ABSTRACT
Human pluripotent stem cells (hPSCs) have shown the ability to self-organize into different types of neural organoids (e.g., whole brain organoids, cortical spheroids, midbrain organoids etc.) recently. The extrinsic and intrinsic signaling elicited by Wnt pathway, Hippo/Yes-associated protein (YAP) pathway, and extracellular microenvironment plays a critical role in brain tissue morphogenesis. This article highlights recent advances in neural tissue patterning from hPSCs, in particular the role of Wnt pathway and YAP activity in this process. Understanding the Wnt-YAP interactions should provide us the guidance to predict and modulate brain-like tissue structure through the regulation of extracellular microenvironment of hPSCs.
ABBREVIATIONS
| 3-D | = | three-dimensional |
| DKK | = | Dickkopf |
| EBs | = | embryoid bodies |
| ECM | = | extracellular matrix |
| ESCs | = | embryonic stem cells |
| FGF | = | fibroblast growth factor |
| GSK 3 | = | glycogen synthase kinase 3 |
| hESCs | = | human embryonic stem cells |
| hiPSCs | = | human induced pluripotent stem cells |
| hPSCs | = | human pluripotent stem cells |
| IWP2 (or 4) | = | inhibitor of Wnt-production 2 (or 4) |
| IWR-1 | = | inhibitor of Wnt-response 1 |
| LRP6 | = | low-density lipoprotein receptor-related protein 6 |
| MSCs | = | mesenchymal stem cells |
| NPCs | = | neural progenitor cells |
| PSCs | = | pluripotent stem cells |
| RA | = | retinoic acid |
| ROCK | = | Rho-associated protein kinase |
| SHH | = | sonic hedgehog |
| TAZ | = | transcriptional coactivator with PDZ-binding motif |
| YAP | = | Yes-associated protein |
INTRODUCTION
Human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), have great potential in drug screening, disease modeling, and potentially in cell therapy.Citation1-3 Recently, it is found that hPSCs can self-assemble into three-dimensional (3-D) organoids, in particular the “mini-brains”, which have the organized structure to mimic human tissue morphogenesis.Citation4-6 Midbrain organoids have also been derived from hPSCs, containing long-lived dopaminergic neurons which otherwise have short-term life span in vitro.Citation7 These mini-organoids provide a novel platform as physiologically relevant human brain tissue models for drug screening and disease modeling.Citation5,8 Wnt signaling is one of the major pathways that serve as extrinsic signaling to regulate stem cells for neural tissue development and regeneration.Citation9 Wnt signaling can switch rostral and caudal brain tissue identity in human neural tissue morphogenesis in a concentration-dependent manner.Citation10 In the meanwhile, Hippo/Yes-associated protein (YAP) signaling has been identified as another important pathway that plays a critical role in organ growth control as well as stem cell propagation and differentiation.Citation11 The interactions of Wnt signaling and YAP expression attract extensive interests in regenerative medicine recently,Citation12,13 but how Wnt-YAP interactions affect neural tissue morphogenesis from hPSCs has been unexplored. Here, the biological significance of Wnt signaling and YAP expression in neural organoid formation from hPSCs is discussed.
THE ROLE OF CANONICAL WNT/β-CATENIN SIGNALING IN HPSC FATE DECISIONS
Wnt signaling has been found to play an important role in regulating hPSC self-renewal and differentiation through intercellular interactions ( and ).Citation14 At the undifferentiated state, hPSCs express high level of β-catenin at cell membrane due to the formation of cadherin-catenin complex with little nuclear localization. Increased E-cadherin expression can sequester β-catenin at the adherent junctions, which results in less β-catenin available for translocation to the nucleus and the down-regulation of Wnt signaling.Citation15 The loss of E-cadherin expression enables β-catenin translocation into nucleus to activate Wnt signaling. During differentiation, the influence of Wnt/β-catenin has the context-dependent effects on hPSC fate decisions.
Figure 1. Illustration of the interactions of extracellular microenvironment (soluble factors, matrices etc.) with Wnt/β-catenin signaling through biochemical regulation. The effects of Wnt activation (e.g., Wnt 3A or CHIR99021) and inhibition (e.g., DKK1 or IWP4) on pluripotent stem cell (PSC) lineage commitment are shown. GSK-3β: glycogen synthase kinase-3β; TCF: T-cell factor; LEF: lymphoid enhancer factor. FGF, fibroblast growth factor; HGF: Hepatocyte growth factor; IGF: Insulin-like growth factor; TGF, transforming growth factor.
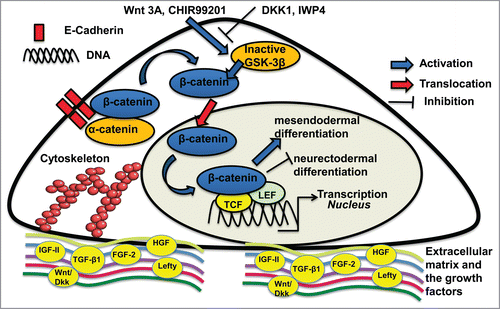
TABLE 1. Summary of the role of Wnt signaling in pluripotent stem cell fate decisions.
The role of Wnt signaling in early stage neural differentiation from hPSCs
During early stage ectoderm differentiation from PSCs, inhibition of Wnt signals promotes anterior character and enhances neurectodermal differentiation, while local activation of Wnt signaling posteriorizes embryoid body (EB) and promotes mesendodermal differentiation.Citation16 Consistently, efficient cardiac differentiation from hPSCs requires temporal modulation of Wnt signaling, i.e., early activation of canonical Wnt signaling followed by the inhibition of Wnt activity.Citation17 Characterization of Wnthigh and Wntlow populations in hESCs reveals that Wnthigh cells mainly differentiate into endodermal and cardiac cells (FOXA2+), while Wntlow cells differentiate into neuroectodermal cells (PAX6+ and Otx2+).Citation9 For neural differentiation of hPSCs using LDN193189 and SB431542 (dual SMAD inhibitors), Wnt inhibition (e.g.,, using XAV-939) in combination with sonic hedgehog (SHH) signaling promotes the telencephalic specification and ventral patterning of telencephalic neural precursors.Citation18
The role of Wnt signaling in neural tissue patterning of hPSCs
Wnt signaling was found to regulate the heterogeneity of hPSC-derived neural progenitor cells (NPCs) and the subsequent neural differentiation along rostral and caudal positional identity. In the presence of high endogenous Wnt signaling, NPCs adopt a posterior neural fate by expressing PITX2, FGF8, IRX3, and HOXB4, the caudal neural markers for hindbrain and spinal cord.Citation19 In contrast, in the absence of endogenous Wnt signaling, NPCs are enriched for rostral forebrain and anterior-specific markers (e.g., FOXG1, DLX2, LHX2 and LHX8) [19]. The intermediate level of Wnt signaling leads to the enrichment of midbrain neurons. Consistently, exogenous activation of Wnt signaling with CHIR99021 increases the expression of posterior markers such as HOXB4, while inhibitor of Wnt-production 2 (IWP2) treatment increases the expression of anterior markers such as FOXG1 [19]. Therefore, Wnt signaling plays a critical role in the regional patterning and positional identity of hPSC-derived NPCs.
Due to the neural patterning effect, Wnt signaling can efficiently promote motor neuron differentiation from hPSCs, in combination with caudalization factor retinoic acid (RA) and ventralization factor SHH.Citation20,21 Wnt signaling may elevate the threshold level of SHH signaling to enrich motor neural progenitors. Using dual SMAD inhibition in the presence of Wnt activator CHIR99021, high purity of motor neural progenitors (>95% Oligo2+) can be generated in 12 days.Citation20 Consistently, high purity of spinal motor neurons (74% ISL1+HB9+ cells, by activating Wnt signaling with CHIR99021 at 3 μM and RA at 1 μM) or cranial motor neurons (52% ISL1+PHOX2B+ cells, by lowering Wnt and RA concentrations with CHIR99021 at 1 μM and RA at 0.01 μM) were generated in 14 days by early exposure to Wnt signaling.Citation22 These studies indicate that Wnt activation induces a caudal fate of neural progenitors that more easily differentiate into motor neurons.
While the mentioned studies demonstrated the effects of Wnt signaling on generating specific types of neural cell populations with high purity at cellular levels, the influence of Wnt signaling on spatial patterning of different neural populations from hPSCs in 3-D culture at tissue/mini-organ levels is still unexplored and needs further investigation.
Wnt activators and inhibitors
To achieve effective Wnt activation or inhibition, various Wnt activators and inhibitors have been developed to modulate Wnt signaling, which may act through different mechanisms ().
TABLE 2. Wnt antagonists and agonists.
Wnt antagonists (inhibitors). Dickkopf (DKK) family and the WISE/SOST family are the well-known Wnt antagonists. DKK1 inhibits Wnt signaling by inducing internalization and degradation of low-density lipoprotein receptor-related protein 6 (LRP6) through transmembrane Kremen proteins.Citation23 Similarly, WISE/SOST also belongs to the LRP5/6 ligands/antagonists that can inhibit Wnt signaling. Inhibitor of Wnt-production 4 (IWP4) prevents palmitoylation of Wnt proteins by Porcupine, thereby blocking Wnt protein secretion and activity.Citation24 Addition of IWP4 in the slow rotary culture was shown to reduce the expression of cardiac genes MLC-2v and αMHC and inhibit cardiac differentiation of PSCs.Citation25 Similarly, IWP2-treated hESCs have high neuroectodermal differentiation potential and express higher levels of Pax6 and Otx2 but lower level of Gata4.Citation9 XAV939 is a Tankyrase inhibitor that inhibits Wnt/β-catenin signaling. XAV939 can block the effect of Wnt3a on the induction of mesoderm and primitive streak.Citation14 Inhibitor of Wnt response (IWR)-1, acting similarly to XAV939, is an antagonist which inhibits Wnt/β-catenin through the stabilization of Axin.
Wnt agonists (activators): Wnt proteins such as Wnt3a promote Wnt/β-catenin signaling by acting as the ligands to Wnt receptors. Inhibition of glycogen synthase kinase 3 (GSK3) (e.g.,, using GSK3 inhibitor CHIR99021) leads to nuclear accumulation of β-catenin, which associates with T-cell factor/lymphoid enhancer-binding factor and activates Wnt-targeted gene transcription.Citation17 CHIR99021 in combination with PD0325901 (a potent mitogen-activated protein kinase inhibitor) supports PSC long-term self-renewal and can significantly improve the reprogramming efficiency of mouse embryonic fibroblasts.Citation26 Activation of Wnt using CHIR99021 also promotes mesoderm differentiation that can lead to the high purity of cardiac lineage and endothelial lineage cells.Citation27
WNT INTERACTIONS WITH EXTRACELLULAR MATRIX AND THE EFFECT OF CULTURE SYSTEMS
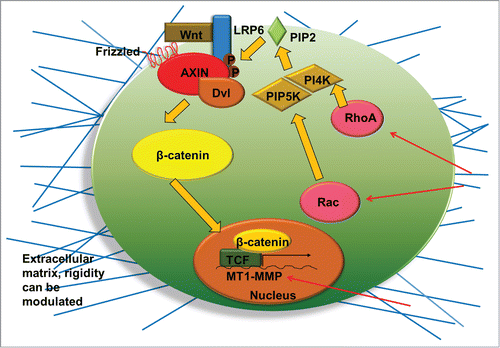
The extracellular control of Wnt signaling depends on the extracellular storage of Wnt proteins and the secretion of Wnt antagonists such as frizzled-related proteins and DKK1.Citation28 In this regard, proteoglycans and glycosaminoglycan chain components may affect Wnt/β-catenin pathway through the interactions with Wnt ligands or inhibitors. Extracellular matrix (ECM) components, such as biglycan, glypican, and heparan sulfate, are able to modulate the activation or the inhibition of Wnt/β-catenin signaling by binding Wnt to the frizzled receptor and its coreceptor LRP6.Citation28 It has been reported that biglycan directly interacts with Wnt3a at C-terminus, which enhances β-catenin/T cell factor mediated transcriptional activity.Citation28 Syndecan-4 was shown to inhibit Wnt/β-catenin signaling through regulation of LRP6 and R-spondin 3.Citation29 The influence of acellular ECMs on β-catenin expression and the response to Wnt activator and inhibitor were demonstrated recently.Citation30 The ECMs derived from PSC-NPCs reduce the expression of β-catenin compared to the ECMs derived from undifferentiated PSCs and spontaneously differentiated EBs. ECM rigidity also affects Wnt signaling through the Rho GTPase signaling ().Citation31 Rho GTPase is a key regulator of intracellular contractility and, thus, allows cells to sense matrix stiffness and respond to mechanical cues. Increased matrix rigidity up-regulates Rho and Rac, which lead to the synthesis of phosphoinositide lipid (PIP2) and the phosphorylation of LRP5/6 coreceptors, and thus indirectly activates canonical Wnt signaling (). Matrix rigidity was also found to influence Wnt signaling by the downregulation of DKK1 protein, which results in elevated membrane type 1 matrix metalloproteinase expression.Citation32 Collectively, these studies demonstrate the important role of ECMs in modulating Wnt signaling.
Figure 2. Illustration of the interactions of extracellular microenvironment (matrix rigidity, geometry etc.) with Wnt/β-catenin signaling through biophysical regulation. Rigid matrix increases the RhoA and Rac which activate phosphatidylinositol 4-phosphate 5-kinase (PIP5K) and phosphatidylinositol 4-kinase (PI4K). These kinases synthesize the phosphoinositide lipid (PIP2) which enhances Wnt signaling. Rigidity also results in elevated membrane type 1 matrix metalloproteinase (MT1-MMP) expression, a transcriptional target of β-catenin/T-cell factor (TCF), and thereby promotes Wnt signaling. Dvl: Dishevelled.
Changes in the spatial localization and phosphorylation state of β-catenin expression can be regulated by the PSC aggregation process through modulating culture systems, e.g., the speed of rotary culture (25–55 rpm) or 3-D microwell culture, which affect the subsequent cardiomyocyte differentiation.Citation15,25 Slower rotary speed (25 rpm) was shown to increase the nuclear accumulation of β-catenin at early time point and the expression of cardiac genes MESP-1 and MEF-2C.Citation25 3-D microwell culture was shown to promote the membrane localization of β-catenin and thus downregulate Wnt signaling. However, the EBs formed from 3-D microwell culture contain more canonical Wnt signaling activity at early stage of differentiation and promote cardiac differentiation of hESCs.Citation15 These studies indicate that modulation of culture systems of PSC aggregates affects Wnt signaling.
THE ROLE OF HIPPO/YAP SIGNALING IN STEM CELL FATE DECISIONS
Hippo/YAP signaling has been found to play a critical role in the effects of substrate mechanics on stem cell propagation and differentiation (). It also affects cell density and organ size control through the interactions with Wnt signaling.Citation33 Hippo pathway promotes cytoplasmic retention of YAP and inhibits nuclear localization of YAP. The downstream YAP activity is the effector in the transcriptional regulation of cellular behaviors of Hippo pathway.
TABLE 3. Summary of the role of YAP in stem cell fate decisions.
Substrate stiffness regulates Hippo/YAP signaling in stem cell differentiation
Using human mesenchymal stem cells (MSCs), stiff ECM (40 kPa) was found to result in cell spreading and active nuclear expression of YAP/transcriptional coactivator with PDZ-binding motif (TAZ) activity, which lead to osteoblast differentiation.Citation34 In contrast, soft substrates (0.7 kPa) inhibit YAP/TAZ and promote adipocyte differentiation. Similarly, stiff surface based on glycosaminoglycan-binding hydrogels was found to promote nuclear localization of YAP, which leads to the self-renewal of hPSCs,Citation35 while compliant surface inhibits nuclear YAP and cannot support the self-renewal of hPSCs. Moreover, activation of YAP can increase reprogramming efficiency and prevent differentiation of PSCs.Citation36
The inhibition of nuclear YAP through the biophysical properties of substrates was found to promote neuronal differentiation from hPSCs. Using polydimethylsiloxane micropost arrays, inhibition of Hippo/YAP signaling (i.e., cytoplasmic YAP expression) on soft surface was observed to accelerate motor neuron differentiation in the presence of soluble neurogenic factors (e.g., higher PAX6+ cells for 5 kPa vs. 1200 kPa: 60–70% vs. 20–30%).Citation37 Similarly, neuronal specification (i.e., GABAergic interneurons) of hPSCs was enhanced in the absence of neurogenic factors on soft substrates (0.7 kPa vs. 3–10 kPa) that inhibited the nuclear localization of YAP.Citation38 While these studies were performed in 2-D cultures, inhibition of nuclear YAP for neural differentiation of hPSCs based on 3-D cultures remains to be revealed.
YAP/TAZ also plays a critical role in the proliferation and differentiation of Sca-1+ adult cardiovascular progenitor cells on substrates with different stiffness.Citation39 Increased stiffness leads to the shuttling of YAP from cytoplasm to nucleus. Nanostructure of the matrix created by thermo-responsive crosslinked polycaprolactone polymers induces cytoplasmic YAP and removing nanopattern on the surface leads to the shift in YAP/TAZ nuclear expression.Citation39 More importantly, YAP silencing correlates to the down-regulation of cardiomyocyte genes and the up-regulation of endothelial-specific genes.Citation39 Consistently, soft matrix leads to cytoplasmic expression of YAP and the ability of the cells to form branching endothelial morphology. Therefore, YAP/TAZ can control the switch between cardiac and endothelial lineages for potential vascularization of neural organoids.
Rho and actin cytoskeleton (stress fibers) are required for the nuclear localization of YAP/TAZ and the mechano-transduction role of YAP/TAZ is found to be related to the interactions between Hippo signaling and the Rho GTPase signaling.Citation40 Stress fibers reduce YAP phosphorylation and promote nuclear YAP accumulation,Citation41 so disruption of stress fibers using cytochalasin D reduces nuclear YAP. Inhibiting myosin II contractility using Blebbistatin or the inhibition of Rho-associated protein kinase (ROCK) signaling using Y27632 to decrease the actomyosin contractility also reduces nuclear YAP.Citation34,39 Therefore, nuclear YAP/TAZ transduces the signals exerted by ECM stiffness and cell morphology to regulate the downstream transcription factors through Rho GTPase activity and the stress fibers.
YAP has been observed to play a central role in “mechanical memory behavior” of stem cells,Citation42 which means that stem cells possess mechanical memory and the past physical environments influence the cell fate. Human MSCs exposed to stiff surface longer (7 days vs. 3 days) have higher tendency to differentiate into osteogenic lineage even when the cells are cultured on soft substrates, appearing to “remember” the stiff environment. In this process, YAP/TAZ acts as the intracellular mechanical rheostat that stores the past information. A threshold mechanical dose can lead to the constitutive expression of nuclear YAP even after the mechanical dose is removed. Given the role of YAP regulation in neural differentiation of hPSCs, it is likely that the mechanical memory behaviors would exist in neural differentiation.
The role of Hippo/YAP pathway in cell density and organ size control
Hippo/YAP pathway plays a critical role in controlling cell density and organ size. At low cell density (cells spread), accumulated nuclear YAP promotes cell proliferation. While at high cell density (cells round up),Citation41 strong Hippo signaling inhibits nuclear YAP (through YAP phosphorylation and cytoplasmic translocation) and suppresses cell proliferation.Citation41 Changes in cell morphology due to different cell density were found to affect YAP localization through stress fibers. In organ growth, when the organ reaches a certain size (i.e., high cell density), the activated Hippo pathway suppresses cell proliferation. The dysregulation of this process leads to abnormal cell growth and cancer. For example, in multiple human cancers, the elevated YAP protein expression and nuclear localization have been observed.Citation11
Cell-cell contact also partially triggers Hippo pathway. It was found that the tight-junction protein complex (consists of PATJ, PALS1/MPDZ and Lin7 proteins) inhibits YAP/TAZ by promoting YAP/TAZ localization to the tight junctions and the YAP/TAZ phosphorylation.Citation11 Disruption of cell-cell junctions in epithelium was found to result in nuclear localization of YAP/TAZ and abnormal growth.Citation43 Therefore, maintaining cell-cell contacts is important for normal Hippo function and organ size control.
RELATIONSHIP OF HIPPO/YAP PATHWAY AND WNT/β-CATENIN PATHWAY
YAP localization regulates Wnt signaling
Hippo pathway promotes cytoplasmic localization of YAP/TAZ and inhibits Wnt signaling through two distinct mechanisms.Citation44 In cell nuclei, YAP and β-catenin can form YAP-β-catenin complex and the activation of YAP due to inhibition of Hippo pathway activates β-catenin-regulated genes (i.e., nuclear YAP promotes Wnt). In cell cytoplasm, cytoplasmic YAP can sequester β-catenin and thus promotes the retention of cytoplasmic β-catenin, which inhibits Wnt signaling (i.e., cytoplasmic YAP inhibits Wnt). α-catenin inhibits YAP activity by forming the trimeric complex of α-catenin, 14-3-3, and YAP, which sequesters YAP in cytoplasm and inhibits YAP nuclear localization.Citation33
The cross-interactions of Hippo pathway and Wnt pathway were found to restrain the proliferation of cardiomyocytes and control heat size.Citation45 Wnt/β-catenin signaling is required for the upregulation of cardiac cell growth. Since nuclear YAP forms complex with β-catenin and promotes β-catenin activity (e.g., upregulating SOX2 and Snail2 genes), Hippo pathway promotes the cytoplasmic retention of YAP and thus inhibits Wnt/β-catenin signaling. Consequently, blocking Hippo pathway was found to generate the oversized heart due to the promoted cell growth.Citation45
Wnt activation regulates YAP localization
It was recently found that Wnt activation leads to the nuclear YAP localization.Citation12 In the absence of Wnt signaling, YAP/TAZ is sequestered to the destruction complex in the cytoplasm, which consists of a central protein named Axin, GSK3, β-catenin, as well as YAP/TAZ. When associated with destruction complex, YAP/TAZ is transcriptionally inactive. This destruction complex is used to degrade β-catenin and also serves as cellular “sink” for YAP/TAZ. The destruction complex interacts with Wnt-activated Fz-LRP6 receptors through Axin/LRP6 interactions. With the activation of Wnt by Wnt3a, progressive dissociation of YAP/TAZ from Axin is observed along with the increased association of Axin with LRP6. In the meanwhile, β-catenin is released from the complex and translocated to the nuclei.
The biological relevance of Wnt activation to regulate YAP localization was demonstrated in the growth of crypt organoids.Citation12 YAP/TAZ was shown as the downstream effector of Wnt activation. Combined deletion of YAP/TAZ blocks the growth of crypt, indicating that YAP and TAZ are required for Wnt-induced response. The biological relevance of Wnt activation to regulate YAP localization is also demonstrated in maintaining undifferentiated mouse ESCs. Cytoplasmic YAP/TAZ inhibits β-catenin which leads to the loss of ESC self-renewal ability and activates differentiation. Loss of YAP/TAZ has the similar effect compared to GSK inhibition (i.e., add CHIR99021) and compensates for the absence of GSK inhibitor. To date, the biological relevance of Wnt activation to regulate YAP localization in neural organoid formation from hPSCs has not been well studied.
An “alternative Wnt-YAP/TAZ signaling axis” was proposed recently, which is independent of canonical Wnt/β-catenin signaling.Citation13 Alternative Wnt ligands Wnt5a/b and other Wnt inhibitors including DKK1, bone morphogenetic protein 4, and insulin like growth factor binding protein 4 were found to be involved in the “alternative Wnt-YAP/TAZ signaling axis.” Treatment on the cells with Wnt5a/b (ligands for non-canonical Wnt signaling) results in the nuclear accumulation of YAP but has no effect on β-catenin. YAP-TAZ was suggested to induce the secretion of Wnt/β-catenin inhibitors (e.g., sFRP1) to inhibit Wnt/β-catenin signaling. In this mechanism, Wnt5a was shown as both an upstream activator and a downstream targeted gene of YAP/TAZ, forming a positive feedback loop between Wnt pathway and YAP/TAZ expression.
Biophysical perturbation of YAP to affect Wnt pathway
Given the bi-directional regulation between Wnt and YAP,Citation46 it is reasonable to hypothesize that biophysical perturbation of YAP protein localization should affect Wnt pathway, and thus neural tissue patterning of hPSCs. Biophysical perturbation of YAP is usually achieved using different small molecules.Citation47 For example, cytochalasin D and latrunculin A are actomyosin cytoskeletal molecules that disrupt F-actin and then inhibit YAP nuclear localization. Blebbistatin is a non-muscle myosin II inhibitor that inhibits YAP nuclear localization. Lysophosphatidic acid, sphingosine-1-phosphate and thrombin activate Rho and actin and thus stimulate YAP nuclear localization. Treating the cells with these small molecules should affect Wnt signaling like Wnt modulators. Biophysical perturbation of YAP can also be achieved by modulating the biophysical microenvironment of the cells,Citation37 such as the stiffness of 3-D substrates, to impact neural organoid formation.
CONCLUSIONS AND PERSPECTIVES
Wnt signaling affects neural tissue patterning of hPSCs by switching rostral and caudal brain tissue identity. Wnt-on condition induces nuclear YAP localization and the caudalization of neural cells. On the other hand, biophysical perturbation of YAP may affect Wnt pathway and thus neural tissue patterning of hPSCs. However, the biological relevance of Wnt-YAP interactions in neural organoid formation from hPSCs remains to be revealed.
There are several approaches to modulate Wnt pathway and Hippo/YAP pathway in order to generate different types of neural organoids from hPSCs. The most straightforward approach is to use soluble Wnt activators or inhibitors, or YAP modulators, which can be presented to the stem cell aggregates at appropriate time windows and locations to generate the 3-D neural organoids from different brain regions (such as forebrain, hippocampus, midbrain, hindbrain etc.). In particular, using the disease-relevance hPSC lines to generate neural organoids should be helpful to understand neurological disease pathology during brain tissue development.Citation48 Since the spatial presentation of the pathway modulators is important, the molecules can be tethered with ECMs or loaded with mciro/nanoparticles to create the in vivo-like gradient of the pathway modulators.
Potential problems and challenges still remain to fully re-create brain-like organoids in vitro. For example, the impact of Wnt signaling is context-dependent. The signaling network of Wnt-YAP with other signaling pathways (such as SHH, RA, fibroblast growth factors) may be required. Moreover, just like any de novo generated organoids, current neural organoids still lack the vascularization structure and fully mimicking specific brain region needs to consider the contributions of many non-neural cell types such as brain microvascular endothelial cells, the supportive brain stromal cells, etc. By better understanding of intracellular signaling pathways and their interactions with extracellular microenvironment, it should be possible in future to increase the complexity and function of hPSC-derived neural organoids.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Funding
We acknowledge grant support from the National Science Foundation (grant No. 1342192) and Florida State University start up fund.
REFERENCES
- Engle SJ, Puppala D. Integrating human pluripotent stem cells into drug development. Cell Stem Cell 2013; 12:669-77; PMID:23746976; http://dx.doi.org/10.1016/j.stem.2013.05.011
- Xu XH, Zhong Z. Disease modeling and drug screening for neurological diseases using human induced pluripotent stem cells. Acta Pharmacol Sin 2013; 34:755-64; PMID:23685955; http://dx.doi.org/10.1038/aps.2013.63
- Yu DX, Marchetto MC, Gage FH. Therapeutic translation of iPSCs for treating neurological disease. Cell Stem Cell 2013; 12:678-88; PMID:23746977; http://dx.doi.org/10.1016/j.stem.2013.05.018
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature 2013; 501:373-9; PMID:23995685; http://dx.doi.org/10.1038/nature12517
- Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 2014;345:1247125; PMID:25035496; http://dx.doi.org/10.1126/science.1247125
- Pasca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park JY, O'Rourke NA, Nguyen KD, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods 2015; 12:671-8; PMID:26005811; http://dx.doi.org/10.1038/nmeth.3415
- Tieng V, Stoppini L, Villy S, Fathi M, Dubois-Dauphin M, Krause KH. Engineering of midbrain organoids containing long-lived dopaminergic neurons. Stem Cells Dev 2014; 23:1535-47; PMID:24576173; http://dx.doi.org/10.1089/scd.2013.0442
- Schwartz MP, Hou Z, Propson NE, Zhang J, Engstrom CJ, Costa VS, Jiang P, Nguyen BK, Bolin JM, Daly W, et al. Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Proc Natl Acad Sci U S A 2015; 112:12516-21; PMID:26392547; http://dx.doi.org/10.1073/pnas.1516645112
- Blauwkamp TA, Nigam S, Ardehali R, Weissman IL, Nusse R. Endogenous wnt signalling in human embryonic stem cells generates an equilibrium of distinct lineage-specified progenitors. Nat Commun 2012; 3:1070; PMID:22990866; http://dx.doi.org/10.1038/ncomms2064
- Suzuki IK, Vanderhaeghen P. Is this a brain which I see before me? Modeling human neural development with pluripotent stem cells. Development 2015; 142:3138-50; PMID:26395142; http://dx.doi.org/10.1242/dev.120568
- Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol 2011; 13:877-83; PMID:21808241; http://dx.doi.org/10.1038/ncb2303
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 2014; 158:157-70; PMID:24976009; http://dx.doi.org/10.1016/j.cell.2014.06.013
- Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, et al. Alternative Wnt aignaling activates YAP/TAZ. Cell 2015; 162:780-94; PMID:26276632; http://dx.doi.org/10.1016/j.cell.2015.07.013
- Davidson KC, Adams AM, Goodson JM, McDonald CE, Potter JC, Berndt JD, Biechele TL, Taylor RJ, Moon RT. Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc Natl Acad Sci U S A 2012; 109:4485-90; PMID:22392999; http://dx.doi.org/10.1073/pnas.1118777109
- Azarin SM, Lian X, Larson EA, Popelka HM, de Pablo JJ, Palecek SP. Modulation of Wnt/β-catenin signaling in human embryonic stem cells using a 3-D microwell array. Biomaterials 2012; 33:2041-9; PMID:22177620; http://dx.doi.org/10.1016/j.biomaterials.2011.11.070
- Ten Berge D, Koole W, Fuerer C, Fish M, Eroglu E, Nusse R. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell 2008; 3:508-18; PMID:18983966; http://dx.doi.org/10.1016/j.stem.2008.09.013
- Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A 2012; 109:E1848-57; PMID:22645348; http://dx.doi.org/10.1073/pnas.1200250109
- Nicoleau C, Varela C, Bonnefond C, Maury Y, Bugi A, Aubry L, Viegas P, Bourgois-Rocha F, Peschanski M, Perrier AL. Embryonic stem cells neural differentiation qualifies the role of Wnt/β-Catenin signals in human telencephalic specification and regionalization. Stem Cells 2013; 31:1763-74; PMID:23818270; http://dx.doi.org/10.1002/stem.1462
- Moya N, Cutts J, Gaasterland T, Willert K, Brafman DA. Endogenous WNT signaling regulates hPSC-derived neural progenitor cell heterogeneity and specifies their regional identity. Stem Cell Reports 2014; 3:1015-28; PMID:25458891; http://dx.doi.org/10.1016/j.stemcr.2014.10.004
- Du ZW, Chen H, Liu H, Lu J, Qian K, Huang CL, Zhong X, Fan F, Zhang SC. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat Commun 2015; 6:6626; PMID:25806427; http://dx.doi.org/10.1038/ncomms7626
- Imaizumi K, Sone T, Ibata K, Fujimori K, Yuzaki M, Akamatsu W, Okano H. Controlling the regional identity of hPSC-derived neurons to uncover neuronal subtype specificity of neurological disease phenotypes. Stem Cell Reports 2015; 5(6):1010-22; PMID:26549851
- Maury Y, Come J, Piskorowski RA, Salah-Mohellibi N, Chevaleyre V, Peschanski M, Martinat C, Nedelec S. Combinatorial analysis of developmental cues efficiently converts human pluripotent stem cells into multiple neuronal subtypes. Nat Biotechnol 2015; 33:89-96; PMID:25383599; http://dx.doi.org/10.1038/nbt.3049
- MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell 2009; 17:9-26; PMID:19619488; http://dx.doi.org/10.1016/j.devcel.2009.06.016
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 2009; 5:100-7; PMID:19125156; http://dx.doi.org/10.1038/nchembio.137
- Kinney MA, Sargent CY, McDevitt TC. Temporal modulation of β-catenin signaling by multicellular aggregation kinetics impacts embryonic stem cell cardiomyogenesis. Stem Cells Dev 2013; 22:2665-77; PMID:23767804; http://dx.doi.org/10.1089/scd.2013.0007
- Li W, Ding S. Small molecules that modulate embryonic stem cell fate and somatic cell reprogramming. Trends Pharmacol Sci 2010; 31:36-45; PMID:19896224; http://dx.doi.org/10.1016/j.tips.2009.10.002
- Lian X, Bao X, Al-Ahmad A, Liu J, Wu Y, Dong W, Dunn KK, Shusta EV, Palecek SP. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Reports 2014; 3:804-16; PMID:25418725; http://dx.doi.org/10.1016/j.stemcr.2014.09.005
- Berendsen AD, Fisher LW, Kilts TM, Owens RT, Robey PG, Gutkind JS, Young MF. Modulation of canonical Wnt signaling by the extracellular matrix component biglycan. Proc Natl Acad Sci U S A 2011; 108:17022-7; PMID:21969569; http://dx.doi.org/10.1073/pnas.1110629108
- Astudillo P, Carrasco H, Larrain J. Syndecan-4 inhibits Wnt/β-catenin signaling through regulation of low-density-lipoprotein receptor-related protein (LRP6) and R-spondin 3. Int J Biochem Cell Biol 2014; 46:103-12; PMID:24275095; http://dx.doi.org/10.1016/j.biocel.2013.11.012
- Yan Y, Martin L, Bosco D, Bundy J, Nowakowski R, Sang QX, Li Y. Differential effects of acellular embryonic matrices on pluripotent stem cell expansion and neural differentiation. Biomaterials 2015; 73:231-42; PMID:26410789; http://dx.doi.org/10.1016/j.biomaterials.2015.09.020
- Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev 2009; 23:265-77; PMID:19204114; http://dx.doi.org/10.1101/gad.1760809
- Barbolina MV, Liu Y, Gurler H, Kim M, Kajdacsy-Balla AA, Rooper L, Shepard J, Weiss M, Shea LD, Penzes P, et al. Matrix rigidity activates Wnt signaling through downregulation of Dickkopf-1 protein. J Biol Chem 2013; 288:141-51; PMID:23152495; http://dx.doi.org/10.1074/jbc.M112.431411
- Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev 2013; 27:355-71; PMID:23431053; http://dx.doi.org/10.1101/gad.210773.112
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature 2011; 474:179-83; PMID:21654799; http://dx.doi.org/10.1038/nature10137
- Musah S, Morin SA, Wrighton PJ, Zwick DB, Jin S, Kiessling LL. Glycosaminoglycan-binding hydrogels enable mechanical control of human pluripotent stem cell self-renewal. ACS Nano 2012; 6:10168-77; PMID:23005914; http://dx.doi.org/10.1021/nn3039148
- Ramos A, Camargo FD. The Hippo signaling pathway and stem cell biology. Trends Cell Biol 2012; 22:339-46; PMID:22658639; http://dx.doi.org/10.1016/j.tcb.2012.04.006
- Sun Y, Yong KM, Villa-Diaz LG, Zhang X, Chen W, Philson R, Weng S, Xu H, Krebsbach PH, Fu J. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat Mater 2014; 13:599-604; PMID:24728461; http://dx.doi.org/10.1038/nmat3945
- Musah S, Wrighton PJ, Zaltsman Y, Zhong X, Zorn S, Parlato MB, Hsiao C, Palecek SP, Chang Q, Murphy WL, et al. Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification. Proc Natl Acad Sci U S A 2014; 111:13805-10; PMID:25201954; http://dx.doi.org/10.1073/pnas.1415330111
- Mosqueira D, Pagliari S, Uto K, Ebara M, Romanazzo S, Escobedo-Lucea C, Nakanishi J, Taniguchi A, Franzese O, Di Nardo P, et al. Hippo pathway effectors control cardiac progenitor cell fate by acting as dynamic sensors of substrate mechanics and nanostructure. ACS Nano 2014; 8:2033-47; PMID:24483337; http://dx.doi.org/10.1021/nn4058984
- Ohgushi M, Minaguchi M, Sasai Y. Rho-signaling-directed YAP/TAZ activity underlies the long-term survival and expansion of human embryonic stem cells. Cell Stem Cell 2015; 17:448-61; PMID:26321201; http://dx.doi.org/10.1016/j.stem.2015.07.009
- Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development 2011; 138:3907-14; PMID:21831922; http://dx.doi.org/10.1242/dev.070987
- Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater 2014; 13:645-52; PMID:24633344; http://dx.doi.org/10.1038/nmat3889
- Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, Wrana JL. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev Cell 2010; 19:831-44; PMID:21145499; http://dx.doi.org/10.1016/j.devcel.2010.11.012
- Guo X, Zhao B. Integration of mechanical and chemical signals by YAP and TAZ transcription coactivators. Cell Biosci 2013; 3:33; PMID:23985334; http://dx.doi.org/10.1186/2045-3701-3-33
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 2011; 332:458-61; PMID:21512031; http://dx.doi.org/10.1126/science.1199010
- Konsavage WM, Jr, Yochum GS. Intersection of Hippo/YAP and Wnt/β-catenin signaling pathways. Acta Biochim Biophys Sin (Shanghai) 2013; 45:71-9; PMID:23027379; http://dx.doi.org/10.1093/abbs/gms084
- Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov 2014; 13:63-79; PMID:24336504; http://dx.doi.org/10.1038/nrd4161
- Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, Amenduni M, Szekely A, Palejev D, Wilson M, et al. FOXG1-dependent dysregulation of GABA/Glutamate neuron differentiation in Autism Spectrum Disorders. Cell 2015; 162:375-90; PMID:26186191; http://dx.doi.org/10.1016/j.cell.2015.06.034
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med 2004; 10:55-63; PMID:14702635; http://dx.doi.org/10.1038/nm979
- Marson A, Foreman R, Chevalier B, Bilodeau S, Kahn M, Young RA, Jaenisch R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell 2008; 3:132-5; PMID:18682236; http://dx.doi.org/10.1016/j.stem.2008.06.019
- Kim H, Wu J, Ye S, Tai CI, Zhou X, Yan H, Li P, Pera M, Ying QL. Modulation of β-catenin function maintains mouse epiblast stem cell and human embryonic stem cell self-renewal. Nat Commun 2013; 4:2403; PMID:23985566
