor pool. However, imperfect tissue matches often lead to rejection and even graft-vs.-host disease, a major hurdle to overcome in many fields of study.
Induced pluripotent stem cells (iPSCs) could theoretically be used to generate HSCs from a patient's own cells, thereby eliminating donor-recipient mismatch. Interestingly, reprogramming terminally differentiated cells into iPS cells induces telomerase reverse transcriptase gene (TERT), leading to telomere elongation and maintenance, in a process that can be described as “reverse aging.” A recent study showed that human senescent cells can successfully undergo genetic reprogramming and become functional once again.Citation62 IPSCs created from senescent and centenarian cells had reset telomere length, gene expression profiles, mitochondrial metabolism, and oxidative stress patterns that were indistinguishable from human embryonic stem cells. In addition, the iPSCs were able to redifferentiate into fully rejuvenated cells.Citation62 Therefore, iPS technology may be the key to the fountain of youth, where any senescent cell could in theory be reborn. Techniques to differentiate HSCs from iPS cells exist, but efficiency and safety are major hurdles that this technology must yet overcome.Citation63 In addition, genetic reprogramming will likely need to take place ex vivo to prevent collapse of organ function in the intermediate, undifferentiated cell state, so repopulation of tissue resident macrophages and lymphocytes will take several weeks or months from a single bone marrow transplantation. Also, effectiveness of rejuvenated HSCs would be limited by thymic output for T-cells and would likely not replace tissue resident macrophages, which are self-sustaining. However, repopulating the bone marrow with autologous IPSC-derived HSCs is a promising approach to rejuvenating the majority of immune system, especially the innate effector response.
Inducing autophagy in aged cells and organisms by caloric restriction has been shown to reduce age-related damage,Citation64 suggesting that decline in cell function may be prevented by maintaining autophagy levels, or potentially even rescued by the induction of autophagy in aged cells. A recent study showed that macrophages from aged mice exhibit reduced autophagic flux compared to young mice, therefore chemically increasing autophagy might be one strategy to reduce aged macrophage dysfunction.Citation65 In addition to caloric restriction, exercise is a known factor that can boost lifespan and health span. One study found that long-term moderate exercise in male BALB/c mice tended to reverse age-associated changes in macrophage function using cells isolated from the lungs and peritoneal cavity.Citation66
Another method to boost macrophage function, especially recognition of foreign pathogens, is to treat with cytokine or growth factor cocktails. Treatment of aged macrophages with IFN-γ Citation67 or with insulin-like growth factor (IGF) Citation68 significantly improved both inflammatory and effector responses to lipopolysaccharide (LPS) stimulation. One challenge to this technique will be in vivo delivery of cytokines and growth factors to relevant anatomical locations and in relevant doses. Alternatively, macrophages could be isolated, stimulated ex vivo, and reinfused to the patient as a living adjuvant to reduce infection.
As mentioned previously, macrophages are heterogeneous cells with behaviors that are heavily influenced by the microenvironment. One group recently demonstrated that effector functions can be restored in macrophages from aged mice by simply removing them from the aged environment.Citation69 Therefore, therapies aimed at altering the environment, rather than the cells themselves, could be an alternative approach to treating aging.
Finally, strategies aimed at macrophage rejuvenation may also influence the T-cell compartment. One study found that IL-2-/CD40-activated macrophages rescue the production of IFN-γ by T-cells in geriatric mice. Therefore, targeting macrophages with specific antibodies may improve both innate and adaptive functions in aging hosts.Citation70 In addition, vitamin E improves T cell responsiveness in old mice mostly by reducing macrophage prostaglandin E2 production.Citation71
Rejuvenating the Thymus
The thymus begins to significantly deteriorate around 10 years of age in humans, and likely plays a role in the decline of the immune system, especially the diversity of the T-cell repertoire, during aging. Rejuvenating or somehow regulating thymic output is an intriguing approach to combat age-related decline of T-cells. Sex hormones have been shown to modulate thymus size and function. In fact castration of 9-month-old mice enhances the number of early thymic progenitors.Citation72 While this technique may have limited application in humans, male patients undergoing sex steroid ablation therapy for prostate cancer have increased circulating naïve T-cells, demonstrating the link between sex hormones and thymic health in humans.Citation73 Bolotin et al. reported an enhancement of thympoiesis after bone marrow transplant with administration of IL-7 in mice.Citation74 Indeed, IL-7 therapy alone has been shown to rejuvenate the thymus in aged mice, although with size and output slightly less than observed in the young mice.Citation75 IL-7 therapy has yet to be tested in humans, and has been shown to have species-specific functions, possibly limiting its efficacy.
Other approaches to replacing or regenerating the thymus include tissue and cell transplantation. Transplantation of cultured thymic tissue from human cadavers into the kidney capsule of patients with DiGeorge syndrome successfully restored immune function for up to 10 years.Citation76 However there are limitations to this approach for treating the aging population due to lack of donated tissue, invasive surgery, and tissue rejection.
Regenerative medicine, including tissue engineering and cell and gene therapy, offer alternative approaches to replacing the thymus. Many groups have identified murine multipotent progenitors, termed thymic epithelial cells (TEC), that can grow into a 3-dimensional thymus and support normal T-cell development when transplanted into the kidney capsule.Citation77 Human TECs have yet to be isolated in sufficient numbers, however protocols to push human embryonic stem cells toward TEC lineage are becoming consistently more efficient.Citation78
In addition to the kidney capsule, lymph nodes have emerged as an intriguing site for ectopic organ formation. Recently, Lagasse's group showed that minced thymuses from newborn mice can be injected into the jejunal lymph nodes of athymic mice and reconstitute functional lymphocytes.Citation79 The ectopic thymuses were organized into epithelial thymus structures with areas corresponding to both the thymic medullary and cortical epithelia and contained maturing CD4/CD8+ double positive T-cells. The transplanted mice rejected both a skin allograft and injected tumor cells, suggesting the newly developed T-cells were functional.
Even more recently, the thymus was the first organ ever to be successfully regenerated in a living organism. Diminished expression of the TEC specific transcription factor, Forkhead box N1 (FOXN1), is implicated in the mechanism of thymus involution. Blackburn's group found that forcing the expression of FOXN1 in the involuted thymus of aged mice caused complete thymus regeneration, characterized by increased thymopoiesis and naïve T-cell output, as a result of robust TEC progenitor responses.Citation80 The study showed that regeneration of an organ in an aged animal can be controlled by manipulation of a single transcription factor, perhaps ushering in a new era of regenerative medicine. The authors do not comment on how this therapy could one day be applied to human patients.
CONCLUSION
Immunosenescence is a complex problem arising from age-associated changes to the cells of the immune system, a process that may increase susceptibility to cancer, infection, autoimmune disease, or simply organ failure as a result of chronic inflammation and build up of non-functional senescent cells. Changes in the function of haematopoietic stem cells, macrophages, and T-cells just scratch the surface of the complexities of immune system decline with age. Cellular and genetic therapies are beginning to make headway in rejuvenating the immune system, which could ultimately improve lifespan and/or health span of the human race. Genetically reprogramming cells into induced pluripotent stem cells can rejuvenate any cell type through telomere elongation, overcoming hurdles of replicative senescence. Activating transcription factors such as FOXN1 may completely regenerate the thymus and reestablish functional naïve T-cells in the elderly. Finally, altering the systemic inflammatory environment through caloric restriction to increase autophagy, exercise, and vitamin supplementation are practical ways to combat immunosenescence right now. Increasing our understanding of immunosenescence using standard cell isolation and culture methods, as well as the increasing the feasibility of cellular and genetic techniques are crucial to push the field forward. Currently genetic reprogramming generally has low efficiency and raises concerns about ethics and safety. It is also unclear how genes can be altered in vivo without detrimental effects. In addition, rejection of cell and tissue transplantation is a major hurdle in many fields, including immunosenescence, which scientists are continuously working to solve. Significant progress in the field of immunosenescence has been made over the last 40 years; it will be remarkable to see what the next few years bring.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Funding
This work was supported by National Institutes of Health awards T32-EB001026 (E.C.S.) as well as K12HD043441 and R03 AG043606 (B.N.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology 2007; 120:435-46; PMID:17313487; http://dx.doi.org/10.1111/j.1365-2567.2007.02555.x
- Jennifer M Ortman VAV, Howard H. An Aging Nation: The Older Population in the United States. No. P25-1140. Issued May 2014. From: http://www.census.gov/content/dam/Census/library/publications/2014/demo/p25-1140.pdf
- Aging NIO. Global Health and Aging Living Longer. NIH publication no. 11-7737. October 2011. From: https://www.nia.nih.gov/research/publication/global-health-and-aging/preface
- Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nature Immunol 2013; 14:428-36; PMID:23598398; http://dx.doi.org/10.1038/ni.2588
- Sebastián C, Lloberas J, Celada A. Molecular and Cellular Aspects of Macrophage Aging. In: Fulop T, Franceschi C, Hirokawa K, Pawelec G, eds. Handbook on Immunosenescence: Springer Netherlands, 2009:919-45
- Mathon NF, Lloyd AC. Cell senescence and cancer. Nat Rev Cancer 2001; 1:203-13; PMID:11902575; http://dx.doi.org/10.1038/35106045
- Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 2013; 123:966-72; PMID:23454759; http://dx.doi.org/10.1172/JCI64098
- Burton DG, Faragher RG. Cellular senescence: from growth arrest to immunogenic conversion. Age 2015; 37:27; PMID:25787341; http://dx.doi.org/10.1007/s11357-015-9764-2
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mechanisms Age Dev 2007; 128:92-105; PMID:17116321; http://dx.doi.org/10.1016/j.mad.2006.11.016
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011; 479:232-6; PMID:22048312; http://dx.doi.org/10.1038/nature10600
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013; 153:1194-217; PMID:23746838; http://dx.doi.org/10.1016/j.cell.2013.05.039
- Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol Series A, Biol Sci Med Sci 2014; 69 Suppl 1:S4-9; PMID:24833586; http://dx.doi.org/10.1093/gerona/glu057
- Petersen LE, Grassi-Oliveira R, Siara T, Dos Santos SG, Ilha M, de Nardi T, Keisermann M, Bauer ME. Premature immunosenescence is associated with memory dysfunction in rheumatoid arthritis. Neuroimmunomodulation 2015; 22:130-7; PMID:24751698; http://dx.doi.org/10.1159/000358437
- Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Ann Rev Med 2011; 62:141-55; PMID:21090961; http://dx.doi.org/10.1146/annurev-med-042909-093756
- Calado RT, Dumitriu B. Telomere dynamics in mice and humans. Seminars Hematol 2013; 50:165-74; PMID:23956466; http://dx.doi.org/10.1053/j.seminhematol.2013.03.030
- Parkin J, Cohen B. An overview of the immune system. Lancet 2001; 357:1777-89; PMID:11403834; http://dx.doi.org/10.1016/S0140-6736(00)04904-7
- Harrison DE, Astle CM, Delaittre JA. Loss of proliferative capacity in immunohemopoietic stem cells caused by serial transplantation rather than aging. J Exp Med 1978; 147:1526-31; PMID:25943; http://dx.doi.org/10.1084/jem.147.5.1526
- de Haan G, Van Zant G. Dynamic changes in mouse hematopoietic stem cell numbers during aging. Blood 1999; 93:3294-301; PMID:10233881
- Rube CE, Fricke A, Widmann TA, Furst T, Madry H, Pfreundschuh M, Rube C. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PloS One 2011; 6:e17487; PMID:21408175
- Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, Weissman IL. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci USA 2011; 108:20012-7
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11:723-37; PMID:21997792; http://dx.doi.org/10.1038/nri3073
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958-69; PMID:19029990; http://dx.doi.org/10.1038/nri2448
- Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging cell 2004; 3:161-7; PMID:15268749; http://dx.doi.org/10.1111/j.1474-9728.2004.00102.x
- Takahashi I, Ohmoto E, Aoyama S, Takizawa M, Oda Y, Nonaka K, Nakada H, Yorimitsu S, Kimura I. Monocyte chemiluminescence and macrophage precursors in the aged. Acta medica Okayama 1985; 39:447-51; PMID:4091040
- Thiers BH, Maize JC, Spicer SS, Cantor AB. The effect of aging and chronic sun exposure on human Langerhans cell populations. J Invest Dermatol 1984; 82:223-6; PMID:6199432; http://dx.doi.org/10.1111/1523-1747.ep12260055
- Wang Y, Wehling-Henricks M, Samengo G, Tidball JG. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell 2015; 14:678-88; PMID:26009878; http://dx.doi.org/10.1111/acel.12350
- Zissel G, Schlaak M, Muller-Quernheim J. Age-related decrease in accessory cell function of human alveolar macrophages. J Investig Med 1999; 47:51-6; PMID:10071481
- Herrero C, Sebastian C, Marques L, Comalada M, Xaus J, Valledor AF, Lloberas J, Celada A. Immunosenescence of macrophages: reduced MHC class II gene expression. Exp Gerontol 2002; 37:389-94; PMID:11772525; http://dx.doi.org/10.1016/S0531-5565(01)00205-4
- Mancuso P, McNish RW, Peters-Golden M, Brock TG. Evaluation of phagocytosis and arachidonate metabolism by alveolar macrophages and recruited neutrophils from F344xBN rats of different ages. Mechanisms Ageing Dev 2001; 122:1899-913; PMID:11557288; http://dx.doi.org/10.1016/S0047-6374(01)00322-0
- Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol 2001; 117:1027-35; PMID:11710909; http://dx.doi.org/10.1046/j.0022-202x.2001.01539.x
- Alvarez E, Machado A, Sobrino F, Santa Maria C. Nitric oxide and superoxide anion production decrease with age in resident and activated rat peritoneal macrophages. Cell Immunol 1996; 169:152-5; PMID:8612288; http://dx.doi.org/10.1006/cimm.1996.0103
- De La Fuente M. Changes in the macrophage function with aging. Comparative Biochem Physiol A, Comparative Physiol 1985; 81:935-8; PMID:2863082; http://dx.doi.org/10.1016/0300-9629(85)90933-8
- Khare V, Sodhi A, Singh SM. Effect of aging on the tumoricidal functions of murine peritoneal macrophages. Natural Immunity 1996; 15:285-94; PMID:9523280
- De la Fuente M, Medina S, Del Rio M, Ferrandez MD, Hernanz A. Effect of aging on the modulation of macrophage functions by neuropeptides. Life Sci 2000; 67:2125-35; PMID:11057762; http://dx.doi.org/10.1016/S0024-3205(00)00799-2
- Ershler WB, Sun WH, Binkley N, Gravenstein S, Volk MJ, Kamoske G, Klopp RG, Roecker EB, Daynes RA, Weindruch R. Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res 1993; 12:225-30; PMID:8218595
- Mariani E, Pulsatelli L, Neri S, Dolzani P, Meneghetti A, Silvestri T, Ravaglia G, Forti P, Cattini L, Facchini A. RANTES and MIP-1alpha production by T lymphocytes, monocytes and NK cells from nonagenarian subjects. Exp Gerontol 2002; 37:219-26; PMID:11772507; http://dx.doi.org/10.1016/S0531-5565(01)00187-5
- Gon Y, Hashimoto S, Hayashi S, Koura T, Matsumoto K, Horie T. Lower serum concentrations of cytokines in elderly patients with pneumonia and the impaired production of cytokines by peripheral blood monocytes in the elderly. Clin Exp Immunol 1996; 106:120-6; PMID:8870709
- Beharka AA, Meydani M, Wu D, Leka LS, Meydani A, Meydani SN. Interleukin-6 production does not increase with age. J Gerontol Series A, Biological Sci Med Sci 2001; 56:B81-8; PMID:11213271; http://dx.doi.org/10.1093/gerona/56.2.B81
- Ahluwalia N, Mastro AM, Ball R, Miles MP, Rajendra R, Handte G. Cytokine production by stimulated mononuclear cells did not change with aging in apparently healthy, well-nourished women. Mechanisms Ageing Dev 2001; 122:1269-79; PMID:11438118; http://dx.doi.org/10.1016/S0047-6374(01)00266-4
- Hayek MG, Mura C, Wu D, Beharka AA, Han SN, Paulson KE, Hwang D, Meydani SN. Enhanced expression of inducible cyclooxygenase with age in murine macrophages. J Immunol 1997; 159:2445-51
- Wu D, Meydani SN. Mechanism of age-associated up-regulation in macrophage PGE2 synthesis. Brain, Behavior, Immunity 2004; 18:487-94; PMID:15331118; http://dx.doi.org/10.1016/j.bbi.2004.05.003
- Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol 2002; 169:4697-701; http://dx.doi.org/10.4049/jimmunol.169.9.4697
- Ashcroft GS, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerontology 2002; 3:337-45; PMID:12510172; http://dx.doi.org/10.1023/A:1021399228395
- Ashcroft GS, Horan MA, Ferguson MW. Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab Invest; J Tech Meth Pathol 1998; 78:47-58; PMID:9461121
- Danon D, Kowatch MA, Roth GS. Promotion of wound repair in old mice by local injection of macrophages. Proc Natl Acad Sci USA 1989; 86:2018-20
- Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, Leibovich SJ. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors. Am J Pathol 2003; 163:711-21; PMID:12875990; http://dx.doi.org/10.1016/S0002-9440(10)63698-X
- Lang A, Nikolich-Zugich J. Functional CD8 T cell memory responding to persistent latent infection is maintained for life. J Immunol 2011; 187:3759-68; http://dx.doi.org/10.4049/jimmunol.1100666
- den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, Willems N, Schrijver EH, Spierenburg G, Gaiser K, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity 2012; 36:288-97; PMID:22365666; http://dx.doi.org/10.1016/j.immuni.2012.02.006
- Gui J, Mustachio LM, Su DM, Craig RW. Thymus Size and Age-related Thymic Involution: Early Programming, Sexual Dimorphism, Progenitors and Stroma. Aging Dis 2012; 3:280-90; PMID:22724086
- Steinmann GG, Klaus B, Muller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scandinavian J Immunol 1985; 22:563-75; PMID:4081647; http://dx.doi.org/10.1111/j.1365-3083.1985.tb01916.x
- Blackman MA, Woodland DL. The narrowing of the CD8 T cell repertoire in old age. Curr Opin Immunol 2011; 23:537-42; PMID:21652194; http://dx.doi.org/10.1016/j.coi.2011.05.005
- Weng NP, Akbar AN, Goronzy J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol 2009; 30:306-12; PMID:19540809; http://dx.doi.org/10.1016/j.it.2009.03.013
- Nguyen DH, Espinoza JC, Taub DD. Cellular cholesterol enrichment impairs T cell activation and chemotaxis. Mechan Ageing Dev 2004; 125:641-50; PMID:15491683; http://dx.doi.org/10.1016/j.mad.2004.08.002
- Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med 1999; 190:1013-24; PMID:10510091; http://dx.doi.org/10.1084/jem.190.7.1013
- Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM, Goronzy JJ. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med 2012; 18:1518-24; PMID:23023500; http://dx.doi.org/10.1038/nm.2963
- Johnson PL, Yates AJ, Goronzy JJ, Antia R. Peripheral selection rather than thymic involution explains sudden contraction in naive CD4 T-cell diversity with age. Proc Natl Acad Sci U S A 2012; 109:21432-7
- Nikolich-Zugich J, Li G, Uhrlaub JL, Renkema KR, Smithey MJ. Age-related changes in CD8 T cell homeostasis and immunity to infection. Seminars Immunol 2012; 24:356-64; PMID:22554418; http://dx.doi.org/10.1016/j.smim.2012.04.009
- Haynes L, Lefebvre JS. Age-related Deficiencies in Antigen-Specific CD4 T cell Responses: Lessons from Mouse Models. Aging Dis 2011; 2:374-81; PMID:22396889
- Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mechan Age Dev 2000; 121:187-201; PMID:11164473; http://dx.doi.org/10.1016/S0047-6374(00)00210-4
- Wikby A, Johansson B, Olsson J, Lofgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol 2002; 37:445-53; PMID:11772532; http://dx.doi.org/10.1016/S0531-5565(01)00212-1
- Center for Disease Control and Prevention. Cytomegalovirus (CMV) and Congenital CMV Infection: Prevention. 28 July 2010 From: http://www.cdc.gov/cmv/prevention.html
- Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Ait-Hamou N, Leschik J, Pellestor F, Ramirez JM, De Vos J, et al. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev 2011; 25:2248-53; PMID:22056670; http://dx.doi.org/10.1101/gad.173922.111
- Suzuki N, Yamazaki S, Yamaguchi T, Okabe M, Masaki H, Takaki S, Otsu M, Nakauchi H. Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol Therapy 2013; 21:1424-31; PMID:23670574; http://dx.doi.org/10.1038/mt.2013.71
- Trepanowski JF, Canale RE, Marshall KE, Kabir MM, Bloomer RJ. Impact of caloric and dietary restriction regimens on markers of health and longevity in humans and animals: a summary of available findings. Nutri J 2011; 10:107; PMID:21981968; http://dx.doi.org/10.1186/1475-2891-10-107
- Stranks AJ, Hansen AL, Panse I, Mortensen M, Ferguson DJ, Puleston DJ, Shenderov K, Watson AS, Veldhoen M, Phadwal K, et al. Autophagy Controls Acquisition of Aging Features in Macrophages. J Innate Immunity 2015; 7:375-91; PMID:25764971; http://dx.doi.org/10.1159/000370112
- Kohut ML, Senchina DS, Madden KS, Martin AE, Felten DL, Moynihan JA. Age effects on macrophage function vary by tissue site, nature of stimulant, and exercise behavior. Exp Gerontol 2004; 39:1347-60; PMID:15489058; http://dx.doi.org/10.1016/j.exger.2004.07.001
- Hayakawa H, Sato A, Yagi T, Uchiyama H, Ide K, Nakano M. Superoxide generation by alveolar macrophages from aged rats: improvement by in vitro treatment with IFN-gamma. Mechan Ageing dev 1995; 80:199-211; PMID:7564571; http://dx.doi.org/10.1016/0047-6374(95)01573-I
- Burgess W, Liu Q, Zhou J, Tang Q, Ozawa A, VanHoy R, Arkins S, Dantzer R, Kelley KW. The immune-endocrine loop during aging: role of growth hormone and insulin-like growth factor-I. Neuroimmunomodulation 1999; 6:56-68; PMID:9876236; http://dx.doi.org/10.1159/000026365
- Stout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev 2005; 205:60-71; PMID:15882345; http://dx.doi.org/10.1111/j.0105-2896.2005.00260.x
- Jackaman C, Radley-Crabb HG, Soffe Z, Shavlakadze T, Grounds MD, Nelson DJ. Targeting macrophages rescues age-related immune deficiencies in C57BL/6J geriatric mice. Aging Cell 2013; 12:345-57; PMID:23442123; http://dx.doi.org/10.1111/acel.12062
- Beharka AA, Wu D, Han SN, Meydani SN. Macrophage prostaglandin production contributes to the age-associated decrease in T cell function which is reversed by the dietary antioxidant vitamin E. Mechan Ageing Dev 1997; 93:59-77; PMID:9089571; http://dx.doi.org/10.1016/S0047-6374(96)01819-2
- Heng TS, Goldberg GL, Gray DH, Sutherland JS, Chidgey AP, Boyd RL. Effects of castration on thymocyte development in two different models of thymic involution. J Immunol 2005; 175:2982-93; http://dx.doi.org/10.4049/jimmunol.175.5.2982
- Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA, Chidgey AP, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol 2005; 175:2741-53; http://dx.doi.org/10.4049/jimmunol.175.4.2741
- Bolotin E, Smogorzewska M, Smith S, Widmer M, Weinberg K. Enhancement of thymopoiesis after bone marrow transplant by in vivo interleukin-7. Blood 1996; 88:1887-94; PMID:8781449
- Aspinall R. T cell development, ageing and Interleukin-7. Mechan Ageing Dev 2006; 127:572-8; PMID:16529797; http://dx.doi.org/10.1016/j.mad.2006.01.016
- Rice HE, Skinner MA, Mahaffey SM, Oldham KT, Ing RJ, Hale LP, Markert ML. Thymic transplantation for complete DiGeorge syndrome: medical and surgical considerations. J Pediatric Surg 2004; 39:1607-15; PMID:15547821; http://dx.doi.org/10.1016/j.jpedsurg.2004.07.020
- Rossi SW, Jenkinson WE, Anderson G, Jenkinson EJ. Clonal analysis reveals a common progenitor for thymic cortical and medullary epithelium. Nature 2006; 441:988-91; PMID:16791197; http://dx.doi.org/10.1038/nature04813
- Su M, Hu R, Jin J, Yan Y, Song Y, Sullivan R, Lai L. Efficient in vitro generation of functional thymic epithelial progenitors from human embryonic stem cells. Scientific Reports 2015; 5:9882; PMID:26044259; http://dx.doi.org/10.1038/srep09882
- Komori J, Boone L, DeWard A, Hoppo T, Lagasse E. The mouse lymph node as an ectopic transplantation site for multiple tissues. Nat Biotechnol 2012; 30:976-83; PMID:23000933; http://dx.doi.org/10.1038/nbt.2379
- Bredenkamp N, Nowell CS, Blackburn CC. Regeneration of the aged thymus by a single transcription factor Development. 2014; 141:1627-37; PMID:24715454; http://dx.doi.org/10.1242/dev.103614
