ABSTRACT
Gonadal differentiation has a determinative influence on sex development in human embryos. Disorders of sexual development (DSD) have been associated with persistent embryonal differentiation stages. Between 1998 and 2015, 139 female patients with various (DSD) underwent operations at the Scientific Center of Obstetrics, Gynaecology and Perynatology in Moscow, Russia. Clinical investigations included karyotyping, ultrasound imaging, hormonal measurement and investigations of gonadal morphology. The male characteristics in the embryo are imposed by testicular hormones. When these are absent or inactive, the fetus may be arrested at between developmental stages, or stay on indifferent stage and become phenotypically female. A systematic analysis of gonadal morphology in DSD patients and a literature review revealed some controversies and led us to formulate a new hypothesis about sex differentiation. Proliferation of the mesonephric system (tubules and corpuscles) in the gonads stimulates the masculinization of gonads to testis. Sustentacular Sertoli cells of the testes are derived from mesonephric excretory tubules, while interstitial Leydig cells are derived from the original mesenchyme of the mesonephros. According of the new hypothesis, the original mesonephric cells (tubules and corpuscles) potentially persist in the ovarian parenchyma. In female gonads, some mesonephric excretory tubules regress and lose the tubular structure, but form ovarian theca interna and externa, becoming analogous to the sustentacular Sertoli cells in the testis. The ovarian interstitial Leydig cells are derived from intertubal mesenchyme of the mesonephros, similar to what occurs in male gonads (testis). Surprisingly, the leading determinative factor in sexual differentiation of the gonads is the mesonephros, represented by the embryonic urinary system.
Epigraph: Sex is correlative concept.
INTRODUCTION
Sex differentiation includes the following distinct sequential stages: genetic, gonadal, hormonal, phenotypic and psychological. At the genetic stage, chromosomal sex is established at fertilization in which XY indicates male and XX denotes female. During the first two months of human gestation, the two sexes develop identically. The gonadal stage is the period during which indifferent gonads develop into either ovaries or testes. The phenotypic stage is induced in response to gonadal differentiation; the internal genital tract and external genitalia develop into characteristic male or female structures.Citation1-4
Gonads appear initially as a pair of longitudinal genital or gonadal ridges at the 4–5th week. Primitive gonads are formed by the proliferation of germ cells, which migrate from the yolk sac and undergo condensation of the underlying mesenchyme in the sixth week. The gonads do not acquire male or female morphological characteristics until the seventh week of development, so they are classified as indifferent.Citation1-4
Mesonephros is primary embryonal kidney, which functions for a short time during the early fetal period (in the fourth week). The mesonephros and mesonephric ducts are derived from intermediate mesoderm. In the 6th week, the mesonephros forms a large ovoid organ on each side of the midline, which is lateral to the gonadal ridges. Laterally, the tubule enters the longitudinal collecting ducts, which are known as the mesonephric ducts. In male embryos, some caudal tubules and the mesonephric ducts persist and participate in the formation of the genital system, while they disappear in females.Citation1-4
Testis
If an embryo is genetically male (46,XY), the indifferent gonad differentiates to testes under influence of the SRY gene on the Y chromosome, which encodes testis-determining factor. The primitive proliferating sex cords penetrate deep into the gonadal medulla to form the medullary cords of the testis. Testis cords are composed of primitive germ cells and sustentacular cells of Sertoli derived from the surface epithelium of the mesonephral ducts.
Interstitial Leydig cells, which are derived from the original mesenchyme of the gonadal ridge, lie between the testis cords.
The mesonephric ducts persist and form the main genital ducts of male embryo. The efferent ductules represent the remaining parts of the mesonephric system excretory tubules. These link the rete testis and mesonephric duct, which become the ductus deferens. The seminiferous tubules join to the rete testis tubules, which in turn enter into the ductuli efferentes.Citation1-4
Ovary
In female embryos with a 46,XX sex chromosome complement, primitive sex cords dissociate into irregular cell clusters. These clusters, which contain groups of primitive germ cells, occupy the medullary part of the ovary. Later, they disappear and are replaced by a vascular stroma that forms the ovarian medulla. These cords split into isolated cell clusters, which each surround one or more primitive germ cells. Germ cells subsequently develop into oogonia, while the surrounding epithelial cells, descendants of the surface epithelium, form follicular cells.Citation1-4
According to Alfred Jost (1976), sex differentiation depends upon testosterone influenced virilization on the mesonephral ducts, urogenital sinus and external genitalia. Jost resolved the controversy surrounding the mechanism of somatic sex differentiation by establishing that male characteristics must be imposed on the fetus by the testicular hormones testosterone and MIS, respectively, which are responsible for the virilization of the mesonephral ducts, urogenital sinus and external genitalia as well as for regression of the Mullerian ducts.Citation4 In the absence or inactivity of these hormones, the development of the fetus is stalled at an indifferent stage; thus, it becomes phenotypically female. By the eighth week of gestation, Leydig cells of the testes begin to produce testosterone and the testes can influence sexual differentiation of the genital ducts and external genitalia. Formation of the external genitalia is completed by the 12th week.Citation1-4
Results
Disorders of sex development (DSD) are congenital conditions in which chromosomal, gonadal, or anatomical sex development is atypical. For a review see refs.Citation5-15
Epidemiological studies have estimated a rate of 2.2 per 10,000 cases with ambiguous genitalia in newborns.Citation16
All DSD patients were classified into three categories that were based on karyotype: with sex chromosome anomalies, with male karyotype 46,XY DSD, and female karyotype 46,XX DSD. For a review see refs.Citation7-14
Gonad removal was performed in female patients with androgen insensitivity syndrome (AIS); 46,XY gonadal dysgenesis, SRY-positive and ovotesticular DSD, to prevent the development of malignancy in adulthood.
Gonadal biopsy allowed for verification of ovarian dysgenesis or streak-gonads in SRY-negative patients with X-monosomy and chimerism. For a review see refs.Citation6-13
Investigations of gonadal morphology revealed patterns that were related to established types of gonadal dysgenesis.
Turner syndrome is caused by partial or complete loss of one of the two X chromosomes or mosaics of 45,X cells with 46,XX cells, 46,XY, or other karyotypes (e.g., cells bearing 46,XdelXp, 46,XiXq). In Turner females, the frequency of mosaicism is estimated to range from 67% to 90%.Citation17
The three fibrous streak types of the gonads distinguished in patients with Turner syndrome.Citation13
Classical fibrous streak-gonads, with a 45,X karyotype. The whitish streak gonads, which histologically contain fibrous tissue, contain no ovarian follicles.
Utero-vaginal aplasia can be detected during laparoscopy. The Mullerian ducts derivates – Fallopian tubes.
The fibrous streak-gonads with occasional ovarian follicles, presenting as ovarian dysgenesis revealed by a 45,X/46,XX mosaic karyotype. The ovaries appear hypoplastic with a higher degree of X-monosomy. In other cases with a prevalence of normal 46,XX cells, they present with an anatomically and functionally normal ovary.
They also present with a hypoplastic normal shaped uterus and vagina.
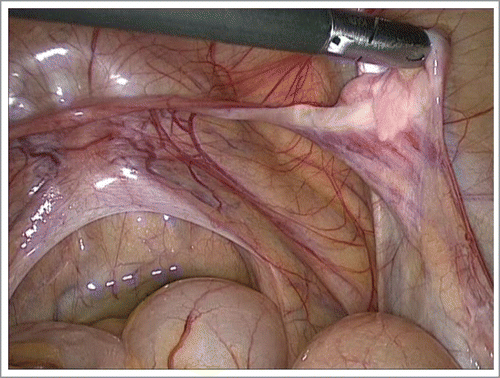
The fibrous streak cord at one gonadal pole and testicular tissue in another, can occur in a patient with 45,X/46,XY mosaicism. The streak pole contains only fibrous tissue without oocytes; the testicular pole contains a dysgenetic structure with anastomosed tubular formations, such as streak-testes.()
FIGURE 1. Patient with 45,X/46,XY mosaicism, laparoscopy. The right gonad contains a whitish fibrous streak cord and dysgenetic testicular structure. Persistent Mullerian ducts derivates detected: Fallopian tube, rudimental uterus.
In cases in which oocytes are present, the patient is defined as ovotesticular DSD.
These patients had persistent Mullerian ducts derivates (PMDS – persistent Mullerian syndrome): Fallopian tubes and uterine rudiments.
Normally, the SRY gene encodes the sex-determining region Y-protein, which is a transcription factor of the testis-determining protein family that initiates male sex determination. Mutation of SRY prevents testes differentiation, resulting gonadal dysgenesis.Citation4,12,15
Patients with 46,XY testicle dysgenesis are characterized by a reduced tubular diameter and an increased presence of Sertoli cells in both gonads.
Patients with the complete form without virilization of the external genitalia (i.e., completely feminine) had a normal shaped hypoplastic uterus, Fallopian tubes and vagina.()
In the incomplete form, patients are characterized by ambiguous genitalia resulting from incomplete masculinization – clitoromegaly and urogenital sinus (Prader III–V degrees); and the persistence of Mullerian structures (hypoplastic uterus, or rudimental uterine horns and Fallopian tubes).
Adrenal insensitivity syndrome (AIS) is most commonly caused by mutations of the androgen receptor gene and may be either partial or complete. The testes of these individuals produce Mullerian inhibitory substance (MIS) and consequently the uterus, Fallopian tubes and vagina are absent.Citation15
FIGURE 2. 46,XY gonadal dysgenesis with PMDS, laparoscopy. Testicle dysgenesis, Fallopian tubes, a normal shaped hypoplastic uterus and vagina. A. 46,XY gonadal dysgenesis with PMDS. Testicle dysgenesis with only Fallopian tube on the right side.
Patients with androgen insensitivity syndrome (AIS) exhibit testicular dysgenesis, which is histologically composed of small seminiferous tubules with a reduced diameter, immature Sertoli cells that are immature, lack a central lumen, and reach the Leydig cells.
In androgen insensitivity syndrome (AIS), depending on the residual activity of the androgen receptor, patients exhibit feminine external genitalia in a complete form, or ambiguous genitalia as a consequence of incomplete masculinization (in the incomplete form).
The ovotesticular gonads present with both testicular and ovarian tissues or as separate gonads. In the bilateral ovotestis, the testicular and ovarian components are adjacent to each other in an end-to-end manner.
Most ovotesticular DSD patients () had a 46,XY karyotype, although some patients had a 46,XX karyotype or exhibited a Y-to X chromosomal translocation and chimerism (i.e., the presence of two or more cell lines derived from different zygotes).
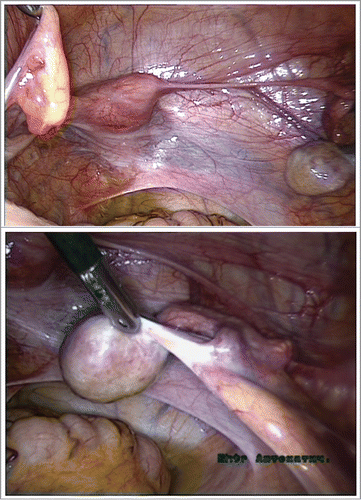
A unique clinical case of ovotesticular DSD with rare form of chimerism was detected in a 1-year-old patient with a 46,XX/46,XY karyotype (). Both gonads presented as ovotestis in which the gonad on the left side () was associated with the Fallopian tube, while on the right side () it was associated with the ductus seminiferous alone. The persistent Mullerian ducts could be detected in the crossing area between the Fallopian tube (on the left) and ductus seminiferous (on the right) with gonadal ridges.
FIGURE 3. (a, b). A unique clinical case of ovotesticular DSD with rare form of 46,XX/46,XY chimerism was detected in a 1-year-old patient. Laparoscopically, the both gonads were ambiguous. Figure 3A. The left ovotestis: predominated ovarian (O) tissue (yellow), with whitish testicular structure on the upper pole, associated with the Fallopian tube (F) only. Figure 3B. The right ovotestis: predominated testicular tissue (T), with small ovarian structure (yellow) on the upper pole (O), associated with the ductus seminiferous (DS) alone. The ovarian (O) part seems like appendix testis.
Persistent Mullerian duct syndrome (PMDS) is a rare form of DSD, in which Mullerian ducts derivatives occur in genotypic 46,XY patients (). The internal genitalia in PMDS patients presents as uterine rudiments or hypoplastic uterine horns and Fallopian tubes. The uterine rudiments in all cases could be detected in the crossing area of the Fallopian tubes with gonadal ridges. The external genitalia were ambiguous because of variable degrees of virilization.
PMDS may result from Sertoli cell-specific dysfunction resulting from mutations in the MIS gene with normal fetal Leydig and Sertoli cell function.
Defective androgen action is associated with female or ambiguous genitalia.
Importantly, in PMDS patients with a 46,XY karyotype and testicular gonads, the Fallopian tubes or ductus deferens persisted separately on the unilateral or bilateral sides. In none of these cases did both types of ducts persist on either side.
Based on these findings, we propose a novel hypothesis which predicts that the Fallopian tubes, similarly to the seminiferous ducts, are analogous and develop from mesonephric ducts.
Conclusions
Systematic clinical analysis and a literature review revealed the following controversies and questions:
What is the embryonal pathogenesis of ambiguous gonads - ovotestis?
Why do most ovotesticular patients have a 46,XY karyotype?
Why do SRY-negative ovotesticular patients with a 46,XX karyotype have testicular components in their gonads?
In 46,XX-male syndrome, why do the SRY-negative patients have testes?
How do some 46,XY DSD patients develop Mullerian ducts derivatives, including Fallopian tubes, uterine rudiments or a hypoplastic uterus and vagina?
A new hypothesis to describe gonadal embryogenesis (, )
FIGURE 4. Gonadal Sex Differentiation: A new hypothesis. Schematic representation of gonadal differentiation from the genital ridge (GR – blue), mesonephros (Ms – red), and mesonephric duct (MD – red). Figure 4A. The indifferent gonad and mesonephros. The gonadal ridge (GR-blue) grows longitudinally. The paramedial side the gonad connects with the mesonephros (Ms). The mesonephric tubules progressively surround the germ cells in gonadal ridge to form the Sertoli cells. The cranial tubules and glomeruli of the mesonephros (dashed horizontal red lines)degenerate and partially disappear. The cranial part of the indifferent gonad becomes ovarian after regression of the mesonephric tubules. In the caudal section, the mesonephric ducts persist (solid horizontal red lines) and participate in the formation of testicular tissue (ductuli semynipherous, rete testis and ductuli efferentes). The mesonephric duct (MD) grows on the lateral side of the mesonephros (Ms), then crosses medially to the gonadal ridge. The area of intersection between the mesonephric duct and the gonadal ridge develops into the uterine fold (U) in females and the prostatic utricle in males. The intermediate stage of gonadal differentiation consists of ovarian (Ov) and testicular (T) tissue, which corresponds to ovotestis. Figure 4B. Female gonad differentiation: SRY-negative, ovary. In the female gonads, germ cells (from gonadal ridge) become oocytes and granulosa cells. Some mesonephric excretory tubules regress, and some lose their tubular structure, but most persist as Sertoli cells and form theca interna and externa, which surround oocytes in primordial follicles. In the female embryo, the upper part of the gonadal ridge (before intersecting with the mesonephric ducts) form ovary and ligamentum ovary; the lower part represents the ligamentum teres uteri. While most mesonephric tubules recede, the mesonephric ducts (MD) become Fallopian tubes. Figure 4C. Male gonad differentiation: SRY-positive, testis. In the male gonads, germ cells become spermatozoids. Mesonephric tubules surround the germ cells in the ductus seminiferous and form Sertoli cells. While most mesonephric tubules persist, the mesonephric ducts become ductus deferens. The Leydig cells are derived from mesonephric intertubular mesenchyme in both gonads. The Fallopian tubes (in females) and the seminiferous duct (in males) are analogous, and both develop from the mesonephral ducts.
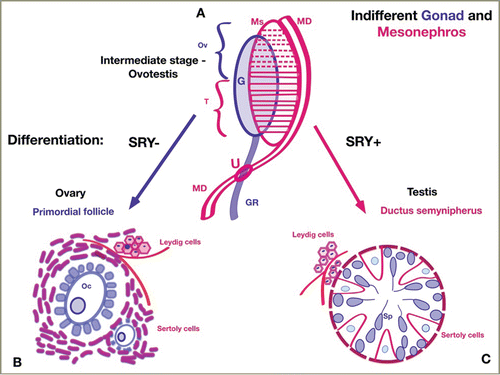
TABLE 1. Gonadal differentiation: key differences between the new hypothesis and contemporary view.
The indifferent gonad () is differentiating into an ovary () or testis () because of its chromosomal sex, which is either 46,XX (SRY-negative) or 46,XY (SRY-positive), respectively.
Male or female pathways of gonadal development are different, but share some common features.
Male gonadal development (testis) ()
The primitive sex cords are proliferating and penetrate deep into the gonadal medulla to form the lobuli of the testis.
The major determinative factor for the testes is SRY-dependent influences of the mesonephric system (mesonephric tubules and corpuscles) on gonadal masculinization.
Mesonephric derivatives persist and form the main genital ducts of the male embryo.
Excretory tubules of the mesonephric system form the seminiferous tubules, which join to the rete testis and develop into the ductuli deferentes. Sustentacular cells of Sertoli are derived from the mesonephric excretory tubules.
The interstitial Leydig cells are derived from the original mesenchyme of the mesonephros.
The mesonephric tubules have inductive influences on the primordial gonads (germ cells) to drive the development of the indifferent gonad into testis.
If the mesonephric tubules recede, the gonads will not differentiate and the indifferent gonads will remain as an ovary.
Female gonadal development (ovary) ()
In contrast to the most prevalent current opinion, the original mesonephric cells (tubules and corpuscles) persist in the ovarian parenchyma.
In the female gonads, some mesonephric excretory tubules recede and then lose their tubular structure; however, most of them persist and form ovarian theca interna and externa. The theca interna and externa support the processes of oocyte proliferation, maturity and ovulation, similarly to the Sertoli cells in the male gonads.
Hence, ovarian theca interna and externa are analogous to the sustentacular Sertoli cells in the testis.
The interstitial Leydig cells in the ovary are derived from the intertubal mesenchyme of the mesonephros, similar to the male gonad (testis).
Reduction of the mesonephric tubular system in the gonads results in female gonadal development. After regression of mesonephric tubules occurs, the mesonephric ducts persist and develop into Fallopian tubes with cranial opening of fimbrial orifice.
The Fallopian tubes and seminiferous ducts are analogs, as they both develop from the mesonephric ducts (in female or male embryos, respectively), which can account for the etiology of persistent Mullerian ducts derivate in 46,XY DSD patients.
The uterus (uterine fold) develops in the area of intersection between the mesonephral ducts and the gonadal ridge.
The major determinative factor in the sexual differentiation of gonads is the mesonephros, represented by the embryonic kidney (provisory urinary system). The excretory ducts of both males (ductus deferens) and females (Fallopian tubes) develop from the mesonephric ducts.
The ovotestis forms during the intermediate stage of normal testis embryogenesis, while the gonads presented with both testicular and ovarian tissue.
Alfred Jost showed that testicular organization is marked by the development of pre-Sertoli cells, which progressively surround germ cells to form seminiferous tubules in the testes only.Citation4,18
Ditewig, Yao (2005) consider the process of ovary organogenesis as the default organ, which develops in the absence of testis-promoting factors. Bisexual development of the gonads is a universal phenomenon in vertebrates.Citation19
Based on our new hypothesis, the interstitial Leydig cells can differentiate into both gonads (testis and ovary) from the intertubal mesenchyme of the mesonephros.
The cranial tubules and glomeruli of the mesonephros degenerate and partially disappear. In the intermediate stage, the gonad exhibits bipotential: the cranial part of the indifferent gonad becomes ovarian after regression of the mesonephric tubules, while in the caudal part the mesonephric ducts persist and participate in forming the testicular tissue.
Consequently, the ovotestis progresses into the intermediate stage of normal testis embryogenesis, in which the gonads are present with both testicular and ovarian tissue.
By contrast, most ovotesticular DSD patients have a 46,XY karyotype (or SRY-positive).
While most mesonephric tubules reduced, the mesonephric ducts become the Fallopian tubes. The excretory ducts of both males and females develop from mesonephric ducts. Therefore, the Fallopian tubes and seminiferous ducts can be considered to be analogues, and both develop from the mesonephric ducts (in female or male embryos, respectively), which could explain the phenomenon of Mullerian duct persistence in genetically 46,XY patients who have testicular dysgenesis.
Over 150 cases have been reported in which SRY-negative 46,XX testicular DSD patients present with small testes and androgen deficiency, and in some cases with ambiguous genitalia.Citation12,13,15
Surprisingly, the leading determinative factor in sexual differentiation of the gonads is the mesonephros, represented by the embryonic kidney (urinary system).
Embryogenesis in the human urogenital system is intimately interwoven with sex differentiation. Proliferation of the mesonephric system (tubules and corpuscles) in the gonads stimulates masculinization of the gonads into testis. Reduction of the mesonephric tubular system in the gonads results in female gonad development.
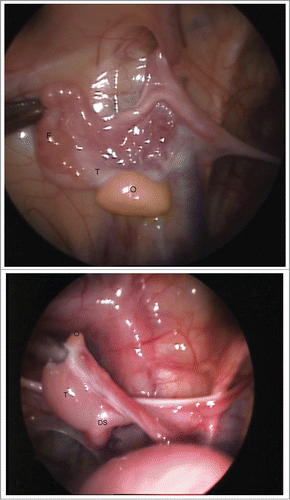
The intermediate locus between the gonadal ridge and mesonephros becomes the mesosalpyngs in female embryos. The paraovarian cysts in mesosalpyngs are derived from mesonephric tubules.
The vestigial structures after regression of mesonephros are persisting in embryo. Probably, the epoophoron and paroophoron are remnant of a few scattered rudimentary mesonephric tubules situated in the mesosalpings in females. The regressed upper pole of gonadal ridge with mesonephric tubules in male embryo may become the rudimental appendix testis ().
If we accept a mesonephric origin of the Fallopian tubes, we can account for the determinative influence of the Fallopian tubes in the ovarian cancer.
Future embryological studies of sex differentiation will of great importance to test the applicability of this new hypothesis.
MATERIALS AND METHODS
Between 1998 and 2015, 139 female patients with various disorders of sexual development (DSD) underwent operations at the Scientific Center of Obstetrics, Gynecology and Perynatology in Moscow, Russia ().
TABLE 2. The 139 patients with various disorders of sex development (DSD).
Management and surgical correction were performed according to the Guidelines of European Consensus Statement in DSD, 2006, 2010, and 2011. For a review see refs.Citation5-11
Clinical assessments included karyotyping, ultrasound imaging, hormone measurements and investigations of gonadal morphology.
All individuals received a female gender assignment after clinical investigations.
Surgical correction for female patients with DSD included laparoscopic gonadal biopsy (46,XX; 45,X/46,XX) or adnexectomy in XY karyotype patients.Citation13
Feminizing clitoroplasty was performed for patients with ambiguous genitalia (in severe III–IV degrees of virilization by Prader).Citation14
Females with AIS and classic Turner syndrome underwent creation of a neovagina.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- Sadler TW. “Langman's Medical Embryology.” IX-th edn, Baltimore:Lippincott Williams&Wilkins 2000; 230-256
- Sadler TW. “Langman's Medical Embryology.” XII-th edn, Baltimore:Lippincott Williams&Wilkins, 2012; 232-259
- Hill MA. Embryology BGD Lecture - Sexual Differentiation. Retrieved October 16, 2014. Available from URL: http://php.med.unsw.edu.au/embryology/index.php?title=BGD_Lecture_Sexual_Differentiation
- Jost A. A new look at the mechanism controlling seх differentiation in mammals. Johns Hopkins Med J 1972; 130:28-36
- Hughes IA, Houk C, Ahmed SF, et al. Consensus statement on management of intersex disorders. Archives of Disease in Childhood 2006; 91:554-63; PMID:16624884; http://dx.doi.org/10.1136/adc.2006.098319
- Brain CE, Creighton SM, Mushtaq I. et al. Holistic management of DSD. Best Practice and Research. Clin Endocrinol Metab 2010; 24:335-54
- Pasterski V, Prentice P, Hughes IA. Consequences of the Chicago consensus on disorders of sex development (DSD): current practices in Europe. Arch Dis Childhood 2010; 95:618-23; http://dx.doi.org/10.1136/adc.2009.163840
- Houk CP, Lee PA. Update on disorders of sex development. Curr Opin Endocrinol Diabetes Obes 2012; 19:28-32; PMID:22157406; http://dx.doi.org/10.1097/MED.0b013e32834edacb
- Ahmed F, Achermann J, Arlt W, et al. UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development. Clin Endocrinol (Oxf) 2011 July; 75(1):12-26; PMID:21521344; http://dx.doi.org/10.1111/j.1365-2265.2011.04076.x
- Achermann JC, Hughes IA, Disorders of sex development. In: Kronenberg HM, Melmed S, Polonsky KS, Larsen PR (eds) Williams Textbook of Endocrinology 11th edition, Philadelphia; 2008; 783-848
- Grumbach MM, Hughes IA, Conte FA. Disorders of sex differentiation. In: Williams Textbook of Endocrinology. 10th ed. Eds. Larsen PR, Kronenberg HM, Melmed S, Polonsky KS. Heidelberg (Germany): Saunders 2003; 842-1002
- Chemes H, Muzulin PM, Venara MC, Mulhmann M, del C, Martinez M, Gamboni M. Early manifestations of testicular dysgenesis in children: pathological phenotypes, karyotype correlations and precursor stages of tumor development. APMIS 2003; 111(1):12-23; PMID:12760349; http://dx.doi.org/10.1034/j.1600-0463.2003.1110104.x
- Nistal M, García-Fernández E, Mariño-Enríquez A, et al. Usefulness of gonadal biopsy in the diagnosis of sexual developmental disorders. Actas Urol Esp 2007; 31(9):1056-75; PMID:18257373; http://dx.doi.org/10.1016/S0210-4806(07)73767-1
- Prader A, Gartner HP. The syndrome of male pseudohermaphroditism in adrenocortical hyperplasia without overproduction of androgens. Helv Pediatric Acta 1955; 10:397-412
- Nathalie J, Rodolfo Rey, Jean-Yves Picard. Testicular Anti-Müllerian Hormone: Clinical Applications in DSD. Semin Reprod Med 2012; 30(05): 364-73; PMID:23044872; http://dx.doi.org/10.1055/s-0032-1324719
- Thyen U, Lanz K, Holterhus PM, Hiort O. Epidemiology and initial management of ambiguous genitalia at birth in Germany. Horm Res 2006; 66(4):195-203
- Bernard C. Turner syndrome and the evolution of human sexual dimorphism. Evol Appl 2008 August; 1(3):449-61
- Josso N. Professor Alfred Jost: the builder of modern sex differentiation. Sex Dev 2008; 2(2):55-63; PMID:18577872; http://dx.doi.org/10.1159/000129690
- Ditewig AC, Yao HH. Organogenesis of the ovary: a comparative review on vertebrate ovary formation. Organogenesis 2005 Apr; 2(2):36-41; PMID:19521565; http://dx.doi.org/10.4161/org.2.2.2491
